Abstract
People with heterozygous status for sickle cell disease (also called “sickle cell trait”) are essentially healthy, but evidence about rare health problems has increased interest in screening adolescents and young adults before athletics or military service. Ironically, almost everyone with sickle cell trait is already identified during routine newborn screening for sickle cell disease, but this identification may never reach the parents. As part of a larger statewide study of communication after newborn screening, we decided to document the amount of labor required to connect sickle cell trait screening results with primary care providers (PCPs). Case review methods examined records and call logs from the first 150 cases in a 42-month project. Our study procedures identified PCPs for 136/150 infants (90.6%); a total of 266 phone calls were needed. We identified nine categories of experiences, ranging from incorrect baby names to restrictions on accepting Medicaid patients. Cases demonstrate that it is possible to connect with most PCPs after newborn screening despite warnings about difficulties with this population. Success was due to persistence, relationships with clinics and hospitals, and Internet search capabilities. If sickle cell trait identification is necessary to protect health, then only modest increases in effort will be needed to reduce disparities in service.
Keywords: Sickle cell trait, Newborn Screening, Genetic screening, Provider-patient communication
Introduction
Newborn infants are routinely screened for sickle cell disease (SCD) as part of newborn screening (NBS), in order to prevent life-threatening infections and other causes of childhood morbidity and mortality1,2. NBS methods also inadvertently identify infants who are heterozygous for the SCD mutation, a genetic status often referred to as “sickle cell trait.” Sickle cell trait is defined by the presence of fetal, adult, and sickle hemoglobin. NBS programs strive to rapidly notify providers and parents of infants with SCD, but recent evidence suggests the possibility of a disparity in reporting NBS results since many sickle cell trait and other NBS results may not be reaching primary care providers (PCPs) or parents at all3–5. There may be several reasons for this disparity, and indeed it is not clear how much effort should be spent on locating providers for infants with sickle cell trait. Recent interest in screening adolescents and young adults for sickle cell trait led us to examine the amount of effort required to locate PCPs of infants with sickle cell trait results identified by NBS, in order to inform future policy decisions by NBS laboratories and other screening programs.
Sickle cell trait status is usually thought of as an essentially benign condition, but notifying families about sickle cell trait results may be important for several reasons. People with sickle cell trait often have mild anemia or hematuria,6 and a very small number may develop more serious problems6–13. People with sickle cell trait may also be at slight risk for sequestration crises or even sudden death after strenuous exercise, severe dehydration, or exposure to altitude or hypoxia6,14–17. Although evidence for these problems is not conclusive, concerns have led to interest in screening for sickle cell trait in athletes18–20 and in certain branches of the military21,22. In fact, the National Collegiate Athletic Association (NCAA) now requires Division I athletes to undergo testing for sickle cell trait, or sign a waiver that would release institutions from liability18–20. However, this policy is not applied uniformly across other NCAA schools, which have many more athletes than the Division I schools.
Another potential reason to notify families about sickle cell trait results is that the status may have implications for reproductive decision-making. Heterozygotes can pass sickle cell trait on to future children. In addition, they may have children with a version of SCD if the other parent also has at least one abnormal hemoglobin gene. Parents may wish to get themselves tested to know if both are heterozygous, which may impact future children. The infant may someday wish to know about his or her sickle cell trait status, in order to be prepared. Members of the extended family may also wish to know if their future children are at risk for having SCD.
On the other hand, controversy exists about whether infants should be screened for the exclusive purpose of informing future reproductive decision-making, in part because the infant cannot be said to have given informed consent23,24. Furthermore, there is some question about whether this type of information actually influences reproductive decision-making25–26, or if it is worth the anxiety and psychosocial complications that some families experience after trait status is identified27–31. As a result, some have argued that screening programs should not inform PCPs or parents about sickle cell trait status27,32–34. This may be ethically questionable, regardless of whether the information is likely to be harmful or beneficial. In this view, the sickle cell trait result is information that belongs to the infant and its parents27,28,35. Not disclosing these results may amount to censorship.
Given the potential importance of assuring that parents and families are made aware of sickle cell trait status, the purpose of this report is to demonstrate the amount and type of labor required to confirm the correct PCPs for sickle cell trait infants in our subject population.
Method
This analysis was done with a subset of data from “the Wisconsin Project on Improvement of Communication Process and Outcomes after Newborn Screening.”3 The project is an ongoing a quality improvement and research effort by the NBS laboratory of the Wisconsin State Laboratory of Hygiene and the Department of Health Services, with the Medical College of Wisconsin as a contracted project agent. The primary purpose of the project is to assess how physicians communicate newborn screening results to parents, what parents understand about newborn screening, and how communication of newborn screening results might be improved. The project began enrolling infants in November 2007 and continues as of this manuscript publication. Data is collected from two interviews: one with the physician shortly after the newborn screening results indicates an infant has sickle cell trait or is a carrier for cystic fibrosis, and an interview with the infant’s parents when the child is 2–3 months old.
Infants enrolled are born in the state of Wisconsin and identified by newborn screening as either having sickle cell trait or are carriers for cystic fibrosis. Infants were excluded from the project if they were less that 35 weeks gestation age, spent more than 5 days in the neonatal intensive care unit, if the parent spoke a language other than English or if the physician did not want us to contact the family for follow-up. Project methods and materials were approved by Institutional Review Boards at the Medical College of Wisconsin and University of Wisconsin, Madison.
As part of this project, we also want to assess how much effort is required to assure that newborn screening results are sent to the correct physician, so that the physician can counsel the parents about the result. The usual NBS practice in Wisconsin is for the laboratory to send results to the provider listed on the NBS card. This listing is supplied by the staff of the nursery at the birthing facility, along with the mother’s last name and the infant’s name and medical record number for the facility. These listings may only reflect what is known at the time the sample is taken. For example, NBS cards may be filled out with the designation “boy” or “girl” instead of a first name, or a nursery attending or neonatologist may be incorrectly designated as the PCP responsible for follow-up 3. In Wisconsin, when the results indicate sickle cell trait, the results are mailed to the provider. There are approximately 650–800 sickle cell trait cases in Wisconsin per year36. It should be noted that Wisconsin statute restricts the NBS laboratory from contacting families about abnormal screening results, requiring it instead to provide information to infants’ care providers to facilitate follow-up testing and referrals. Wisconsin’s NBS laboratory does not follow up with providers regarding sickle cell trait results, and does not confirm if the sickle cell trait results were ever disclosed to the parents.
An illustration of the methods for the Wisconsin Project on Improvement of Communication Process and Outcomes after Newborn Screening is shown in Figure 1. When the NBS laboratory identified an infant with a sickle cell trait result, the result report was sent to our project team in addition to the provider listed on the NBS card. Shortly after receiving those results, our study team sent an introductory fax and a copy of the NBS result to the provider listed on the report. After the fax was sent, the project coordinator called the listed provider’s clinic to confirm that our fax was received and to verify with the provider’s nurse or medical assistant that the provider listed was the one who will be following up with the infant’s family. In some cases, we would speak directly to the listed clinician to try to confirm who the infant’s provider was going to be. Additional phone calls and Internet queries were made as needed until we were able to identify the correct PCP. Phone calls to the clinics were limited to every other day so we would not create an extra burden to the clinic staff with our phone queries.
Figure 1. Usual practice (left) and Project methods (right) after newborn screening identifies sickle cell trait.
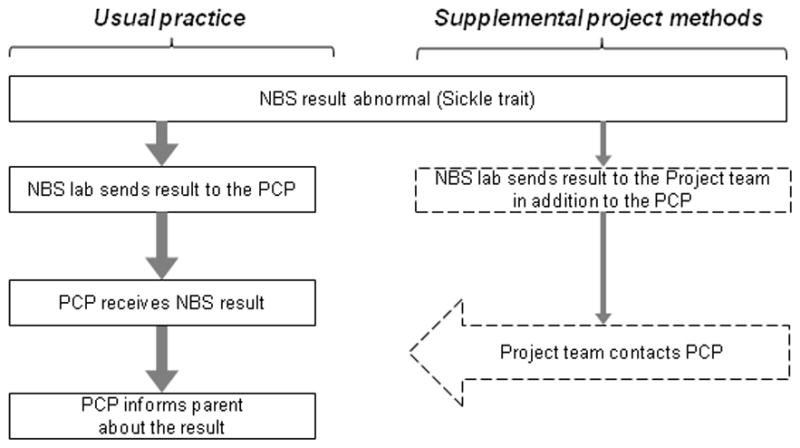
*Abbreviations: NBS, newborn screening; PCP, the infant’s primary care provider.
Adapted with permission from: Farrell et al. “Improving communication between doctors and patients after newborn screening.” Wisconsin Medical Journal, 2011; 110 (5). Copyrighted © 2011, Wisconsin Medical Association. All rights reserved.
After identifying the correct PCP, we spoke with that provider on the telephone. During this call, we asked the PCP if he or she had any questions about the NBS result, and also when he or she planned to discuss the result with the infant’s parent(s). These data and other information gathered during the project will be reported elsewhere.
The interviewers kept detailed logs about the number of phone calls, effort required, and other issues that came up in identifying the infants’ providers. After the first 150 sickle cell infants, we pulled the search logs to analyze the amount of effort required to identify and contact the infants’ physicians, including the date we made our first phone call and the date we confirmed the PCP. When we started the project, there were concerns that the project was not sustainable because we would not be able to find these infants and their physicians in a systematic way. This analysis was done to demonstrate the long-term viability of the larger quality improvement project. We read the logs to look for common themes and experiences in looking for the PCPs, and to count telephone calls for comparison. As will be described in the Results section, details in these records allowed us to define nine categories that described the effort required to find an infant’s PCP.
Results
We succeeded in confirming the PCPs for 136/150 infants (90.6%). This included 101 individual PCPs, as some of the providers had a primary care role for more than one infant in our sample.
We found nine different categories of recruitment experiences during our searches for the infants’ PCPs. These categories are described in Table 1, along with the average number of telephone calls for each category (not including faxes and calls necessary to confirm fax numbers). Overall, a total of 266 phone calls were needed to confirm PCPs for the 136 infants. It should be noted that this number includes PCPs for 45 more infants than would have otherwise received their NBS result if usual practice methods had been followed (reported by us elsewhere as 60.6%, or what would have been 91/150) 3. The additional cases included 15 infants where a provider was sent a result and initially denied knowing the patient because an incomplete name was listed on the NBS report.
Table 1.
Details about the search and amount of labor needed to confirm the correct PCPs for the first 150 sickle trait infants
| Description of our search for the infant’s PCP | Number of calls per infant* | ||
|---|---|---|---|
| Median | SD | ||
| Rapid success = 91 | |||
| 85 infants | The listed provider knew that he or she was the infant’s PCP | 1 | .3 |
| 6 infants | The listed provider was the PCP or a partner, but a nurse or other staff person had been delegated to inform the parent about the result. | 1 | .4 |
| Eventual success = 45 | |||
| 18 infants | The listed provider was not the PCP but knew the correct PCP | 2 | 1.4 |
| 5 infants | We identified the PCP after a call to the birthing facility’s nursery or medical records department | 3 | .9 |
| 2 infants | We identified the PCP after a second NBS report was sent to us with a different PCP | 4 | 0 |
| 15 infants | The listed provider initially denied knowing the infant, but we learned later that the parent subsequently called the PCP’s office for an appointment | 3 | .5 |
| 5 infants | The listed provider initially claimed to be the PCP, but we later discovered that the infant had been moved to another PCP’s practice | 3 | 1.5 |
| Failure = 14 | |||
| 5 infants | PCP search ended when an exclusion criterion was identified** | 2 | 1.3 |
| 9 infants | PCP was not found using protocol criteria*** | 4 | .8 |
Number of calls does not include faxes and calls needed to verify fax numbers or other contact information
Exclusion criteria were if the infant was less than 35 weeks gestation age, spent more than 5 days in the NICU, or had a parent that spoke a language other than English.
The IRB protocol specifically prohibited us from contacting the parents directly to ask who their infant’s physician is. We could only seek this information from clinic, hospital or insurance records.
As shown in Table 1, the number of telephone calls required to confirm the PCP was greater when the birthing facility’s listing was inaccurate or the NBS laboratory’s database was outdated. More effort was also required to confirm PCPs when the primary care responsibility was ambiguous or changed over time. The calls were made by one full-time project coordinator. Per our protocol, we only made query phone calls to the clinics every other day. Thus the cases where it took 3–4 phone calls to identify the correct PCP may have taken 5–7 business days.
Our protocol for finding the infant’s physicians was limited by a few restrictions made by the IRB. The primary restriction was that we could not attempt to contact parents directly to ask them who they planned on taking their infant to for medical care. We were limited to searching for this information in hospital, clinic and insurance records.
Many of our experiences involved interactions with nurses and office staff, at least as often as the PCPs themselves. The following illustrative cases provide some examples of the complexity sometimes necessary to identify the correct PCP, and provide additional insight into procedures that would be necessary to connect sickle cell trait results with PCPs.
Illustrative cases
Example 1 – Hospital provides correct first and last name to identify patient
For many of the infants, the clinics did not recognize the infant because the name on the NBS card was “Boy” or “Girl” followed by the mother’s last name, whereas the PCP’s clinic knew the infant by an actual first name and the father’s last name. In these cases, the date of birth was not enough for the clinic staff to connect the NBS result with the correct infant. To clear up the confusion, it was necessary for us to call the medical records office at the hospital where the infant was born. The medical records personnel were able to provide the infants’ names by their medical record number, which is provided on the NBS card. We then called the listed provider’s office staff with the correct name, and they did have that infant listed as a patient.
Example 2 – Provider initially denied knowing infant
In this example, the office staff for the listed provider had not heard of the infant and suggested that the nursery might have confused their provider with another provider in the same city who had a similar last name. When we called the second provider we found that he could not be the PCP because he was no longer accepting new patients, being close to retirement. The interviewer then called the birthing facility’s medical records department to clarify both the provider’s name and the infant’s actual name. Several calls were needed to keep in touch with these and other providers, but eventually the infant’s mother called the first provider to make an appointment.
Example 3- Listed provider knew the infant, but was not able to see the infant and was unsure if there would be a different PCP
In this case, the office staff for the listed provider said the infant had an appointment in 4 months, because of clinic restrictions on new Medicaid patients. The staff was unsure if the infant was going to be seen somewhere else in the meantime. A week later, a repeat call by us found that the infant’s appointment had been moved up to 2 months. The nurse commented that this was far enough out that the infant would likely be seeing another PCP. We later learned that the other provider had never been identified, and the original provider kept the NBS result for when infant came in at 2 months.
Example 4 – Listed provider had been covering for another provider, who was confused about the infant’s original name
In this case, a nurse at the listed provider’s office did not know the infant. At our prompting, the nurse asked the listed provider if he knew the infant. It turned out that the provider did know the infant, but added that he had been covering for another provider who would serve as the infant’s PCP. When we called that second provider, he initially denied knowing the infant based on the information on the NBS card. We called the hospital to get infant’s full name, and then called the second provider again. Once he had heard the infant’s full name, the second provider was able to confirm that he was the infant’s PCP.
Discussion
Experts have debated whether sickle cell trait results should be reported to parents23,24, but most NBS laboratories at least notify infants’ PCPs37–38 or contact the families directly37–39. Given recent interest in screening for sickle cell trait prior to athletics18–20 and military service21,22, and our recent data about possible disparities with sickle cell trait results reaching PCPs and parents3, we decided to publish our experiences quantifying the amount of labor needed to reach PCPs of sickle cell trait infants.
When we first proposed our statewide research Project, some hematologists and NBS experts across the country warned us that it is very difficult to reach families of sickle cell trait infants because they can change residences and often have irregular health care utilization. One SCD expert from another state even commented that families of sickle cell trait infants are a “nearly unservable population.” Fortunately, finding the correct PCPs has been easier than we were told to expect. Our successes have largely been a result of persistence, personal contacts, and patience with busy staff in medical records offices. We have also benefitted from more modern searching methods such as the use of resources on the Internet.
Similar steps may help NBS laboratories who find it challenging to verify that sickle cell trait results have reached the correct provider. Our illustration of the amount of effort and resources that need to be allocated may help NBS laboratories and other screening programs to ensure the results get to the correct PCP. Ensuring that sickle cell trait screening results get to the correct PCP is important so the PCP can disclose those results to families. Making sure parents have these results may also reduce the need for testing later in adolescence or young adulthood, as organizations like the NCAA require testing for sickle cell trait in athlete physicals. It may also be that having those sickle cell trait results may inform family members’ future decision-making, or help them to prepare for the possibility of a future child with SCD.
Our findings may also be of some value to NBS efforts outside of SCD screening, especially for future genetic tests like those for 22q11.2 deletion syndrome40. Unless sufficient resources are allocated for follow-up efforts, it may be that infants with other NBS conditions will also become lost to follow-up.
Limitations
Although our statewide quality improvement project was done on a public health scale, our individual research methods have some limitations and this project had to operate under restrictions imposed by our Institutional Review Boards. The primary limitation was that we were not allowed to contact the parents directly to identify the infants’ PCPs or to ask which clinic the parents planned to attend. This, however, is not solely a limitation of the current study, but is also a structural limitation of the standard practice for the newborn screening program in Wisconsin and elsewhere. In the future, if NBS programs implement similar follow-up procedures, they will need to consider carefully whether it might be more appropriate to contact some parents directly, or if sickle cell trait results should be deferred to hematologists or public health nurses when a PCP cannot be identified.
In contrast to other follow-up projects where a public health nurse counsels families about sickle cell trait41, with the procedures in this project, we were able to confirm that the correct results did reach the correct provider, but discussion and disclosure of those results to the families remained the responsibility of the provider. Though not reported in this manuscript, we later followed up with the families to discuss their understanding of the newborn screening result and when and how the physicians discussed the result with the family42. It is still unclear what the impact of this follow-up in child’s infancy will have on long-term knowledge of newborn screening and sickle cell trait.
This project has conducted as a part of a larger quality improvement project with the Wisconsin Newborn screening program. Other states, especially those with more sickle cell trait cases than Wisconsin, may not have the resources to conduct a quality improvement project on this scale. However, the results from this analysis demonstrate that it is feasible to assure that sickle cell trait results reach the correct physician with a small investment in staff time.
Some might be concerned that 150 infants is a small sample size to draw conclusions about NBS over an entire state. We limited this report’s sample size because it gave us the ability to describe specific cases for readers, and because the follow-up success did not differ significantly for the subsequent hundreds of infants born later in the Project.3
Recommendations
In our view, given the potential clinical importance of sickle cell trait results and existing disparities in results disclosure, it is not a major burden to increase the number of calls in order to connect PCPs with NBS results. To better facilitate the dissemination of results from the NBS laboratory to the appropriate provider, the single most important action we can take is to assure that birthing facilities include accurate information with the NBS card, including the infant’s full name, the mother’s full name and intended PCP. Accurate infant identification can help health care professionals convey information to parents in a timely manner.
To maximize the effectiveness of NBS follow-up and to make sure the screening results get to the correct PCP, it will be necessary for NBS programs to be funded with enough resources to locate PCPs. Then, in order for NBS to remain a viable and valuable service, the next responsibility lies with PCPs to continue to provide information regarding the results to parents in a timely and effective manner. We would hope that PCPs not regard sickle cell trait results as an incidental finding, but should regard it with the same level of importance as other abnormal screening results. In addition, establishing an ongoing relationship and discussion about sickle cell trait may make future discussions more productive. We are quite concerned about national disparities in health care and public health, and grateful that NBS is often able to serve families without regard to background or ethnicity. With cooperation between NBS laboratories, birthing facilities, PCPs, and parents, this service can improve still further. If sickle cell trait identification continues to be regarded as beneficial for the health of adolescents and young adults, our successful experience finding and confirming PCPs, suggests that nobody should ever again refer to families of sickle cell trait infants as “unservable.”
Acknowledgments
This project was funded by grants from the National Health Lung and Blood Institute of the National Institutes of Health (K01-HL072530 and R01-HL086691). We gratefully acknowledge the contribution of Karen Kennedy-Parker and Gary Hoffman at the Wisconsin Newborn Screening Laboratory at the Wisconsin State Laboratory of Hygiene.
Footnotes
Financial disclosure statement: The authors have no financial relationships to disclose.
Contributor Information
Stephanie A. Christopher, Email: schristopher@mcw.edu.
Jenelle L. Collins, Email: jlcollins@mcw.edu.
Michael H. Farrell, Email: mfarrell@mcw.edu.
References
- 1.Pass K, Harris K, Lorey F, Choi R, Kling S. Update: newborn screening for sickle cell disease--California, Illinois, and New York, 1998. MMWR. 2000;49(32):729–731. [PubMed] [Google Scholar]
- 2.Farrell PM, Lai HJ, Li Z, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: enough is enough! J Pediatr. 2005 Sep;147(3 Suppl):S30–36. doi: 10.1016/j.jpeds.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Farrell MH, Christopher SA, Tluczek A, et al. Improving communication between doctors and patients after newborn screening. Wisc Med J. 2011 Oct;110(5):221–227. [PMC free article] [PubMed] [Google Scholar]
- 4.Abhyankar S, Lloyd-Puryear MA, Goodwin R, et al. Standardizing newborn screening results for health information exchange. Paper presented at: American Medical Informatics Association 2010 Symposium; Washington DC. [PMC free article] [PubMed] [Google Scholar]
- 5.Desposito F, Lloyd-Puryear MA, Tonniges TF, Rhein F, Mann M. Survye of pediatrician practices in retrieving statewide authorized newborn screening results. Pediatrics August. 2001;108(2):E22. doi: 10.1542/peds.108.2.e22. [DOI] [PubMed] [Google Scholar]
- 6.Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med Jun. 2009;122(6):507–512. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Ashcroft M, Miall W, Milner P. A comparison between the characteristics of Jamaican adults with normal hemoglobin and those with sickle cell trait. Am J Epidemiol. 1969;90(3):236. doi: 10.1093/oxfordjournals.aje.a121066. [DOI] [PubMed] [Google Scholar]
- 8.Tuck S, Studd J, White J. Pregnancy in sickle cell disease in the UK. Br J Obstet Gynaecol. 1983;90(2):112. doi: 10.1111/j.1471-0528.1983.tb08893.x. [DOI] [PubMed] [Google Scholar]
- 9.Hakimi A, Koi P, Milhoua P, et al. Renal medullary carcinoma: the Bronx experience. Urology. 2007;70(5):878. doi: 10.1016/j.urology.2007.06.1124. [DOI] [PubMed] [Google Scholar]
- 10.Bruno D, Wigfall D, Zimmerman S, Rosoff P, Wiener J. Genitourinary complications of sickle cell disease. J Urol. 2001;166(3):803. [PubMed] [Google Scholar]
- 11.Austin H, Key N, Benson J, et al. Sickle cell trait and the risk of venous thromboembolism. Blood. 2007;110(3):908. doi: 10.1182/blood-2006-11-057604. [DOI] [PubMed] [Google Scholar]
- 12.Bennett N, Mulhall J. Sickle cell disease status and outcomes of African-American men presenting with priapism. J Sex Med. 2008;5(5):1244. doi: 10.1111/j.1743-6109.2008.00770.x. [DOI] [PubMed] [Google Scholar]
- 13.Tita A, Biggio J, Chapman V, Neely C, Rouse D. Perinatal and maternal outcomes in women with sickle or hemoglobin C trait. Obstet Gynecol. 2007;110(5):1113. doi: 10.1097/01.AOG.0000285995.41769.83. [DOI] [PubMed] [Google Scholar]
- 14.Anzalone ML, Green VS, Buja M, Sanchez LA, Harrykissoon RI, Eichner ER. Sickle cell trait and fatal rhabdomyolysis in football training: a case study. Med Sci Sports Exerc. Jan;42(1):3–7. doi: 10.1249/MSS.0b013e3181ae0700. [DOI] [PubMed] [Google Scholar]
- 15.Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med. 1987 Sep 24;317(13):781–787. doi: 10.1056/NEJM198709243171301. [DOI] [PubMed] [Google Scholar]
- 16.Thogmartin JR, Wilson CI, Palma NA, Ignacio SS, Shuman MJ, Flannagan LM. Sickle Cell Trait-Associated Deaths: A Case Series with a Review of the Literature. J Forensic Sci. 2011 Sep;56(5):1352–1360. doi: 10.1111/j.1556-4029.2011.01774.x. [DOI] [PubMed] [Google Scholar]
- 17.Scoville SL, Gardner JW, Magill AJ, Potter RN, Kark JA. Nontraumatic deaths during U.S. Armed Forces basic training, 1977–2001. Am J Prev Med. 2004 Apr;26(3):205–212. doi: 10.1016/j.amepre.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Hosick MB. Protocol decided for sickle cell testing. The NCAA News. 2010 Apr 13; [Google Scholar]
- 19.Stein R. Colleges mandate sickle cell testing. Washington Post. 2010 Sep 20;:A05. [Google Scholar]
- 20.Thomas K, Zarda B. N.C.A.A., question of bias over a test for a genetic trait. The New York Times. 2010 Apr 12;:D1. [Google Scholar]
- 21.Uddin DE, Dickson LG, Brodine CE. Screening of military recruits for hemoglobin variants. JAMA. 1974 Mar 25;227(12):1405–1407. [PubMed] [Google Scholar]
- 22.Scoville SL. Recruit Medicine. Washington, DC: Office of The Surgeon General, United States Army, Borden Institute Walter Reed Army Medical Center; 2006. Chapter 25: Recruit Mortality. [Google Scholar]
- 23.Davis DS. Discovery of children’s carrier status for recessive genetic disease: some ethical issues. Genetic Testing. 1998;2(4):323–327. doi: 10.1089/gte.1998.2.323. [DOI] [PubMed] [Google Scholar]
- 24.Nelson RM, Botkin JR, Kodish ED, et al. Ethical issues with genetic testing in pediatrics. Pediatrics Jun. 2001;107(6):1451–1455. doi: 10.1542/peds.107.6.1451. [DOI] [PubMed] [Google Scholar]
- 25.Gallo AM, Wilkie D, Suarez M, et al. Reproductive decisions in people with sickle cell disease or sickle cell trait. West J Nurs. 2010 Res;32(8):1073–1090. doi: 10.1177/0193945910371482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asgharian A, Anie KA. Women with sickle cell trait: reproductive decision-making. Journal of Reproductive & Infant Psychology. 2003;21(1):23–34. [Google Scholar]
- 27.Oliver S, Dezateux C, Kavanagh J, Lempert T, Stewart R. Disclosing to parents newborn carrier status identified by routine blood spot screening. Cochrane Database of Systematic Reviews. 2004;(4):CD003859. doi: 10.1002/14651858.CD003859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller FA, Hayeems RZ, Bombard Y, et al. Clinical obligations and public health programmes: healthcare provider reasoning about managing the incidental results of newborn screening. J Med Ethics. 2009;35:626–634. doi: 10.1136/jme.2009.030346. [DOI] [PubMed] [Google Scholar]
- 29.Laird L, Dezateux C, Anionwu EN. Neonatal screening for sickle cell disorders: what about the carrier infants? BMJ. 1996 Aug 17;313(7054):407–411. doi: 10.1136/bmj.313.7054.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kai J, Ulph F, Cullinan T, Qureshi N. Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice. Health Technol Assess. 2009 Nov;13(57):1–82. iii. doi: 10.3310/hta13570. [DOI] [PubMed] [Google Scholar]
- 31.Miller FA, Paynter M, Hayeems RZ, et al. Understanding sickle cell carrier status identified through newborn screening: a qualitative study. Eur J Hum Genet. 2010 Mar;18(3):303–308. doi: 10.1038/ejhg.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markel H. The stigma of disease: implications of genetic screening. Am J Med. 1992;93(2):209–215. doi: 10.1016/0002-9343(92)90052-d. [DOI] [PubMed] [Google Scholar]
- 33.McNeil TF, Harty B, Thelin T, Aspegren-Jansson E, Sveger T. Identifying children at high somatic risk: long-term effects on mother- child interaction. Acta Psychiatr Scand Dec. 1986;74(6):555–562. doi: 10.1111/j.1600-0447.1986.tb06284.x. [DOI] [PubMed] [Google Scholar]
- 34.Farrell MH, Certain L, Farrell PM. Genetic counseling and risk communication services of newborn screening programs. Arch Ped Adol Med. 2001;155(2):120–126. doi: 10.1001/archpedi.155.2.120. [DOI] [PubMed] [Google Scholar]
- 35.Miller FA, Robert JS, Hayeems RZ. Questioning the consensus: managing carrier status results generated by newborn screening. Am J Public Health Feb. 2009;99(2):210–215. doi: 10.2105/AJPH.2008.136614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Newborn Screening and Genetics Resource Center. [Accessed July 28, 2011.];National Newborn Screening Information System (NNSIS) 2008 report and ongoing reports from 2010. 2010 http://nnsis.uthscsa.edu/xreports.aspx?XREPORTID=5.
- 37.Kavanagh PL, Wang CJ, Therrell BL, Sprinz PG, Bauchner H. Communication of positive newborn screening results for sickle cell disease and sickle cell trait: variation across states. Am J Med Genet C Semin Med Genet. 2008 Feb 15;148C(1):15–22. doi: 10.1002/ajmg.c.30160. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Lloyd-Puryear MA, Tonniges TF. Examination of communication practices between state newborn screening programs and the medical home. Pediatrics February. 2003;111(2):e120–e126. doi: 10.1542/peds.111.2.e120. [DOI] [PubMed] [Google Scholar]
- 39.Georgia Department of Public Health. [Accessed July 28, 2011.];Newborn screening for metabolic and sickle cell disorders program. 2011 http://health.state.ga.us/programs/nsmscd/diag_hemoglobin.asp.
- 40.Bales A, Zaleski C, McPherson E. Newborn screening programs: Should 22q11 deletion syndrome be added? Genet Med. 2010;12(3):135–144. doi: 10.1097/GIM.0b013e3181cdeb9a. [DOI] [PubMed] [Google Scholar]
- 41.Day SW, Brunson GE, Wang WC. Successful newborn sickle cell trait counseling program using health department nurses. Pediatr Nurs. 1997 Nov-Dec;23(6):557–561. [PubMed] [Google Scholar]
- 42.La Pean A, Collins JL, Christopher SA, et al. A Qualitative Secondary Evaluation of Statewide Follow-Up Interviews for Abnormal Newborn Screening Results for Cystic Fibrosis and Sickle Cell Hemoglobinopathy. Genet Med. 2011 doi: 10.1038/gim.0b013e31822dd7b8. [DOI] [PMC free article] [PubMed] [Google Scholar]


