Abstract
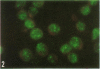
A brilliant, coarsely granular nuclear antigen was detected by anti-complement immunofluorescence in the nuclei of acute myeloid leukemia myeloblasts. Designated as LANA (leukemia-associated nuclear antigen), the reactivity differs from that of the Epstein-Barr-virus-determined nuclear antigen (EBNA) in immunological specificity and morphological appearance, although it is visualized by the same method. Serum from acute myeloid leukemia patients gave positive reactions in 73% of the cases. In acute lymphatic leukemia, chronic myeloid leukemia, chronic lymphatic leukemia, and Burkitt's lymphoma the sera were positive in 35, 14, 19, and 24%, respectively. Two of five polycythemia and two of eleven myeloma sera were also positive. Among 61 healthy controls, 58 were negative, whereas three showed a diffuse nuclear staining with a different pattern. Among 24 carcinoma patients, 18 were negative, whereas six gave a nuclear staining with a different, diffuse pattern. Sera from 20 patients who had recovered from infectious mononucleosis were all negative. In addition to the blasts of acute myeloid leukemia, a similar reactivity was seen with two Epstein-Barr virus DNA and EBNA-negative African lymphoma biopsies and in a short-lived tissue culture line derived from one of them. LANA could be a fetal or tissue-specific antigen, a virally determined antigen, or a specific form of anti-nuclear reactivity.
Keywords: immunology, neoplasms, anti-complement fluorescence, myeloblasts, lymphomas
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Epstein M. A., Achong B. G., Barr Y. M., Zajac B., Henle G., Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst. 1966 Oct;37(4):547–559. [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Hoekstra J., Deinhardt F. Demonstration of Herpesvirus saimiri-associated antigens in peripheral lymphocytes from infected marmosets during in vitro cultivation. J Natl Cancer Inst. 1972 Feb;48(2):523–530. [PubMed] [Google Scholar]
- GOLDWASSER R. A., SHEPARD C. C. Staining of complement and modification of fluorescent antibody procedures. J Immunol. 1958 Feb;80(2):122–131. [PubMed] [Google Scholar]
- HINUMA Y., HUMMELER K. Studies on the complement-fixing antigens of poliomyelitis. III. Intracellular development of antigen. J Immunol. 1961 Oct;87:367–375. [PubMed] [Google Scholar]
- HINUMA Y., OHTA R., MIYAMOTO T., ISHIDA N. Evaluation of the complement method of fluorescent antibody technique with myxoviruses. J Immunol. 1962 Jul;89:19–26. [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Clifford P., Klein E., Stjernswärd J. Search for tumor-specific immune reactions in Burkitt lymphoma patients by the membrane immunofluorescence reaction. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1628–1635. doi: 10.1073/pnas.55.6.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Nilsson K. High-frequency establishment of human immunoglobulin-producing lymphoblastoid lines from normal and malignant lymphoid tissue and peripheral blood. Int J Cancer. 1971 Nov 15;8(3):432–442. doi: 10.1002/ijc.2910080311. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Huang C. H., Pagano J. S., Klein G., Singh S. DNA of Epstein-Barr virus detected in tissue of Burkitt's lymphoma and nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3265–3268. doi: 10.1073/pnas.70.11.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. S., O'Conor G. T., Lorenz D. E., Kirschstein R. L., Legallais F. Y., Tralka T. S. Lymphoid cell-culture line derived from lymph node of marmoset infected wtih Herpesvirus saimiri--preliminary report. J Natl Cancer Inst. 1971 May;46(5):1099–1109. [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]