Abstract
Aim
During type 1 diabetes (T1D), the medullary thick ascending limb (mTAL) displays an NADPH oxidase-dependent increase in sodium transport, in concert with increased NO production by NO synthase 1 (NOS1) and NOS2. We hypothesized that NOS1- and/or NOS2-derived NO blunts T1D-induced activation of sodium transport in the mTAL.
Methods
T1D was induced by streptozotocin injection (STZ rats); sham rats received vehicle. Three-to-four weeks later, mTAL were isolated from both groups for assay of nitrite and superoxide production, and O2 consumption in the absence or presence of various inhibitors.
Results
Apocynin (NADPH oxidase inhibitor) normalized superoxide production and ouabainand furosemide-sensitive O2 consumption by mTALs from STZ rats, without altering O2 consumption by mTALs from sham rats. Apocynin also unmasked a T1D-induced increase in nitrite production. NOS inhibition did not alter superoxide production in either group. In sham mTAL, total NOS inhibition, but not isoform-specific inhibition of NOS1 or NOS2, increased ouabain- and furosemide-sensitive O2 consumption, confirming a tonic inhibitory impact of NOS3 on sodium transport. In contrast, neither total nor isoform-specific NOS inhibition altered O2 consumption by STZ mTAL. Apocynin-treatment of STZ mTAL unveiled the ability of isoform-specific NOS inhibition to significantly increase O2 consumption, without further increase in O2 consumption with total NOS inhibition.
Conclusion
Under normal conditions, NOS3-derived NO inhibits sodium transport in the mTAL. T1D dismantles the impact of NOS-mediated inhibition of sodium transport as a result of NADPH oxidase-dependent NO scavenging. Inhibition of NADPH oxidase to preserve NO bioavailability reveals an inhibitory impact of NOS1- and NOS2-derived NO on sodium transport in the mTAL.
Keywords: Streptozotocin-induced diabetes, nitric oxide, nitric oxide synthase, superoxide, NADPH oxidase, sodium transport
INTRODUCTION
In patients with uncomplicated, moderately hyperglycemic type 1 diabetes mellitus (T1D), sodium balance is altered resulting in net sodium retention that is evidenced by increases in both extracellular fluid volume and exchangeable sodium (Nørgaard & Feldt-Rasmussen 1994, Feldt-Rasmussen et al. 1987, O'Hare et al. 1986, Roland et al. 1986). Rats with streptozotocin-induced T1D are not able to excrete sodium to the level sufficient to match dietary sodium intake (DiPetrillo et al. 2003), nor can they efficiently excrete an acute saline load (Patel 1997). T1D enhances the activity and protein expression of several renal sodium transporters (Wald et al. 1993, Ku et al. 1986, Khadouri et al. 1987). Moreover, both absolute and fractional reabsorption of sodium by the loop of Henle are increased during the early hyperfiltration stage of T1D, independent of sodium-glucose cotransport and osmotic diuresis (Pollock et al. 1991). A role for altered sodium balance as a contributing factor in the pathogenesis of diabetic nephropathy is indicated by the effect of dietary sodium restriction to reduce renal hypertrophy, hyperfiltration and albuminuria in an animal model of T1D (Allen et al. 1997).
The renal medullary thick ascending limb (mTAL) plays a key role in regulating sodium balance. This region of the nephron participates in the fine-tuned regulation of tubule fluid solute concentration by reabsorbing up to 30% of the filtered load of sodium (Burg 1982). Sodium transport in the mTAL is controlled, in part, by the balance of nitric oxide (NO) and superoxide anion, in such a manner as to maintain sodium homeostasis. Under normal physiological conditions, NO can directly inhibit sodium transport in the mTAL (Plato et al. 1999), which expresses all three NO synthase (NOS) isoforms. Using NOS isoform-specific knockout mice, Plato et al. (2000) provided evidence that NOS3-derived NO is responsible for inhibition of chloride reabsorption in the mTAL, an effect mediated by inhibition of the furosemide-sensitive Na+-K+-2Cl− cotransporter (NKCC2) (Ortiz et al. 2001). Conversely, superoxide anion promotes sodium chloride reabsorption in isolated perfused mTAL (Ortiz & Garvin 2002b).
Despite the available literature on the role of NO and superoxide in the control of sodium reabsorption by the mTAL under normal conditions, little information is available regarding the impact of T1D-induced alterations in NO and/or superoxide production on sodium transport by the mTAL. NOS1- and NOS2-derived NO production, as well as NADPH oxidase-derived superoxide production, are increased in the mTAL during T1D (Foster et al. 2009, Yang et al. 2010). Although the balance of NO and superoxide in the mTAL is altered during T1D, the functional interaction of these two physiologically opposing effects on Na transport during T1D has not been clearly defined. In light of our recent report that NADPH oxidase-derived superoxide mediates increased sodium transport in the mTAL during T1D (Yang et al. 2012), we hypothesized that NOS1- and/or NOS2-mediated NO production blunts the T1D-induced activation of sodium transport in the mTAL. Accordingly, the aims of this study were to 1) verify that endogenous NOS3 activity results in tonic inhibition of sodium reabsorption in the rat mTAL under normal physiological conditions, 2) determine if NOS activity in the mTAL from T1D rats produces superoxide, and 3) elucidate whether or not NOS activation in T1D exerts an inhibitory impact on sodium transport in mTAL and, if so, establish the NOS isoform(s) involved.
MATERIALS AND METHODS
Animal Model
All animal studies were approved by the Institutional Animal Care and Use Committee at the Georgia Regents University or the University of Nebraska Medical Center, and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (250 g body wt, Harlan Laboratories) were randomly divided into two groups: sham (vehicle treatment) and STZ (streptozotocin-induced type 1 diabetes). On the first day of the study, the rats were weighed, initial blood glucose measurements were obtained via a small nick in the tail (Accu-Check III model 766; Boehringer Mannheim, Indianapolis, IN) and, under isoflurane anesthesia (2% in oxygen at 1 L min−1), either STZ (65 mg kg−1; Sigma, St. Louis, MO) or vehicle was injected intravenously. One day later, after ensuring hyperglycemia in the STZ group, the animals were again anesthetized with isoflurane for subcutaneous placement of sustained-release insulin implants (Linplant; Linshin Canada, Scarborough, Ontario) were subcutaneously placed in order to maintain moderate hyperglycemia (400-500 mg dl−1, unfasted) for the length of the study. Sham animals received palmitic acid vehicle implants. Blood glucose levels and body weights were measured approximately every 3 days. Terminal studies were performed 3-4 wk after STZ or vehicle injection, as previously described (Foster et al. 2009).
mTAL suspensions
. mTAL suspensions were prepared from anesthetized rats according to the method originally described by Chamberlin et al. (1984), with modifications as described previously (Foster et al. 2009; Yang et al. 2009). Freshly isolated mTAL from sham and STZ rats were suspended in HBSS containing 5 or 20 mM glucose, respectively, and incubated in the presence/absence of various inhibitors prior to measurement of superoxide production, nitrite production or O2 consumption.
Superoxide production
Lucigenin chemiluminescence was utilized as a measure of intracellular and extracellular superoxide production. mTAL suspensions were incubated with lucigenin (5 μM in HEPES-buffered media) in the presence and absence of 100 μM apocynin (NADPH oxidase inhibitor, Sigma) or 250 μM l-NAME (total NOS inhibitor, Sigma) for 30 min at 37 °C as previously described (Yang et al. 2009). Blank corrected relative light units (RLU) sec−1 averaged during the final 90 sec of each 5 min measurement sequence were used to quantify superoxide production. Final values were normalized to total protein concentration.
Nitrite production
After filtering and briefly centrifuging, the mTAL suspensions were incubated with 250 μM l-arginine (NOS substrate; Sigma) in the presence or absence of 100 μM apocynin for 30 min at 37 °C. mTAL were pelleted and the incubation buffer was collected for determining nitrite production. The concentration of nitrite, a metabolite of NO, in the incubation buffer was measured with a dedicated HPLC system (ENO-20; Eicom, Kyoto, Japan), as previously described (Foster et al. 2009). Nitrite was normalized to total protein concentration.
Oxygen consumption
O2 consumption was measured using the BD™ Oxygen Biosensor System (OBS method) as previously described (Yang et al. 2012). This alternative method to the Clark electrode allows for high-throughput analysis of O2 consumption (Guarino et al. 2004, Wang et al. 2005). The OBS method employs an O2-sensitive fluorescent dye, Tris (4,7-diphenyl-1,10-phenanthroline) ruthenium (II) chloride, embedded in a gas-permeable silicon polymer matrix affixed to each well bottom of a 96-well microplate (Wodnicka et al. 2000). O2 quenches the fluoroprobe; hence, as the mTAL consume O2, thereby decreasing O2 levels in the well, fluorescence emission increases. The microplate wells were pre-loaded with vehicle or inhibitors, equilibrated to 37 °C, and mTAL added (15 μg protein per well). Final concentrations of inhibitors were as follows: 2 mM ouabain (Na+-K+-ATPase inhibitor, Sigma), 500 μM furosemide (NKCC2 inhibitor, Sigma), 100 μM apocynin, 1 μM N5-(1-imino-3-butenyl)-l-ornithine (VNIO; NOS1 inhibitor; Alexis), and either 100 nM or 100 μM N-[[3-(aminomethyl)phenyl]-methyl]-ethanimidamide dihydrochloride (1400W; Cayman). At a concentration of 100 nM, 1400W is a highly selective inhibitor of NOS2 (Ki = 7 nM) versus NOS1 or NOS3 (Ki = 2 or 50 μM, respectively), while 100 μM 1400W inhibits all three NOS isoforms (Thomsen et al. 1997). The OBS plate was read (440 nm excitation; 620 nm emission at 37 °C) in kinetic mode every 2 min for 90 min on a Molecular Devices Spectramax M5 spectrophotometer. A 2-step normalization to ambient air was applied to the fluorescence intensity data for each sample, as detailed previously (Yang et al. 2012). The resulting dimensionless normalized relative fluorescence (NRF) values were used to quantify O2 consumption based on the linear change in NRF evident during the 30-to-50-min time frame (e.g. after a 30 min pretreatment), expressed as △NRF min−1 mg protein−1. Inter-assay variability was less than 15%. Ouabain- and furosemide-sensitive O2 consumption were considered to reflect Na+-K+-ATPase (NKA)- and NKCC2-dependent sodium transport, respectively. None of the inhibitors listed above altered the fluorescence generated by the positive control (100 mM sodium sulfite), indicating that the compounds are specific for their intended use. However, 250 μM l-NAME substantially interfered with the fluorescent signal generated by the positive control (data not shown), which precluded its use in the OBS assay. Therefore, 100 μM 1400W was utilized in experiments to inhibit all NOS isoforms in the O2 consumption measurements. Tempol actively quenches fluorescence through its actions as a nitroxide (Matko 1992), and preliminary experiments confirmed that it interfered with the OBS assay for measurement of O2 consumption (data not shown).
Statistics
All values are reported as means ± SE. Statistical comparisons were performed using an unpaired t-test or one-way ANOVA followed by the Newman-Keuls Multiple Comparison Test. Values of P < 0.05 were considered significant.
RESULTS
The STZ model of T1D was utilized in this study. During the 3-4 wk period after injection of STZ or vehicle, blood glucose levels were significantly higher in STZ rats (452 ± 13 mg dl−1) than in sham rats (96 ± 4 mg dl−1). While rats in both groups gained weight over the course of the study, the STZ rats gained significantly less weight than the sham rats. At the time of the terminal study, body weights of STZ and Sham rats averaged 274 ± 8 and 309 ± 9 g, respectively.
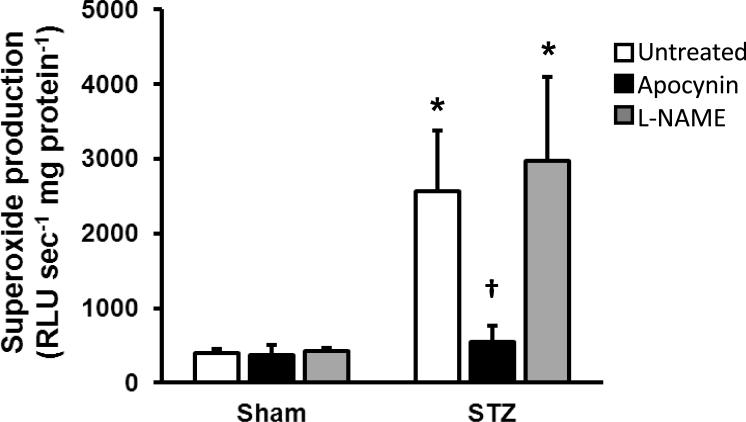
Superoxide production by mTAL from STZ rats was approximately 4-times greater than sham mTAL (Fig. 1). The potential role of uncoupled NOS as a source of superoxide during T1D was explored based on the effect of 250 μM l-NAME; however, this treatment did not alter superoxide production by either sham or STZ mTAL. In contrast, incubation of mTAL from STZ rats with 100 μM apocynin reduced superoxide production to levels that did not differ from sham mTAL in the absence/presence of apocynin (Fig. 1). Moreover, after 30 min mTAL incubation in the presence of exogenous l-arginine, nitrite accumulation in the incubation buffer averaged 53.8 ± 3.7 pmol nitrite mg protein−1 for untreated STZ mTAL. Apocynin significantly increased nitrite accumulation in the incubation buffer (STZ+apocynin: 128.7 ± 15.4 pmol nitrite mg protein−1; P < 0.05), indicating an increase in NO bioavailability during NADPH oxidase inhibition.
Fig 1.
Effects of total NOS inhibition (250 μM l-NAME) and NADPH oxidase inhibition (100 μM apocynin) on superoxide production by mTAL from sham and STZ rats. Values are mean ± SE (n = 5-8 rats). *P < 0.05 vs. sham untreated, †P < 0.05 vs. STZ + L-NAME.
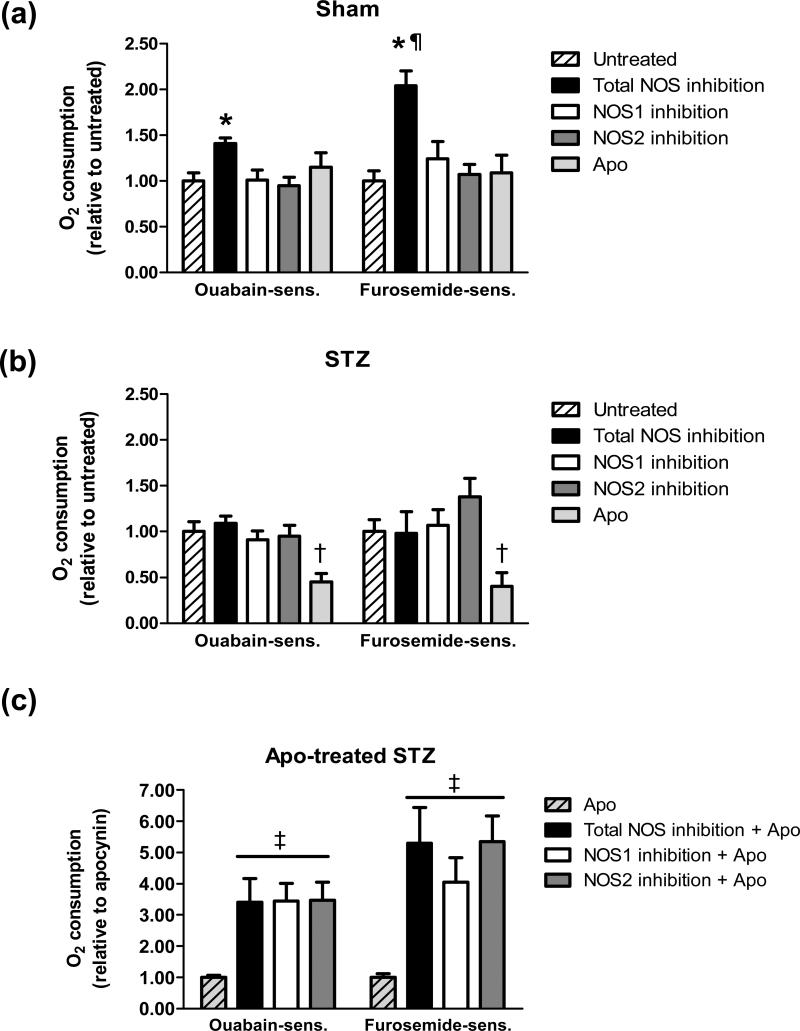
Similar to our previous report (Yang et al. 2012), total, ouabain-sensitive, and furosemide-sensitive O2 consumption by mTAL from STZ rats was significantly greater than that of mTAL from sham rats (total O2 consumption: sham, 0.17 ± 0.04 △NRF min−1 mg protein−1; STZ, 0.35 ± 0.05 △NRF min−1 mg protein−1; ouabain-sensitive O2 consumption: sham, 0.08 ± 0.02 △NRF min−1 mg protein−1; STZ, 0.22 ± 0.04 △NRF min−1 mg protein−1; and, furosemide-sensitive O2 consumption: sham, 0.05 ± 0.02 △NRF min−1 mg protein−1; STZ, 0.17 ± 0.03 △NRF min−1 mg protein−1; each P < 0.05 STZ vs sham). To evaluate the effects of various NOS and NADPH oxidase inhibitors on O2 consumption in Sham and STZ mTAL, the data were normalized to untreated baseline values for presentation in Figure 2.
Fig 2.
(a) Effect of NOS inhibition or NADPH oxidase inhibition on O2 consumption by mTAL from sham rats. Values are normalized to sham untreated (ouabain-sensitive: 0.12 ± 0.01 △NRF min−1 mg protein−1; furosemide-sensitive: 0.10 ± 0.01 △NRF min−1 mg protein−1) and expressed as mean ± SE (n = 6 rats). *P < 0.05 vs. sham untreated; ¶ P < 0.05 vs. ouabain-sensitive. (b) Effect of NOS inhibition or NADPH oxidase inhibition on O2 consumption by mTAL from STZ rats. Values are normalized to STZ untreated (ouabain-sensitive: 0.16 ± 0.02 △NRF min−1 mg protein−1; furosemide-sensitive: 0.13 ± 0.02 △NRF min−1 mg protein−1; n=5-6 rats). †P < 0.05 vs. STZ untreated. (c) Effect of NOS inhibition in the presence of NADPH oxidase inhibition on O2 consumption by mTAL from STZ rats. Values are normalized to STZ + apocynin (ouabain-sensitive: 0.06 ± 0.01 △NRF min−1 mg protein−1; furosemide-sensitive: 0.05 ± 0.02 △NRF min−1 mg protein−1), expressed as mean ± SE (n = 6-10 rats). ‡P < 0.05 vs. STZ + apocynin. NOS1 inhibition (1 μM VNIO), NOS2 inhibition (100 nM 1400W), and total NOS inhibition (100 μM 1400W); NADPH oxidase inhibition (100 μM apocynin).
Neither ouabain- nor furosemide-sensitive O2 consumption by mTAL from sham rats was affected by treatment with the NOS1 inhibitor (1 μM VNIO) or the NOS2 inhibitor (100 nM 1400W) (Fig. 2a). In contrast, inhibition of all NOS isoforms by treatment with a high concentration (100 μM) of 1400W resulted in a significant increase in ouabain- and furosemide-sensitive O2 consumption by greater than 30% and 100%, respectively, compared to untreated sham mTAL. NADPH oxidase inhibition (100 μM apocynin) did not alter O2 consumption by sham mTAL (Fig. 2a). These data indicate an inhibitory impact of endogenous NOS3 on O2 consumption by the sham mTAL, with no apparent tonic impact of NOS1, NOS2 or NADPH oxidase on O2 consumption under these conditions.
In mTAL from STZ rats, ouabain- and furosemide-sensitive O2 consumption was significantly reduced in the presence of the NADPH oxidase inhibitor apocynin (Fig 2b), consistent with our previous report (Yang et al. 2012). In contrast, neither ouabain- nor furosemide-sensitive O2 consumption was influenced by inhibition of NOS1, NOS2, or all NOS isoforms (Fig 2b). Because NADPH oxidase inhibition significantly increased nitrite production by STZ mTALs, we tested whether NOS activity in STZ mTALs is capable of exerting a functional impact on O2 consumption during these conditions of sustained NO bioavailability. In the presence of apocynin, both ouabain- and furosemide-sensitive O2 consumption were significantly increased in the presence of NOS1- or NOS2-selective inhibitors (Fig 2c); however, total NOS inhibition did not further increase O2 consumption in the presence of NADPH oxidase inhibition (Fig. 2c).
DISCUSSION
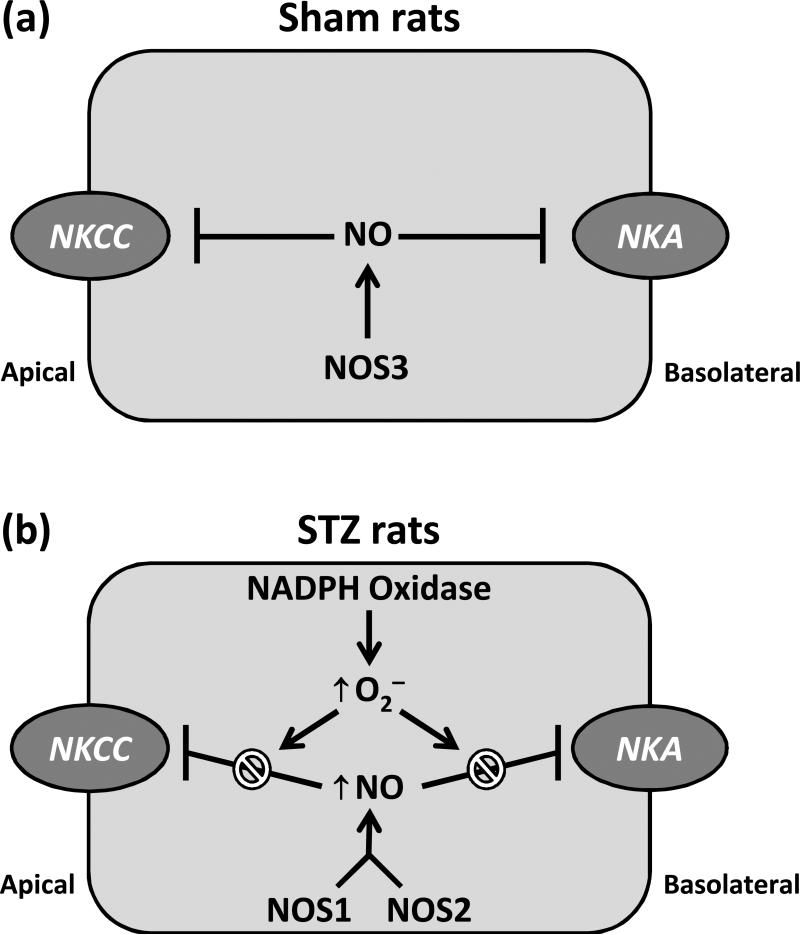
The major findings of this study demonstrate that T1D dismantles NOS-dependent inhibition of sodium transport in the rat mTAL. There is no evidence of NOS-dependent superoxide production (NOS uncoupling) by the mTAL during T1D; rather, increased NADPH oxidase-dependent superoxide production under these conditions reduces NO bioavailability despite increased NOS activity. In accord with previous results (Foster et al. 2009), NO produced by the mTAL during T1D appears to be derived from activation of NOS1 and NOS2 (but not NOS3, which is prominent under normal physiological conditions), and this NO is functionally capable of influencing sodium transport if superoxide production is inhibited. A cartoon scheme summarizing these findings is presented in Fig. 3.
Fig 3.
(a) Under normal conditions, sodium transport-related O2 consumption in the mTAL is blunted by NOS3-derived NO, reflecting its ability to inhibit the Na+-K+-2Cl− cotransporter (NKCC2) and the Na+-K+ -ATPase (NKA). (b) During type 1 diabetes, activation of NOS1 and NOS2 (but not NOS3) leads to an increase in NO production; however, the inhibitory impact of this NO on sodium transport is prevented by NADPH oxidase-dependent superoxide (O2−) production.
More than a decade ago, studies utilizing an NO donor, spermine NONOate, and the NOS substrate, l-arginine, demonstrated that both exogenously- and endogenously-derived NO inhibit NaCl reabsorption by the mTAL (Plato et al.1999, Ortiz et al. 2001). The process of sodium reabsorption in the mTAL is driven by the basolateral NKA, with apical sodium influx occurring via either the NKCC2 or the Na+/H+ exchanger. More than 80% of the O2 consumed by the mTAL is utilized to provide the ATP needed to fuel the NKA (Tejedor et al. 1988); therefore, the rate of ouabain-sensitive O2 consumption is an indicator of active sodium transport in the mTAL. Furosemide-sensitive O2 consumption can be considered to reflect transepithelial sodium transport via the apical NKCC2, which is responsible for the bulk of the apical sodium influx in these cells. In the present study, neither NOS1- nor NOS2-specific inhibition altered O2 consumption in mTAL suspensions from sham rats. Because there are no known NOS3-specific inhibitors, we blocked all three NOS isoforms and found increased ouabain- and furosemide-sensitive O2 consumption indicating that NOS3 controls sodium transport. This is in agreement with previous data showing that the majority of NO production in the mTAL is derived from NOS3 (Foster et al. 2009). Plato et al. (2000), utilizing NOS isoform knockout mice, found that NOS3-derived NO was responsible for inhibition of sodium reabsorption in the normal mTAL. Thus, these results in rats agree with previous literature in mice demonstrating that NOS3-derived NO inhibits sodium transport primarily through NKCC2 in mTAL suspensions under normal conditions (Plato et al. 2000). We previously reported (Foster et al. 2009) that both NOS1 and NOS2 isoforms are expressed in the mTAL, although we detected minimal NO production from NOS1 or NOS2 under normal conditions, thus requiring further work to decipher the functional role of these enzymes in the mTAL.
The results of the present study support the contention that NADPH oxidase activity does not tonically influence sodium transport in the normal mTAL, as evidenced by no effect of apocynin on either ouabain- or furosemide-sensitive O2 consumption, similar to our previous report (Yang et al. 2012). Moreover, Persson et al. (2012) demonstrated that acute inhibition of NADPH oxidase with apocynin was without any affect on proximal tubular sodium transport in normal rats. In contrast, NADPH oxidase has been reported to mediate increased transepithelial Cl− transport, an index of sodium reabsorption by the mTAL under normal conditions (Ortiz & Garvin 2002b). The reason for this discrepancy is unclear; however, transepithelial Cl− transport may be a more sensitive method for detecting a subtle regulatory impact of NADPH oxidase under normal conditions.
We recently provided evidence of increased NOS1 and NOS2 activities in the rat mTAL during T1D, although NO bioavailability was reduced (Foster et al. 2009). Many investigations have found that NOS is capable of producing superoxide as a result of NOS uncoupling under T1D conditions (Förstermann & Münzel 2006); however, the results of this study utilizing a panel of NOS inhibitors revealed no role of NOS in the T1D-induced superoxide production by the mTAL. Rather, as we previously reported (Yang et al. 2010) and confirm in this investigation, T1D induces superoxide production via NADPH oxidase in the mTAL. T1D triggers upregulation of Nox2, Nox4 and p47phox in the rat mTAL (Yang et al. 2010), and preliminary observations indicate that mTAL mitochondria exhibit increased Nox2 and Nox4 levels and superoxide production during T1D (Yang et al. 2011). The current data confirm that T1D increases total, ouabain- and furosemide-sensitive O2 consumption by the mTAL via an NADPH oxidase-dependent mechanism (Yang et al. 2012). Moreover, although NO exerts a tonic inhibitory influence on sodium transport under normal conditions, T1D results in loss of the functional impact of NOS on sodium transport in the mTAL, as shown by a lack of a response with any NOS inhibitor.
The increased NADPH oxidase-dependent superoxide production, in concert with a loss of NOS-dependent regulation of sodium transport, suggested that reduced NO bioavailability might play a role in the loss of a functional impact of NOS during T1D. Thus, we postulated that NADPH oxidase inhibition would promote increased NO bioavailability and restore the ability of NOS activty to impact sodium transport. Indeed, utilizing apocynin to inhibit NADPH oxidase, increased NO production was confirmed in mTAL from diabetic rats. By implicating NADPH oxidase in this phenomenon, this observation extends our previous observation that tempol treatment unveils an increase in NOS1- and NOS2-dependent (but NOS3-independent) NO production by the mTAL during T1D (Foster et al. 2009). To determine whether the increased NOS1- and/or NOS2-dependent NO production is capable of influencing sodium transport in the mTAL during T1D, we assessed the effects of NOS inhibition on ouabain- and furosemide-sensitive O2 consumption during NADPH oxidase inhibition. NOS1-selective inhibition increased ouabain and furosemide-sensitive O2 consumption by STZ mTALs under these conditions, indicating that NO produced by this NOS isoform is functionally able to regulate sodium transport. In agreement with these results, Palm et al. (2010) reported that NOS1-derived NO has a pivotal role in maintaining control of renal tissue oxygenation under in rats with streptozotocin-induced T1D. NOS2-selective inhibition also increased ouabain- and furosemide-sensitive O2 consumption, similar to NOS1-selective inhibition. No further increase in O2 consumption was evident when all three NOS isoforms were blocked, suggesting that NOS3 does not play a functional role in regulation of sodium transport in the mTAL under diabetic conditions even when NO bioavailability is maintained.
At this point, it is unclear what mediates the T1D-induced increase in NOS1 and NOS2 activities in the mTAL, as well as the loss of NOS3-dependent control of sodium transport. Because PKCα mediates the increased O2 consumption and superoxide production in the mTAL from STZ rats (Yang et al. 2012), we speculate that PKC is a possible regulator of NOS activity. As PKC regulation of NOS1 and NOS2 has not been reported previously, further work is necessary to elucidate this association. PKC has been shown to directly phosphorylate NOS3 constitutively at threonine 495 in endothelial cells, with PKC inhibition associated with an increase in endothelial NO production (Fleming et al. 2001). Studies of this phosphorylation site on NOS3 by immunohistochemical staining revealed expression of phosphoThr495 NOS3 expression at the apical membrane of mTAL in normal rats, but not in STZ rats, suggesting a dysfunctional phosphorylation and subcellular localization of NOS3 in the mTAL during T1D (Lee et al. 2005). Studies are being initiated to test the hypothesis that T1D-induced PKCα mediates NOS3 dysfunction in the mTAL.
Previous studies have demonstrated that the effect of superoxide production on O2 consumption by the mTAL involves a direct enhancement of NKA- and NKCC2-mediated sodium reabsorption, as well as an indirect effect involving scavenging of NO, thereby reducing its tonic inhibitory impact on reabsorption (Juncos & Garvin 2005, Ortiz & Garvin 2002a). In rats with T1D, acute NADPH oxidase inhibition improves cortical and medullary O2 tension and reduces tubular sodium transport, while chronic inhibition of this enzyme normalizes O2 consumption by proximal tubular cells and reduces proteinuria (Persson et al. 2012). Further research is needed to determine whether the improvements in renal function observed with NADPH oxidase inhibition during T1D are due, in part, to facilitating NO bioavailability, thereby allowing increased NOS1- and NOS2-dependent NO production to impact sodium transport and other parameters. Taken together with our findings, we propose that NADPH oxidase-generated superoxide production enhances O2 consumption in the mTAL during T1D at least in part via NO scavenging , although a direct effect on NKA and NKCC2 might also occur.
In conclusion, the increased NADPH oxidase-dependent superoxide production and the consequent effect to increase sodium reabsorption in the mTAL cancels out NOS-mediated control of sodium transport, while inhibition of NADPH oxidase re-establishes NOS control of sodium transport in the mTAL. NOS3 activity is the predominantly active NOS isoform regulating sodium transport in the mTAL under normal, physiological conditions, but is not functionally active during T1D conditions in the presence or absence of NADPH oxidase inhibition. T1D activates NOS1 and NOS2 that, in the presence of NADPH oxidase inhibition, allows NOS functionality to control sodium transport in the mTAL. It is interesting to speculate that specific inhibition of NADPH oxidase in T1D could restore control of mTAL function and sodium balance, thereby dampening the deleterious consequences of diabetes on renal function.
ACKNOWLEDGMENTS
The authors acknowledge the financial support from AHA (JMF: Southeast AHA predoctoral fellowship; CDM: Southeast AHA postdoctoral fellowship) and NIH (JSP: HL60653). We gratefully thank Dr. Frank Spradley for helpful assistance throughout these studies.
Footnotes
Dr. Jan Foster's current affiliation: Assistant Professor, North Greenville University, Travelers Rest, SC.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Allen TJ, Saldron MJ, Casley D, Jerums G, Cooper ME. Salt restriction reduces hyperfiltration, renal enlargement, and albuminuria in experimental diabetes. Diabetes. 1997;46:119–124. doi: 10.2337/diabetes.46.1.19. [DOI] [PubMed] [Google Scholar]
- Burg MB. Thick ascending limb of Henle's loop. Kidney Int. 1982;22:454–464. doi: 10.1038/ki.1982.198. [DOI] [PubMed] [Google Scholar]
- Chamberlin ME, LeFurgey A, Mandel LJ. Suspension of medullary thick ascending limb tubules from the rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F955–F964. doi: 10.1152/ajprenal.1984.247.6.F955. [DOI] [PubMed] [Google Scholar]
- DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol. 2003;284:F113–F121. doi: 10.1152/ajprenal.00026.2002. [DOI] [PubMed] [Google Scholar]
- Feldt-Rasmussen B, Mathiesen ER, Deckert T, Giese J, Christensen NJ, Bent-Hansen L, Nielsen MD. Central role for sodium in the pathogenesis of blood pressure changes independent of angiotensin, aldosterone, and catecholamines in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987;30:610–617. doi: 10.1007/BF00277316. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/Calmodulin dependent endothelial nitric oxide synthase activity. Circulation Research. 2001;88:e68–e75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Foster JM, Carmines PK, Pollock JS. PP2B-dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol. 2009;297:F471–F480. doi: 10.1152/ajprenal.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino RD, Dike LE, Haq TA, Rowley JA, Pitner JB, Timmins MR. Method for determining oxygen consumption rates of static cultures from microplate measurements of pericellular dissolved oxygen concentration. Biotechnol Bioeng. 2004;86:775–787. doi: 10.1002/bit.20072. [DOI] [PubMed] [Google Scholar]
- Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol. 2005;288:F982–F987. doi: 10.1152/ajprenal.00348.2004. [DOI] [PubMed] [Google Scholar]
- Khadouri C, Barlet-Bas C, Doucet A. Mechanism of increased tubular Na-K-ATPase during streptozotocin-induced diabetes. Pflugers Arch. 1987;409:296–301. doi: 10.1007/BF00583479. [DOI] [PubMed] [Google Scholar]
- Ku DD, Sellers BM, Meezan E. Development of renal hypertrophy and increased renal Na,K-ATPase in streptozotocin-diabetic rats. Endocrinology. 1986;119:670–679. doi: 10.1210/endo-119-2-672. [DOI] [PubMed] [Google Scholar]
- Lee DL, Sasser JM, Hobbs JL, Boriskie A, Pollock DM, Carmines PK, Pollock JS. Posttranslational regulation of NO synthase activity in the renal medullar of diabetic rats. Am J Physiol Renal Physiol. 2005;288:F82–F90. doi: 10.1152/ajprenal.00127.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matko J, Ohki K, Edidin M. Luminescence quenching by nitroxide spin labels in aqueous solution: studies on the mechanism of quenching. Biochemistry. 1992;31:703–711. doi: 10.1021/bi00118a010. [DOI] [PubMed] [Google Scholar]
- Nørgaard K, Feldt-Rasmussen B. Sodium retention and insulin treatment in insulin-dependent diabetes mellitus. Acta Diabetol. 1994;31:19–25. doi: 10.1007/BF00580755. [DOI] [PubMed] [Google Scholar]
- O'Hare JP, Roland JM, Walters G, Corrall RJ. Impaired sodium excretion in response to volume expansion induced by water immersion in insulin-dependent diabetes mellitus. Clin Sci (Lond) 1986;71:403–409. doi: 10.1042/cs0710403. [DOI] [PubMed] [Google Scholar]
- Ortiz PA, Garvin JL. Interaction of O2–2 and NO in the thick ascending limb. Hypertension. 2002a;39:591–596. doi: 10.1161/hy0202.103287. [DOI] [PubMed] [Google Scholar]
- Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol. 2002b;283:F957–F962. doi: 10.1152/ajprenal.00102.2002. [DOI] [PubMed] [Google Scholar]
- Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl– cotransporter activity. Am J Physiol Renal Physiol. 2001;281:F819–F825. doi: 10.1152/ajprenal.2001.281.5.F819. [DOI] [PubMed] [Google Scholar]
- Patel KP. Volume reflex in diabetes. Cardiovasc Res. 1997;34:81–90. doi: 10.1016/s0008-6363(97)00012-6. [DOI] [PubMed] [Google Scholar]
- Palm F, Fasching A, Hansell P, Källskog O. Nitric oxide originating from NOS1 controls oxygen utilization and electrolyte transport efficiency in the diabetic kidney. Am J Physiol Renal Physiol. 2010;298:F416–F420. doi: 10.1152/ajprenal.00229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson P, Hansell P, Palm F. NADPH oxidase inhibition reduces tubular sodium transport and improves kidney oxygenation in diabetes. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1443–R1449. doi: 10.1152/ajpregu.00502.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plato CF, Shesely E, Garvin JL. eNOS mediates L-arginine-induced inhibition of thick ascending limb chloride flux. Hypertension. 2000;35:319–323. doi: 10.1161/01.hyp.35.1.319. part 2. [DOI] [PubMed] [Google Scholar]
- Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol. 1999;276:F159–F163. doi: 10.1152/ajprenal.1999.276.1.F159. [DOI] [PubMed] [Google Scholar]
- Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol. 1991;260:F946–F952. doi: 10.1152/ajprenal.1991.260.6.F946. [DOI] [PubMed] [Google Scholar]
- Roland JM, O'Hare JP, Walters G, Corrall RJ. Sodium retention in response to saline infusion in uncomplicated diabetes mellitus. Diabetes Res. 1986;3:213–215. [PubMed] [Google Scholar]
- Tejedor A, Noel J, Vinay P, Boulanger Y, Gougoux A. Characterization and metabolism of canine proximal tubules, thick ascending limbs, and collecting ducts in suspension. Can J Physiol Pharmacol. 1988;66:997–1009. doi: 10.1139/y88-164. [DOI] [PubMed] [Google Scholar]
- Thomsen JJ, Scott JM, Topley P, Knowles RG, Keerier AJ, Frend AJ. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. [PubMed] [Google Scholar]
- Wald H, Scherzer P, Rasch R, Popovtzer MM. Renal tubular Na-K-ATPase in diabetes mellitus: relationship to metabolic abnormality. Am J Physiol Endocrinol Metab. 1993;265:E96–E101. doi: 10.1152/ajpendo.1993.265.1.E96. [DOI] [PubMed] [Google Scholar]
- Wang W, Upshaw L, Strong DM, Robertson RP, Reems J. Increased oxygen consumption rates in response to high glucose detected by a novel oxygen biosensor system in non-human primate and human islets. J Endocr. 2005;185:445–455. doi: 10.1677/joe.1.06092. [DOI] [PubMed] [Google Scholar]
- Wodnicka M, Guarino RD, Hemperly JJ, Timmins MR, Stitt D, Pitner JB. Novel fluorescent technology platform for high throughput cytotoxicity and proliferation assays. J Biomol Screen. 2000;5:141–152. doi: 10.1177/108705710000500306. [DOI] [PubMed] [Google Scholar]
- Yang J, Lane PH, Pollock JS, Carmines PK. PKC-dependent superoxide production by the renal medullary thick ascending limb from diabetic rats. Am J Physiol Renal Physiol. 2009;297:F1220–F1228. doi: 10.1152/ajprenal.00314.2009. [DOI] [PubMed] [Google Scholar]
- Yang J, Lane PH, Pollock JS, Carmines PK. Protein kinase C-dependent NAD(P)H oxidase activation induced by type 1 diabetes in renal medullary thick ascending limb. Hypertension. 2010;55:468–473. doi: 10.1161/HYPERTENSIONAHA.109.145714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lane PH, Pollock JS, Carmines PK. Mitochondrial PKC, NAD(P)H oxidase and superoxide anion in the renal medullary thick ascending limb during type 1 diabetes (Abstract). FASEB J. 2011;25:664.12. [Google Scholar]
- Yang J, Pollock JS, Carmines PK. NADPH oxidase and PKC contribute to increased Na transport by the thick ascending limb during type 1 diabetes. Hypertension. 2012;59:431–436. doi: 10.1161/HYPERTENSIONAHA.111.184796. [DOI] [PMC free article] [PubMed] [Google Scholar]