Abstract
Experimental autoimmune encephalomyelitis (EAE) is a CD4+ T cell-mediated disease of the CNS. Serum amyloid P component (SAP) is a highly conserved plasma protein named for its universal presence in amyloid deposits. Here we report SAP transgenic mice had unexpectedly attenuated EAE due to impaired encephalitogenic responses. Following induction with myelin oligodendroglial glycoprotein (MOG) peptide 35–55 in CFA, SAP transgenic mice showed reduced spinal cord inflammation with lower severity of EAE attacks as compared with control C57BL/6 mice. However in SAP-KO mice, the severity of EAE is enhanced. Adoptive transfer of Ag-restimulated T cells from wild-type to SAP transgenic mice or transfer of SAP transgenic Ag-restimulated T cells to control mice induced milder EAE. T cells from MOG-primed SAP transgenic mice showed weak proliferative responses. Furthermore, in SAP transgenic mice, there is little infiltration of CD45-positive cells in the spinal cord. In vitro, SAP suppressed the secretion of IL-2 stimulated by P-selectin, and blocked P-selectin binding to T cells. Moreover, SAP could change the affinity between α4-integrin and T cells. These data suggested that SAP could antagonize the development of the acute phase of inflammation accompanying EAE by modulating the function of P-selectin.
Keywords: Experimental autoimmune encephalomyelitis (EAE), Serum amyloid P component (SAP), P-Selectin
INTRODUCTION
Serum amyloid component P (SAP) is a member of the pentraxin family of proteins secreted by hepatocytes in response to inflammatory challenges. It has been widely used as a clinical indicator for inflammation.1–5 It is a highly conserved plasma protein named for its universal presence in amyloid deposits, and a normal component of a number of basement membranes, including the glomerular basement membrane.6 It is the single normal circulating protein with specific calcium dependent binding to DNA and chromatin in physiological conditions.7 When bound to ligand, SAP forms a stable decamer with two pentameric rings.8 SAP interacts with nuclear ligands, including chromatin and snRNP, is important in the handling of chromatin exposed by cell death, and has a role in the clearance of nuclear ligands from apoptotic and necrotic cells.9–11 SAP binds in vivo both to apoptotic cells, the surface blebs of which bear chromatin fragments, and to nuclear debris released by necrosis.11
The human disease multiple sclerosis (MS) 12 and its animal model, experimental autoimmune encephalomyelitis (EAE), are mediated by inflammatory cells recruited from the circulation into the CNS. Disease is initiated when activated autoreactive T cells cross the blood brain barrier into the healthy CNS. When Ag is encountered, these cells initiate inflammation and the recruitment of inflammatory effector cells to the CNS, resulting in devastating demyelination and axon destruction. EAE can be induced either by immunizing animals with myelin components (actively induced EAE) or by transferring encephalitogenic T cells (adoptive transfer EAE). 13,14 Different strains of small animals are differentially susceptible to EAE.15 C57BL/6 mice develop a monophasic disease upon myelin oligodendroglial glycoprotein (MOG) 35–55 challenge, with extensive demyelination and inflammation in the CNS, and MOG35–55-induced EAE is frequently used in experiments using gene-targeted mice.16
It is generally accepted that α4-integrins play a predominant role in T cell trafficking across the BBB during EAE, as they mediate both T cell rolling and firm adhesion of T cells to the BBB.17 In contrast, the adhesion molecules generally mediating leukocyte rolling along the vascular wall, such as E- and L-selectin, are not required for the development of EAE in C57BL/6 and SJL mice.18–20 Similarly, P-selectin has been shown to play no role in the recruitment of inflammatory cells across the BBB during EAE;18 nevertheless, its role in EAE pathogenesis remains controversial.21,22
The current study was designed to address the role of SAP in the pathogenesis of EAE, using both SAP transgenic mice and SAP-deficient mice. Our data showed SAP deletion increased behavioral and pathological changes of EAE, and overexpression of SAP decreased behavioural and pathological changes of EAE in mice. Furthermore, our studies demonstrated that the function of SAP suppressing EAE is related to modulating the function of P-selectin.
RESULTS
SAP-transgenic mice develop milder EAE
To study the effects of SAP on EAE related immunopathology, we compared the susceptibility of control and SAP-transgenic mice to EAE induction with subcutaneous injection of 200μg peptide consisting of amino acids 35–55 of myelin oligodendrocyte glycoprotein (MOG 35–55) in complete Freund’s adjuvant (CFA) and pertussis toxin, control mice developed a monophasic disease characterized by ascending paralysis 10–18 d after immunization, and SAP-transgenic mice showed resistant to the development of EAE. Table 1 summarizes the neurobehavioral features of MOG35–55-induced EAE in control and SAP-transgenic mice. In the initial experiment, When 200μg MOG35–55 was injected, 15 of 15 (100%) wild-type mice developed fatal EAE, whereas only 12 of 15 (80%) SAP-transgenic mice developed EAE. EAE induced with 200 μg of MOG35–55 was more severe weakness and paralysis in wild-type mice than in SAP-transgenic mice, with higher peak scores and imperfect recovery, with more residual neurological impairment (Table 1 and Figure 1). Wild-type mice had higher EAE scores and greater weight change in day 30 p.i. than did SAP-transgenic animals (Figure 1), indicating more extensive tissue injury. These results indicated that increase of SAP suppresses the severity of EAE in mice.
Table 1.
EAE in wild-type and SAP-Tg mice
| Mouse genotype | Incidence | Day of onset (mean ± s.d.) | Maximum clinical score (mean ± s.d.) |
|---|---|---|---|
|
| |||
| Wild type | 15 out of 15 (100%) | 14.08 (±2.33) | 2.95 (±0.68) |
| SAP-Tg | 12 out of 15 (80%) | 14.91 (±2.43) | 1.71 (±0.34)* |
Abbreviations: EAE, experimental autoimmune encephalomyelitis; SAP, serum amyloid P component; Tg, transgenic. Results are cumulative data from three different experiments, * p<0.05, as compared with corresponding wild-type controls.
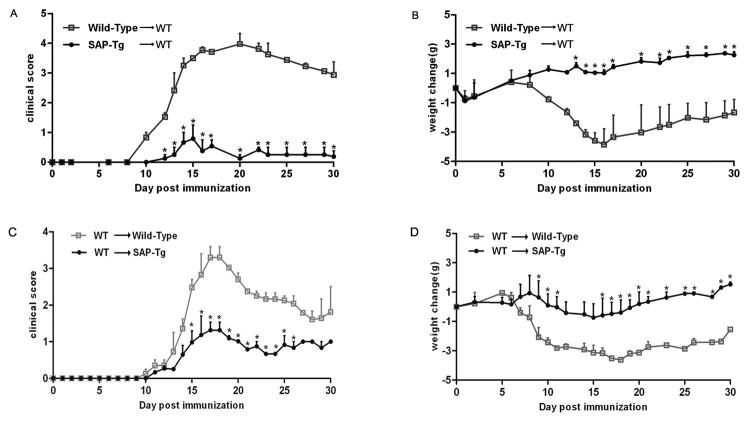
Figure 1.
SAP transgenic mice develop distinguishable EAE from C57BL/6 wild-type mice. SAP transgenic and wild type mice were immunized with 200 μg MOG35–55. The results of average disease scores (A) and average weight change (B) assessed daily following immunization with MOG35-55 are shown; Data are shown as mean ±SD; n = 15 for each group (a combination of three separate experiments with five mice per group).* P <0.05 (Student’s t-test).
SAP-deficient mice develop exaggerated EAE
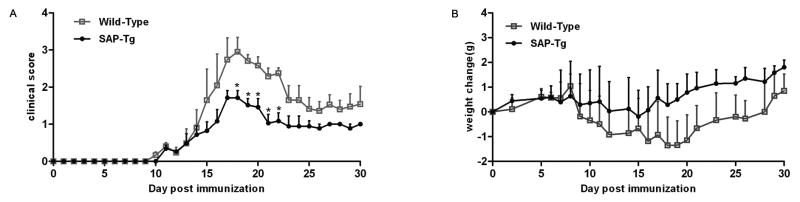
To further determine how SAP deficiency influences in EAE, we immunized wild-type mice and SAP-deficient mice with 100μg MOG35–55 and monitored them daily. Although most of mice in both groups developed EAE, neurologic dysfunction in SAP-KO mice was markedly more severe. Table 2 shows clinical scores over a 30-day period from the above mice. 13 of 15 (87%) SAP-deficient mice developed EAE, whereas only 11 of 15 (73%) wild-type mice developed EAE. In the SAP-deficient mice, the mean clinical score was 2.29(±0.83) vs 1.25(±0.76) in wild-type mice, and the mean weight decrease was greater (data not shown). SAP-deficient mice had higher EAE scores in day 30 p.i. compared with wild-type mice (Supplementary Figure 1).
Table 2.
EAE in wild-type and SAP-Knockout mice
| Mouse genotype | Incidence | Day of onset (mean ± s.d.) | Maximum clinical score (mean ± s.d.) |
|---|---|---|---|
|
| |||
| Wild type | 11 out of 15 (73%) | 14.91 (±3.42) | 1.25 (±0.76) |
| SAP-Knockout | 13 out of 15 (87%) | 13.91 (±2.54) | 2.29 (±0.83)* |
Abbreviations: EAE, experimental autoimmune encephalomyelitis; SAP, serum amyloid P component.
Results are cumulative data from three different experiments, *P<0.05, as compared with corresponding wild-type controls.
Adoptive transfer of EAE resistance
To determine whether SAP overexpression on Ag-specific T cells were beneficial to EAE, we transferred EAE using MOG-sensitized T cells from SAP transgenic mice. We induced EAE by adoptively transferring MOG-sensitized T cells from control mice and SAP transgenic mice to wild-type mice. The result was little development of disease in recipient mice injected by encephalitogenic cells from SAP-transgenic mice. As expected, transfer of Ag-specific T cells from control mice to wild-type mice as an internal control resulted in clinical signs of EAE (Figure 2A–B). So the result indicated cells from MOG-immunized SAP-transgenic mice induced EAE poorly in C57BL/6 animals as compared with those from wild-type mice. We also induced EAE by adoptively transferring MOG-sensitized T cells from wild-type mice to control mice and SAP transgenic mice. We observed an attenuated development of disease in SAP transgenic recipient mice while control mice readily developed EAE (Figure 2C–D). The result indicated SAP transgenic mice were resistant to the MOG-sensitized T cells from wild-type mice. So these experiments showed that the severity of EAE is closely related to cellular immunity of T cells; however, the enriched environment of SAP also could inhibit the severity by suppressing the cellular immunity of T cells.
Figure 2.
SAP transgenic mice develop passive EAE distinguishable from C57BL/6 wild type mice. Encephalitogenic cells prepared from wild type and SAP Tg mice were injected into sublethally irradiated wild type mice. Average disease scores (A) and average weight change (B) assessed daily following immunization with Encephalitogenic cells are shown. Encephalitogenic cells prepared from wild type were injected into sublethally irradiated SAP Tg mice and wild type mice. Average disease scores (C) and average weight change (D) assessed daily following immunization with Encephalitogenic cells are shown. Data are shown as mean ±SD; n = 10 for each group (a combination of two separate experiments with five mice per group). *P <0.01 (Student’s t-test).
Cellular infiltration in SAP- transgenic mice with EAE
We next performed histopathological analysis on spinal cords of wild-type and SAP-transgenic mice with active EAE to determine the extent and nature of the cellular infiltrate between the two groups of mice. Representative spinal cord sections from wild-type mice obtained at peak days after disease induction had extensive cellular infiltration in the meninges and white matter (Figure 3A, C, E and G). Sections obtained from SAP- transgenic mice had little cellular infiltration and inflammation throughout the spinal cord, compared with wild-type mice (Figure 3B, D, F and H). The result indicated that SAP inhibited the inflammation of CNS induced by EAE.
Figure 3.
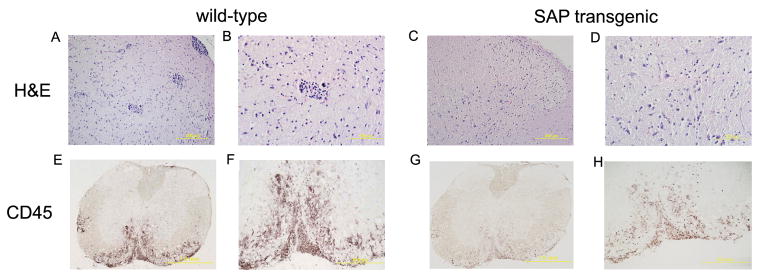
SAP transgenic mice develop milder EAE with decreased infiltrates in CNS tissues. The distribution and extent of H&E (×200 for A and C, ×400 for B and D) and CD45 (×40 for E and G, ×100 for F and H ) immunoreactivity were evaluated on axial sections of the lumbar spinal cord from wild-type and SAP transgenic mice at the peak of EAE. Representative cross sections of the spinal cord from one of four mice per group are shown.
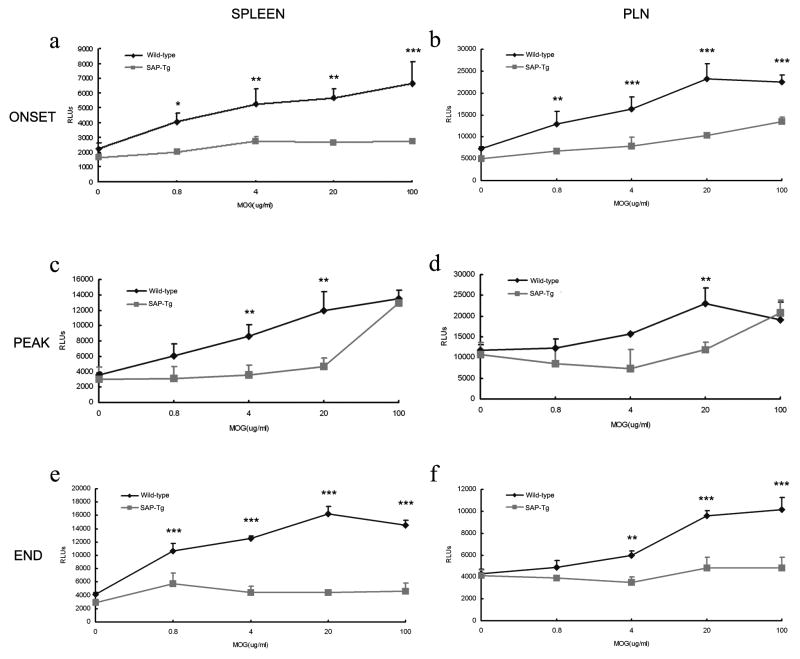
SAP-transgenic T cells have reduced proliferative capacity compared with wild-type T cells
SAP-transgenic mice showed poorly EAE severity than did wild-type mice suggested the possibility of altered T cell effector function. To test the possibility that attenuated active and transferred EAE in SAP-transgenic mice could be due to a poor proliferative capacity of SAP- transgenic T cells, we performed in vitro antigen-recall proliferation assays with lymphocytes isolated from draining PLN and spleen. In C57BL/6 wild-type mice, a prominent dose-dependent T cell proliferation response to MOG35–55 was detected after immunization in the draining PLN and a lesser degree in the spleen (Figure 4). In contrast, in SAP-transgenic mice, the MOG35–55-specific T cell proliferation was limited and maintained at an obviously low level to wild-type mice in the onset, peak and end of the EAE (Figure 4).
Figure 4.
T cell proliferative response to MOG35–55 is impaired in SAP transgenic mice. A decreased proliferative response toward MOG35–55 restimulation was observed in T cells from SAP transgenic mice compared with wild-type mice. Spleen (A, C and E) and lymph node (B, D and F) cells were isolated from SAP transgenic and wild type mice on the onset (A and B), peak (C and D) and end (E and F) of the EAE, restimulated in triplicate (1× 105 cells/well) with the indicated concentrations of MOG35–55 for 72 h. T cell proliferative response was measured by ViaLight Plus Kit. The background RLUs values were subtracted. Each curve (±SD) represents the mean RLUs of three wells. Data shown were pooled from two mice per group and are representative of three independent experiments. **P <0.01, ***P <0.001 (Student’s t-test).
SAP-transgenic cells have an altered cytokine profile compared with wild-type T cells
T helper type1(Th1) cells play an essential role for the development and progression of EAE through the production of Th1 cytokines of such as interferon-γ(INF-γ) and tumor necrosis factor-α (TNF-α);Th17 cells also play an important role in the development of EAE. So we used flow cytometry to monitor the relative populations of Th1 cells and Th17 cells in lymph node cells at the peak of EAE, flow cytometric analysis (Supplementary Figure 2) showed there was an remarkable increase of the INF-γ and IL-17 proportion in WT mice than SAP transgenic mice, that means the Th1 and Th17 reaction were inhibited during the peak of EAE in SAP transgenic mice.
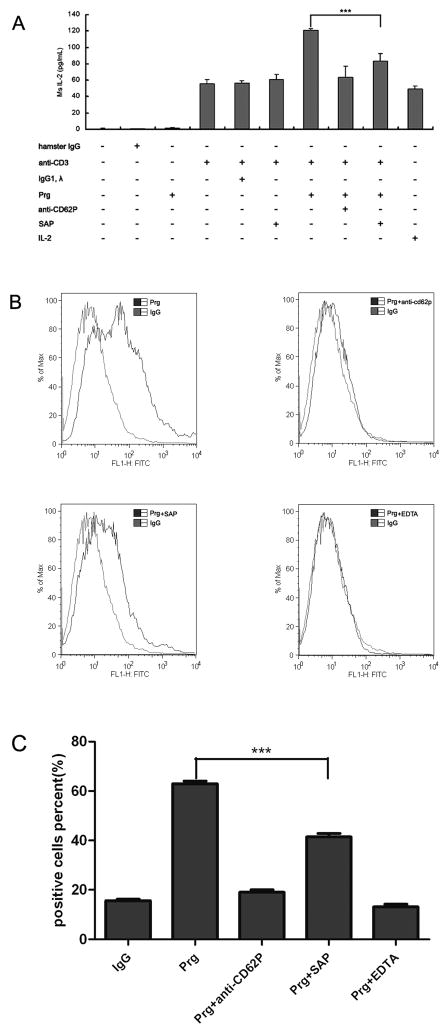
SAP suppress the activation of P-selectin to T cells
In Kubes’s study, 21,22 it has been proved there are important overlapping roles for both P-selectin and α4 integrin in mediating leukocyte-endothelial cell interactions in the EAE. So we wonder if SAP resists the severity of EAE by modulating the function of P-selectin or α4 integrin. To figure out this, we performed following assays with T lymphocytes isolated from draining PLN. In the first, we measured the secretion of IL-2 using ELISA assay. The result showed in the presence of anti-mouse CD3, T lymphocytes secreted more IL-2 under the stimulation of 20 μg P-selectin. However when 10μg mSAP added, the secretion of IL-2 decrease obviously (Figure 5A). The result implies mSAP can suppress the function of P-selectin to stimulate T lymphocytes. And then the binding assay was performed. The result showed mP-Rg can bind to T cells in vitro. However when SAP added, the binding of mP-Rg to mouse T cells was clearly inhibited (Figure 5B and C). Our data demonstrate the capacity of SAP to inhibit the binding and stimulation of P-selectin to T lymphocytes.
Figure 5.
mSAP inhibited the activity of mP-Rg to T cells. CD4+ T cells were isolated from pooled spleens of mice according to protocol, the secretion of IL-2 was measured using ELISA assay after stimulation (A), data are shown as mean ±SD; The binding of mP-Rg to T cells was analysed by FACS (B), and the combined data were analyzed (C). Results are representative of three separate experiments. ***P <0.001.
SAP can change the conformational states of α4-integrin
Because flow cytometers have the ability to discriminate between free and bound fluorescent ligand in a homogeneous assay, so we constructed a novel fluorescent peptide derived from the high affinity LDV sequence to measure binding affinities in real time of α4-integrin on leukocytic cells.23 In these experiments, we preincubated cells with the peptide for 10 min, then IgG, SAP or Mn2+ was added as stimulator. Flow cytometric analysis was performed continuously for up to 500 s to measure the peptide binding affinity. Figure 6 shows an analysis of the Binding characteristics of Fluorescent LDV following addition. We found SAP as a stimulator can obviously change the binding affinity of LDV than its IgG control does, Mn2+ as a positive control. That means SAP can change the conformational states of the α4-integrin to affect its function.
Figure 6.
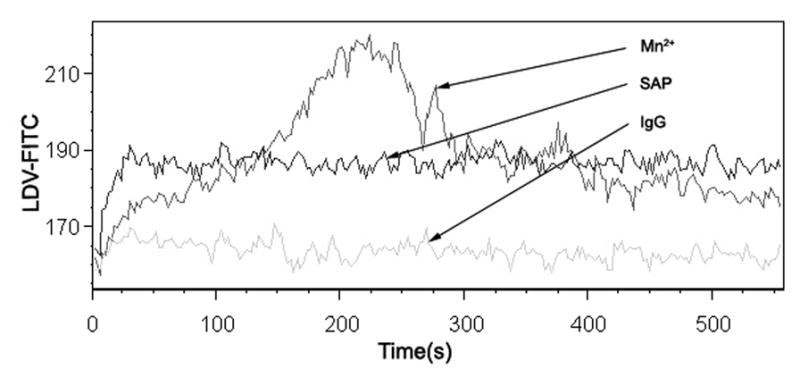
mSAP changed the conformational states of α4-integrin. Cell suspensions were incubated with 3 μM fluorescent VLA-4-specific peptide and stimulated with IgG, SAP or Mn2+. Binding is shown as mean channel fluorescence versus time.
DISCUSSION
In this study, we examined the role of SAP in the pathogenesis of EAE using both SAP transgenic mice and SAP-deficient mice. Our data showed increased severity of EAE in SAP-deficient mice and decreased severity of EAE in SAP transgenic mice. And meanwhile, adoptive transfer of Ag-restimulated T cells from wild-type to SAP transgenic mice or transfer of SAP transgenic Ag-restimulated T cells to control mice induced weaker symptoms of EAE. Moreover, lymph node and spleen cells from MOG-primed SAP transgenic mice showed weak proliferative responses and TH-1 and TH-17 responses; Furthermore, the result of immunohistochemistry showed there is little infiltration of the spinal cord by CD45 and H&E in SAP transgenic mice suffered from EAE. All the results indicate SAP can effectively suppress the development of EAE.
SAP belongs to the pentraxin family of proteins, which are endowed with Ca2+-dependent lectin-like binding activity. Native human SAP is arranged as a flat cyclic pentamer consisting of five noncovalently associated identical subunits of 25,462 kDa each.24, 25 Among the pentraxins, SAP and C-reactive protein (CRP) are secreted proteins with a similar molecular mass, a high degree of conservation throughout evolution, and a comparable genomic organization. Both SAP and CRP are produced and catabolized in the liver of humans and rodents, 25, 26 but they show considerable species differences in their regulation. CRP is highly inducible in humans but not in mice during inflammation. In contrast, SAP is the major acute-phase protein in mice.27
Multiple sclerosis is considered a classical T cell-mediated autoimmune disease with a complex genetic background. The mechanisms involved in producing the acute lesion of multiple sclerosis seem to share similarities with those described for EAE. Over the past decade, progress has been made in elucidating the immunological events in particular. Despite such progress, however, many important critical issues remain unknown.
In EAE model, Subcutaneous injection of myelin antigen into animals or adoptive transfer of purified MOG-specific T cells obtained from EAE mice into naive mice can both induce the disease. Our results of active EAE showed the increase of SAP suppressed the severity of EAE (Figure 1 and supplementary Figure 1), and the adoptive transfer experiments (Figure 2A–B) showed that the milder disease was directly related to SAP transgenic mice donor cells, and disease severity was mediated by decreased cellular immunity. Additionally, the proliferative capacity of SAP transgenic T cells was suppressed in the onset, peak and end of EAE (Figure 4). All these results suggest the suppressed T cellular activity of SAP transgenic mice induced the decreased severity of the EAE. Moreover, we also observed an attenuated development of disease in SAP transgenic recipient mice injected by encephalitogenic cells from wild-type mice (Figure 2C–D). This suggests the enriched environment of SAP also can suppress the cellular immunity of T encephalitogenic cells in SAP transgenic mice conversely. These results indicated SAP suppressed the development of EAE by regulating the activity of T cellular activity.
IL-2, a 15 kDa α-helical cytokine, is produced almost exclusively by activated T cells and promotes proliferation of lymphocytes.28,29 It is important for the differentiation of CD4+ T cells into Th1 and Th2 effector subsets.30–32 P-selectin (CD62P) is a member of the selectin family of cell adhesion molecules, which interacts with P-selectin glycoprotein ligand-1 (PSGL-1, CD162) for leukocyte rolling on stimulated endothelial cells and heterotypic aggregation of activated platelets onto leukocytes.33 Some study have proved that P-selectin can mediate the recruitment of Th1 into inflamed tissues,34 here we first report that P-selectin is able to stimulate the secretion of IL-2, which is neutralized by SAP (Figure 5A). The result suggests that P-selectin can affect the development of EAE by modulating the recruitment of Th1 and SAP can bind to P-selectin to suppress this effect.
Researchers have demonstrated that T cell recruitment across the BBB during EAE is unique due to the predominant involvement of α4 integrins. After the seminal study by Yednock et al.35 demonstrating that Abs blocking α4 integrin inhibit EAE by blocking T cell interaction with the BBB, numerous following studies confirmed and extended the predominant involvement of α4 integrin/VCAM-1 in inflammatory cell recruitment into the CNS in different EAE models in a number of species.36–39 However, the role of P-selectin in CNS autoimmunity has been controversial. Some studies have reported that anti-Pselectin Abs40 had not any impact on the incidence, severity, or development of EAE. Despite this, a number of studies making direct observations in vivo have demonstrated a central role for P-selectin in mediating leukocyte rolling in the CNS vasculature in a number of inflammatory models 41–44 including EAE.45 Our own observations are in complete agreement with these previous studies. Indeed, according to some researcher’s hypothesis, only by combining the blockade of P-selectin with blockade of α4 integrin could recruitment be sufficiently reduced to impact disease and reveal the role of P-selectin in disease development. This is evidenced by dual treatment translated into a significant inhibition of disease development as demonstrated by not only a delay in the onset of clinical signs of disease, but also the significant recovery following the initial attack. Our data showed SAP can not only suppress the function of P-selectin to stimulate the secretion of IL-2 and bind to T cells, but also change the affinity between α4-integrin and T cells. So we have proved SAP suppresses the development of EAE by modulating the function of α4 integrin and P-selectin. We also noticed that SAP has ability to activate microglia, 46 however the result of our research showed this ability has not induced obvious inflammation in the CNS (Figure 3). It indicates that SAP may be considered as a potential disease-modifying agent in EAE. In summary, these discoveries provide a basis for further investigation of SAP in the treatment and prevention of EAE and MS.
METHODS
Peptides and Antibodies
Rat MOG35–55 peptides were obtained from Biosynth International and purified by HPLC, and the purity of the peptide was >95%. The sequence of MOG35–55 were MEVGWYRSPFSRVVHLYRNGK. Murine serum amyloid P component (mSAP) was isolated from mouse ascites to homogeneity according to a previously published protocol.47 Purified Hamster Anti-Mouse CD3e, Rat Anti-Mouse IL-17A, Rat Anti-Mouse CD62P and FITC-conjugated Rat Anti-Mouse CD4 were purchased from BD Pharmingen (Basel, Switzerland); Rat anti-mouse CD45 was purchased from AbD Serotec (Kidlington, UK). Rat anti-Mouse IFN-γ was purchased from invitrogen (Camarillo, CA).
Mice
C57BL/6J mice were obtained from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). SAP transgenic mice were constructed by our lab. SAP-deficient mice on a C57BL/6 background were kindly provided by Dr. Mark B. Pepys (Royal Free and University College Medical School, London, UK). The genotype of mice was confirmed by PCR before and after the experiments. The procedure for animal surgery was performed in accordance with the Guidelines of Animal Care and Use Committee of the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences (SIBS), Chinese Academy of Sciences (CAS).
SAP transgenic mice were constructed according to the method described below. The wild-type, full-length murine SAP cDNA was cloned by reverse transcriptase-polymerase chain reaction (RT-PCR) from mRNAs isolated from mouse livers, and its sequence was confirmed by DNA sequencing. For construction of murine SAP transgene (mSAP), the forward primer, 5′-GTCTGCGGATCCATGGACAAGCTACTGCTT-3′, and the reverse primer, 5′-ACAGTACTCGAGATCCCAGACACGGGGCCT-3′, were used to amplify the insert from murine SAP cDNA. After restriction enzyme digestion with BamHI/XhoI, it was ligated into pCMV-Tag5A (Invitrogen). For high expression in vivo, it was cut with BciVI to generate a 2659 bp fragment, prior to injection into the pronuclei of mouse 129 embryonic stem cells. Transgenic offsprings (5 male and 7 female in total) were verified and subsequently screened by PCR employing the following primers: 5′-TACAGTGACCTTTCCCGCTCTCAGAGTCT-3′ and 5′-AC GACTCACTATAGGGCGAATTGGGTACAC-3′ or 5′-CGTGGATAGCGGTTTGACTCACGGGGATT-3′ and 5′-GAGAGCGGGAAAGGTCACTGTAGGTTCGGAAA-3′. Positive progenies were further confirmed by Southern blotting of the isolated DNAs with the 32P-labeled fragment of murine SAP plasmid. Two transgenic lines with homogeneous mSAP expression were established, among which founder A was expanded for further investigation and is referred to hereafter as SAP transgenic mice. The animals used in these experiments had been backcrossed to C57BL/6J (C57) mice for 12 generations.
Induction and analysis of EAE
Active immunization
Induction of EAE was performed as previously described.48 Briefly, mice were s.c. injected at two sites with 100 or 200 μg of rat myelin oligodendroglial glycoprotein (MOG) peptide 35–55 (MEVGWYRSPFSRVVHLYRNGK; >95% purity) (Bio-Synthesis) emulsified in CFA containing 400 μg of Mycobacterium tuberculosis (Difco Laboratories). On the same day (day 0) and on day 2 postimmunization (p.i.), mice were i.v. injected with 200 ng of pertussis toxin (Sigma-Aldrich). All mice were weighed, examined, and graded daily for neurological signs in a blinded manner as follows: 0, no disease; 1, decreased tail tone or slightly clumsy gait; 2, tail atony and moderately clumsy gait and/or poor righting ability; 3, limb weakness; 4, limb paralysis; and 5, moribund state. Average disease scores were assessed daily. Additionally, in the EAE model we documented the weight changes during the disease course as we have found this to be a valuable additional measure for disease activity. And only mice with a score of at least 2 for >2 consecutive days were judged to have fully developed EAE. The maximum clinical score achieved by each animal during the 30-day observation period was used to calculate average maximum clinical scores for each experimental group. To study the time course of disease development, average clinical scores were calculated daily for each group of mice and plotted. The day of EAE onset was calculated by adding the first day of clinical signs for individual mice and dividing by the number of mice in the group. The day of peak EAE was calculated by determining the first day of maximum EAE score for individual mice and dividing by the number of mice in the group. Mean maximum score was calculated by adding peak scores of individual mice and dividing by the number of mice. Cumulative EAE score was calculated by adding total EAE scores from onset until day 30 p.i. for individual mice and dividing by the number of mice. Active immunization with MOG35–55 induced monophasic EAE in B6 mice and was followed for 30 days. Animals were euthanized if scores were worse than grade 4.
Adoptive transfer
To prepare encephalitogenic cells for adoptive transfer of EAE, mice were immunized with MOG/CFA in the same fashion as for active EAE. Spleens and lymph nodes were collected 8 days p.i., single cell suspensions were prepared, and RBCs were lysed. Cells (6×106 cells/ml) were cultured in RPMI 1640 medium (supplemented with 10% FBS, 2mML-glutamine, 1mM sodium pyruvate, 100 IU/ml penicillin/streptomycin, and 2×10−5M 2-ME (Invitrogen Life Technologies)) with MOG35–55 (20 μg/ml) and IL-12 (30 ng/ml) (R&D Systems). Three days after initiation of culture, the cells were harvested, washed in PBS, and injected into recipient mice that were irradiated sublethally (500 rad) within 16 h before cells injection. All mice were weighed, examined, and graded daily after cell transfer.49
Immunohistochemistry
For immunohistochemical analysis of CNS tissues at different stages of EAE, mice were sacrificed by intracardiac perfusion with ice-cold PBS, followed by 4% paraformaldehyde solution, under anesthesia. Spinal cords were rapidly dissected; 6μm cryostat sections were prepared, rinsed in PBS, incubated with 0.3% hydrogen peroxide, blocked by incubation with 10% BSA at 37°C for 1 h, then incubated overnight at 4°C with primary Abs at the dilution indicated: rat anti-mouse CD45 mAbs, 1/1000 (clone MCA 1388; Serotec); On day 2, tissues were incubated with appropriate biotinylated secondary Abs (goat anti-rat) at 37°C for 1 h, then with DAB. Sections were washed thrice with PBS after each incubation step (except for BSA). All Abs were diluted in 1% BSA in PBS. Tissues were rinsed in double distilled H2O, dehydrated, and mounted. Negative controls were incubated with preimmune IgG.
T cell purification and In vitro T cell proliferation
CD4+ T cells were isolated from pooled spleens of mice using Mouse T Cell CD4 Subset Column Kit as per the manufacturer’s protocol (R&D Systems). Briefly, Spleen Leukocyte suspensions are incubated with a mixture of monoclonal antibodies and then loaded onto T Cell Subset Columns. The resulting column eluate contains a highly enriched T cell subset population. For T cell proliferation, draining lymph node cells from MOG35–55-immunized mice were incubated in a 96-well plate (1×105/well) in the presence of MOG35–55 or Con A (Sigma-Aldrich) at the indicated concentration or in medium alone (background). For all experiments, culture medium was RPMI 1640, supplemented with 10% FBS (Invitrogen Life Technologies), 2 mM L-glutamine, 1 mM sodium pyruvate, 100 IU/ml penicillin/streptomycin, and 2×10−5 M 2-ME (Invitrogen Life Technologies). After a total 72 h in culture, Remove the culture plate from the incubator and add 50 μl of Cell Lysis Reagent to each well and wait 10 minutes. Add 100 μl of AMR PLUS to each appropriate well and incubate the plate for 2 minutes at room temperature. Then analyzed with luminometer(Bio-Tek).
Flow cytometry
Single-cell suspensions (1 × 106 cells/ml) from lymph nodes obtained were washed and resuspended in PBS buffer. Cells were stained for surface markers with primary Abs and appropriate FITC-conjugated secondary Abs in FACS buffer at 4°C for 40 min. Cells were washed twice and resuspended in the 200–400 μl of PBS for flow cytometry analysis as described before.50, 51 Analysis was performed with a FACSCalibur (BD Biosciences) equipped with CellQuest software (BD Biosciences), and 10,000 events were acquired. Data were analyzed with FlowJo software (Tree Star).
For the binding experiments, CD4+ cells were preincubated with mouse IgG or P-selectin Rg and Ca2+, and respectively with mouse SAP anti-CD62P or EDTA for 60 min. Then samples were incubated with FITC-conjugated Abs to mouse IgG and analyzed with FACSCalibur (BD Biosciences).
ELISA assay
For analysis of IL-2, CD4+ cells were preincubated with different stimulator (hamster IgG, anti-mouse CD3e, mouse IgG, P-selectin Rg, 10 μg mouse SAP and 20 μg anti-CD62P). Supernatants from triplicate cultures were harvested after 24 h. IL-2 was quantified by using mouse IL-2 enzyme-linked immunosorbent assay (ELISA) kit (BioSource International Inc.).
Kinetic Analysis of Peptide Binding by FACS Analysis
The affinity states of VLA-4 can be recognized by mAbs sensitive to its molecular conformation. Recently, it has also been shown that the conformations of VLA-4 can be evaluated with a peptide ligand derived from the LDV sequence of the VLA-4-binding region of fibrinogen. Peptide binding affinity has been measured by flow cytometric analysis. Single-cell suspensions from lymph nodes obtained were washed and resuspended in PBS buffer (1–2×106 cells/ml). Then cells were preincubated with the 3 μM fluorescent VLA-4-specific peptide for 10 min at 37 °C and 500 rpm stirring. Flow cytometric analysis was performed: The samples were analyzed for 10–20 s to establish a base line, then The stimulus (IgG, SAP or Mn2+) was added, and FACS acquisition was immediately re-established, losing 5–10 s of the total time course. Flow cytometric analysis was performed continuously for up to 500 s. The resulting data were analyzed using FlowJo software (Tree Star).23
Statistical analysis
Statistical analysis was assessed by one way ANOVA followed by Student–Newman–Keuls analyses. An unpaired t test was used for the analysis of quantitative data of cell counting in the brain. Data were presented as means ± SD. Difference in which p < 0.05 was considered statistically significant.
Supplementary Material
SAP knockout mice develop distinguishable EAE from C57BL/6 wild-type mice. SAP knockout and wild type mice were immunized with 100 μg MOG35–55. The results of average disease scores (A) and average weight change (B) assessed daily following immunization with MOG35-55 are shown; Data are shown as mean ± SD; n = 15 for each group (a combination of three separate experiments with five mice per group). *P <0.05 (Student’s t-test).
Th1 and Th17 cell reaction is impaired in SAP transgenic mice suffered EAE. Lymph node cells were isolated from wild-type and SAP transgenic mice at the peak of EAE, and the frequency of IFN-γ (A) and IL-17 (B) producing cells were measured by FACS. Numbers in quadrants indicate percent cells in each. And the combined data were analyzed (C). Data are representative of at least three different experiments with groups of four or five mice per condition
Acknowledgments
We thank Mark B. Pepys for SAP-deficiency mice and Xinhui Pei for helping construct SAP-transgenic mice. This study was supported by the National Science Foundation of China (30801031 to Z. J.) and China Postdoctoral Science Foundation (to Z. J.).
Footnotes
Conflict of interest:
The authors declare no financial or commercial conflict of interest.
Supplementary information is available at Immunology and Cell Biology’s website.
References
- 1.Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Phil Trans R Soc Lond B. 2001;356:203–211. doi: 10.1098/rstb.2000.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 3.Bickerstaff MCM. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nature Med. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 4.Pepys MB. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Natl Acad Sci USA. 1994;91:5602–5606. doi: 10.1073/pnas.91.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahedi K. Characterization of the binding of serum amyloid P to laminin. J Biol Chem. 1997;272:2143–2148. [PubMed] [Google Scholar]
- 6.Bickerstaff MCM, Botto M, Hutchinson W, Herbert J, Tennent GA, Bybee A. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 7.Pepys MB, Booth DR, Hutchinson WL, Gallimore JR, Collins PM, Hohenester E. Amyloid P component. A critical review. Amyloid Int J Exp Clin Invest. 1997;4:274–295. [Google Scholar]
- 8.Pepys MB, Herbert J, Hutchinson WL, Tennent GA, Lachmann HJ, Gallimore JR. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417:254–259. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]
- 9.Emsley J, White HE, O’Hara BP, Oliva G, Srinivasan N, Tickle IJ. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 10.Bharadwaj D, Mold C, Markham E, DuClos TW. Serum amyloid P component binds to Fcgamma receptors and opsonizes particles for phagocytosis. J Immunol. 2001;166:6735–6741. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- 11.Du Clos TW. The interaction of C-reactive protein and serum amyloid P component with nuclear antigens. Med Biol Rep. 1996;23:253–260. doi: 10.1007/BF00351177. [DOI] [PubMed] [Google Scholar]
- 12.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504. [PubMed] [Google Scholar]
- 13.Lassmann H, Wisniewski HM. Chronic relapsing experimental llergic encephalomyelitis: clinicopathological comparison with multiple sclerosis. Arch Neurol. 1979;36:490–497. doi: 10.1001/archneur.1979.00500440060011. [DOI] [PubMed] [Google Scholar]
- 14.Bernard CC, Leydon J, Mackay IR. T cell necessity in the pathogenesis of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 1976;6:655–660. doi: 10.1002/eji.1830060912. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda T, Tsumita T, Nagai Y, Mitsuzawa E, Ohtani S. Experimental allergic encephalomyelitis (EAE) in mice. I. Induction of EAE with mouse spinal cord homogenate and myelin basic protein. Jpn J Exp Med. 1975;45:423–427. [PubMed] [Google Scholar]
- 16.Mendel I, Kerlero dR, Ben Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor Vβexpression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 17.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Engelhardt B, Vestweber D, Hallmann R, Schulz M. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood-brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459–4472. [PubMed] [Google Scholar]
- 19.Uboldi C, Doring A, Alt C, Estess P, Siegelman M, Engelhardt B. L-Selectin-deficient SJL and C57BL/6 mice are not resistant to experimental autoimmune encephalomyelitis. Eur J Immunol. 2008;38:2156–2167. doi: 10.1002/eji.200838209. [DOI] [PubMed] [Google Scholar]
- 20.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerfoot SM, Kubes P. Overlapping roles of P-selectin and α4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J Immunol. 2002;169:1000–1006. doi: 10.4049/jimmunol.169.2.1000. [DOI] [PubMed] [Google Scholar]
- 22.Kerfoot SM, Norman MU, Lapointe BM, Bonder CS, Zbytnuik L, Kubes P. Reevaluation of P-selectin and alpha 4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:6225–34. doi: 10.4049/jimmunol.176.10.6225. [DOI] [PubMed] [Google Scholar]
- 23.Chigaev A, Blenc AM, Braaten JV, Kumaraswamy N, Kepley CL, Andrews RP, et al. Real Time Analysis of the Affinity Regulation of α4-Integrin. J Biol Chem. 2001;276:48670–48678. doi: 10.1074/jbc.M103194200. [DOI] [PubMed] [Google Scholar]
- 24.Pepys MB, Rademacher TW, Amatayakul-Chantler S, Williams P, Noble GE, Hutchinson WL, et al. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Natl Acad Sci USA. 1994;91:5602–5606. doi: 10.1073/pnas.91.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson WL, Hohenester E, Pepys MB. Human serum amyloid P component is a single uncomplexed pentamer in whole serum. Mol Med. 2000;6:482–493. [PMC free article] [PubMed] [Google Scholar]
- 26.Pepys MB, Dash AC, Markham RE, Thomas HC, Williams BD, Petrie A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol. 1978;32:119–124. [PMC free article] [PubMed] [Google Scholar]
- 27.Steel DM, Whitehead AS. The major acute phase reactants: Creactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 28.Walker E, Leemhuis T, Roeder W. Murine B lymphoma cell lines release functionally active interleukin 2 after stimulation with Staphylococcus aureus. J Immunol. 1988;140:859–865. [PubMed] [Google Scholar]
- 29.Ho IC, Kim JI, Szabo SJ, Glimcher LH. Tissuespecific regulation of cytokine gene expression. Cold Spring Harb Symp Quant Biol. 1999;64:573–584. doi: 10.1101/sqb.1999.64.573. [DOI] [PubMed] [Google Scholar]
- 30.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Laurence A. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Kryczek I. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 33.Chen M, Geng JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp. 2006;54:75–84. doi: 10.1007/s00005-006-0010-6. [DOI] [PubMed] [Google Scholar]
- 34.Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 35.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 36.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of α4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steffen BJ, Butcher EC, Engelhardt B. Evidence for involvement of ICAM-1 and VCAM-1 in lymphocyte interaction with endothelium in experimental autoimmune encephalomyelitis in the central nervous system in the SJL/J mouse. Am J Pathol. 1994;145:189–201. [PMC free article] [PubMed] [Google Scholar]
- 38.Kent SJ, Karlik SJ, Cannon C, Hines DK, Yednock TA, Fritz LC, et al. A monoclonal antibody to α4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:1–10. doi: 10.1016/0165-5728(94)00165-k. [DOI] [PubMed] [Google Scholar]
- 39.Engelhardt B, Laschinger M, Schulz M, Samulowitz U, Vestweber D, Hoch G. The development of experimental autoimmune encephalomyelitis in the mouse requires α4-integrin but not α4β7-integrin. J Clin Invest. 1998;102:2096–2105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelhardt B, Vestweber D, Hallmann R, Schulz M. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood-brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459–4472. [PubMed] [Google Scholar]
- 41.Battistini L, Piccio L, Rossi B, Bach S, Galgani S, Gasperini C, et al. CD8+ T cells from patients with acute multiple sclerosis display selective increase of adhesiveness in brain venules: a critical role for P-selectin glycoprotein ligand-1. Blood. 2003;101:4775–4782. doi: 10.1182/blood-2002-10-3309. [DOI] [PubMed] [Google Scholar]
- 42.Piccio L, Rossi B, Scarpini E, Laudanna C, Giagulli C, Issekutz AC, et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric Gi-linked receptors. J Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho-Tavares J, Hickey M, Hutchison JJ, Michaud J, Sutcliffe IT, Kubes P. A role for platelets and endothelial selectins in tumor necrosis factor-α-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–1148. doi: 10.1161/01.res.87.12.1141. [DOI] [PubMed] [Google Scholar]
- 44.Yong T, Zheng MQ, Linthicum DS. Nicotine induces leukocyte rolling and adhesion in the cerebral microcirculation of the mouse. J Neuroimmunol. 1997;80:158–164. doi: 10.1016/s0165-5728(97)00151-3. [DOI] [PubMed] [Google Scholar]
- 45.Kerfoot SM, Kubes P. Overlapping roles of P-selectin and α4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J Immunol. 2002;169:1000–1006. doi: 10.4049/jimmunol.169.2.1000. [DOI] [PubMed] [Google Scholar]
- 46.Piet E, Rob V, Atoosa F, Jeroen JMH, Willem AVG, Annemieke JMR. Neuroinflammation in Plaque and Vascular β-Amyloid Disorders: Clinical and Therapeutic Implications. Neurodegener Dis. 2008;5:190–193. doi: 10.1159/000113699. [DOI] [PubMed] [Google Scholar]
- 47.Pepys MB. Isolation of serum amyloid P-component (protein SAP) in the mouse. Immunology. 1979;37:637–641. [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Huang D, Matsui M, He TT, Hu T, DeMartino J, et al. Severe disease, unaltered leukocyte migration, and reduced IFN-γ production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176:4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- 49.Marusic S, Leach MW, Pelker JW, Azoitei ML, Uozumi N, Cui J, et al. Cytosolic phospholipase A2α-deficient mice are resistant to experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia YF, Liu LP, Zhong CP, Geng JG. NF-κB activation for constitutive expression of VCAM-1 and ICAM-1 on B lymphocytes and plasma cells. Biochem Biophys Res Commun. 2001;289:851–856. doi: 10.1006/bbrc.2001.6067. [DOI] [PubMed] [Google Scholar]
- 51.Liu LP, Xia YF, Yang L, DiDonato JA, DiCorleto PE, Zhong CP, et al. B lymphocytes and plasma cells express functional E-selectin by constitutive activation of NF-κB. Biochem. Biophys Res Commun. 2001;286:281–291. doi: 10.1006/bbrc.2001.5344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SAP knockout mice develop distinguishable EAE from C57BL/6 wild-type mice. SAP knockout and wild type mice were immunized with 100 μg MOG35–55. The results of average disease scores (A) and average weight change (B) assessed daily following immunization with MOG35-55 are shown; Data are shown as mean ± SD; n = 15 for each group (a combination of three separate experiments with five mice per group). *P <0.05 (Student’s t-test).
Th1 and Th17 cell reaction is impaired in SAP transgenic mice suffered EAE. Lymph node cells were isolated from wild-type and SAP transgenic mice at the peak of EAE, and the frequency of IFN-γ (A) and IL-17 (B) producing cells were measured by FACS. Numbers in quadrants indicate percent cells in each. And the combined data were analyzed (C). Data are representative of at least three different experiments with groups of four or five mice per condition