Abstract
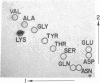
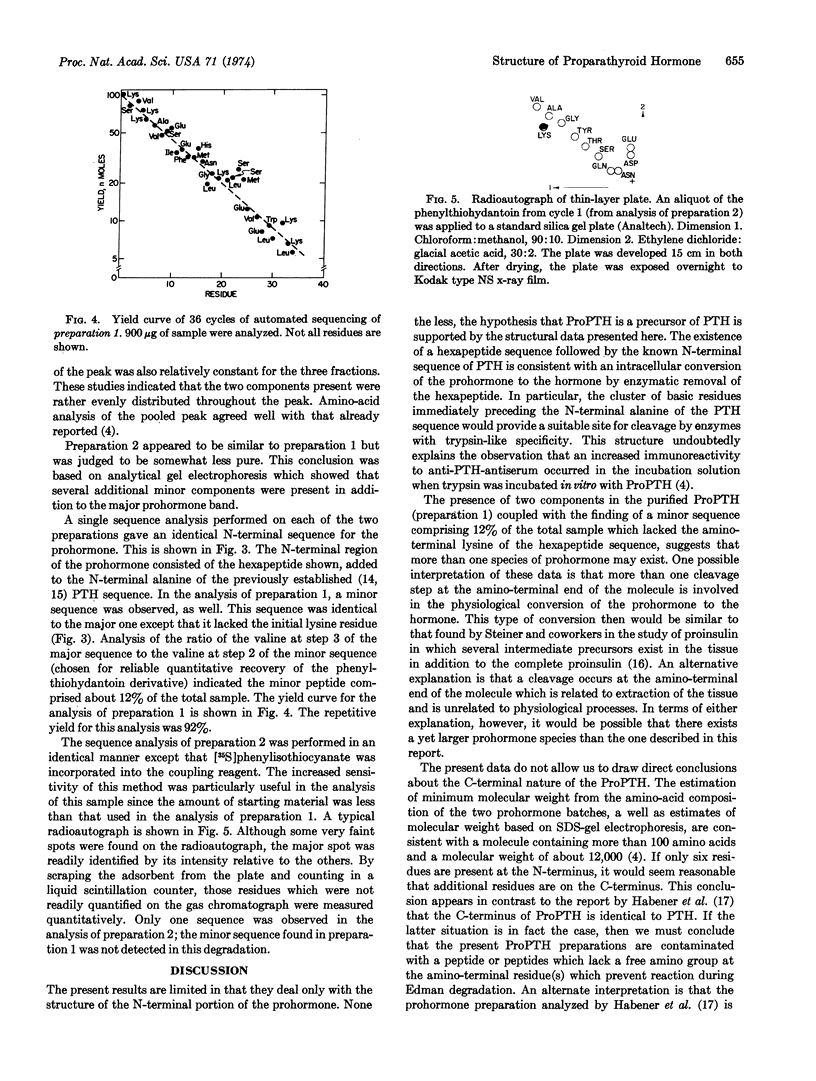
Proparathyroid hormone (calcemic fraction-A) is a biosynthetic precursor of parathyroid hormone in bovine glands. Limited amounts of the isolated prohormone have been obtained for the purpose of initial structural studies. The results of automated sequence analysis on two separate preparations indicate that the N-terminal region of the prohormone consists of the sequence Lys-Ser-Val-Lys-Lys-Arg followed by a sequence exactly corresponding to residues 1 to 34 of bovine parathyroid hormone. Also observed was a minor sequence (about 12% of the total) in which the N-terminal lysine was absent. These data suggest that more than one species of prohormone may exist. Due to the small quantities of sample available, the analyses were restricted to the N-terminal portion of the prohormone only. However, because the amino-acid composition of the prohormone indicates it to be a molecule containing more than 100 amino acids, the possibility remains that additional residues occur at the C-terminus. Thus, the prohormone structure based on these data is believed to consist of the hexapeptide sequence above, followed by the known sequence of the 84 residues in parathyroid hormone, possibly followed by an additional sequence of 10-15 residues.
Keywords: parathyroid hormone, protein structure, prohormone
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer H. B., Jr, Ronan R. Bovine parathyroid hormone: amino acid sequence. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1862–1869. doi: 10.1073/pnas.67.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L. L., MacGregor R. R., Anast C. S., Hamilton J. W., Cohn D. V. Studies on the biosynthesis of rat parathyroid hormone and proparathyroid hormone: adaptation of the parathyroid gland to dietary restriction of calcium. Endocrinology. 1973 Oct;93(4):915–924. doi: 10.1210/endo-93-4-915. [DOI] [PubMed] [Google Scholar]
- Chu L. L., MacGregor R. R., Liu P. I., Hamilton J. W., Cohn D. V. Biosynthesis of proparathyroid hormone and parathyroid hormone by human parathyroid glands. J Clin Invest. 1973 Dec;52(12):3089–3094. doi: 10.1172/JCI107508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. L., Steiner D. F. Insulin biosynthesis in the rat: demonstration of two proinsulins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):278–285. doi: 10.1073/pnas.62.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. V., Macgregor R. R., Chu L. L., Kimmel J. R., Hamilton J. W. Calcemic fraction-A: biosynthetic peptide precursor of parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1521–1525. doi: 10.1073/pnas.69.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Kemper B., Potts J. T., Jr, Rich A. Bovine proparathyroid hormone: structural analysis of radioactive peptides formed by limited cleavage. Endocrinology. 1973 Jan;92(1):219–226. doi: 10.1210/endo-92-1-219. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Kemper B., Potts J. T., Jr, Rich A. Proparathyroid hormone: biosynthesis by human parathyroid adenomas. Science. 1972 Nov 10;178(4061):630–633. doi: 10.1126/science.178.4061.630. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Macgregor R. R., Chu L. L., Cohn D. V. The isolation and partial purification of a non-parathyroid hormone calcemic fraction from bovine parathyroid glands. Endocrinology. 1971 Dec;89(6):1440–1447. doi: 10.1210/endo-89-6-1440. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Potts J. T., Jr, Rich A. Proparathyroid hormone: identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Mar;69(3):643–647. doi: 10.1073/pnas.69.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Chu L. L., Hamilton J. W., Cohn D. V. Partial purification of parathyroid hormone from chicken parathyroid glands. Endocrinology. 1973 May;92(5):1312–1317. doi: 10.1210/endo-92-5-1312. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Keutmann H., Sauer R., Hogan M., Dawson B., Aurbach G., Potts J., Jr The amino acid sequence of bovine parathyroid hormone I. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1586–1588. [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]