Abstract
Asthma is a chronic inflammatory disease in which airway epithelial cells are the first line of defense against exposure of the airway to infectious agents. Src homology protein (SHP)-1, a protein tyrosine phosphatase, is a negative regulator of signaling pathways that are critical to the development of asthma and host defense. We hypothesize that SHP-1 function is defective in asthma, contributing to the increased inflammatory response induced by Mycoplasma pneumoniae, a pathogen known to exacerbate asthma. M. pneumoniae significantly activated SHP-1 in airway epithelial cells collected from nonasthmatic subjects by bronchoscopy with airway brushing but not in cells from asthmatic subjects. In asthmatic airway epithelial cells, M. pneumoniae induced significant PI3K/Akt phosphorylation, NF-κB activation, and IL-8 production compared with nonasthmatic cells, which were reversed by SHP-1 overexpression. Conversely, SHP-1 knockdown significantly increased IL-8 production and PI3K/Akt and NF-κB activation in the setting of M. pneumoniae infection in nonasthmatic cells, but it did not exacerbate these three parameters already activated in asthmatic cells. Thus, SHP-1 plays a critical role in abrogating M. pneumoniae-induced IL-8 production in non-asthmatic airway epithelial cells through inhibition of PI3K/Akt and NF-κB activity, but it is defective in asthma, resulting in an enhanced inflammatory response to infection.
Asthma is a chronic inflammatory disease in which Th2 cells and their cytokines, such as IL-4, IL-5, and IL-13 (1, 2), are critical to asthma pathobiology. Infectious agents are known to contribute to the exacerbation of asthma and, thus, contribute to asthma morbidity (3–6). Mycoplasma pneumoniae is an atypical bacterium that causes asthma exacerbations, in part through increased airway inflammation and mucus hypersecretion (7). Recent studies show that 50% of patients presenting with asthma demonstrate an acute M. pneumoniae airway infection (8). The mechanisms that determine the increased susceptibility of asthmatic airways to M. pneumoniae and other infectious agents remain largely unknown. A lack of understanding of the mechanisms resulting in exacerbations in asthma has been a critical barrier to progress in the knowledge of asthma pathobiology.
Airway epithelial cells are the first line of defense against exposure of the airway to inflammatory stimuli and Ags, and epithelial activation is one of the characteristics of asthma. Epithelial cells play an important role in the innate immune response by killing or neutralizing microorganisms through the production of enzymes, permeabilizing peptides, collectins, and protease inhibitors (9). Airway epithelial cells are also crucial in regulating adaptive immune responses by expressing pattern-recognition receptors to trigger host defense responses, by interacting with dendritic cells to regulate Ag sensitization, and by releasing cytokines to recruit effector cells (9, 10). Therefore, airway epithelial cells act as initiators, mediators, and regulators in innate and adaptive immune responses and modulate the transition from innate to adaptive immunity. Because of these important functions, airway epithelial cells may be valuable therapeutic targets for discovery and development of new drugs or new therapeutic strategies to treat asthma.
Our previous work demonstrated that M. pneumoniae, a microorganism known to exacerbate asthma (7, 8), induced significant MUC5AC production and secretion in airway epithelial cells from asthmatic subjects, and the induction was dependent on TLR2 binding (11). The TLR2-signaling pathway is critical in mycoplasma-induced inflammatory responses (12, 13), as well as those induced by other ligands, such as Gram-positive bacteria (14, 15). Several forms of lipoprotein or peptide, macrophage-activating lipopeptide 2, P48, M161Ag, N-ALP1, and N-ALP2, have been identified as TLR2 binding sites by mycoplasma (16–18). TLR2 binding results in activation of ERK1/2-, P38-, and JNK-signaling pathways and subsequent IL-8 production in the setting of mycoplasma membrane exposure to BEAS-2B cells (19). However, it has not been reported whether the PI3K/Akt-signaling pathway is involved in M. pneumoniae-induced IL-8 production in human airway epithelial cells.
Src homology protein (SHP)-1 is a 68-kDa, nontransmembrane protein tyrosine phosphatase containing two tandem Src homology domains (20), a catalytic domain, and a C-terminal tail of ∼100 aa residues (20–23). SHP-1 is mainly expressed in hematopoietic cells but is found in many other cell types, such as epithelial cells, and usually functions as a negative regulator of signal transduction. SHP-1 negatively controls cell growth, injury, and inflammation through associating with many cytokines and immunoreceptors, including IL-4Rα, IL-13Rα1, BCR, TCR, and erythropoietin receptor (24–28). Once phosphorylated and activated, SHP-1 binds to and dephosphorylates its target molecules and terminates the signaling. For instance, the downstream signaling of IL-4Rα, IL-5R, and IL-13Rα1, which are critical for the development of the asthma phenotype, are inhibited by SHP-1 through inactivation of STAT proteins (29).
Defective SHP-1 expression was shown to be associated with the occurrence of several human diseases (30–34). It was recently reported that SHP-1 has an inhibitory role in the development of airway inflammation in a mouse model of asthma (35–37). Kamata et al. (35) reported significantly increased production of Th2 cytokines in heterozygous SHP-1–deficient mice after OVA sensitization and challenge. After challenge with OVA, eosinophilic inflammation, mucus secretion, and airway hyperresponsiveness were enhanced compared with treatment with saline. Spontaneous Th2-like inflammatory responses in the lung were further shown in SHP-1–deficient mice, along with increased airway resistance (37).
SHP-1 was shown to play an important role in regulating oxidative stress, which has also been demonstrated in asthma (37). Increased intracellular oxidative stress and lack of SHP-1 in the presence of Th2-prone cellular activation may lead to the development of allergic airway inflammation. However, there is no report that links SHP-1 deficiency or dysfunction in cells or tissues directly with allergic airway disease in humans. Although the mechanism of SHP-1 in modulating inflammation has been studied in hematopoietic cells of mice, little is known about the molecular mechanisms of SHP-1 in the regulation of innate immunity of airway epithelial cells in human asthma, especially the mechanisms by which SHP-1 regulates TLR2-mediated proinflammatory cytokine production after M. pneumoniae infection.
In the current study, we hypothesize that, in airway epithelial cells from well- characterized asthmatic subjects, dysfunction of SHP-1, due to quantitative and functional deficiencies in the protein, is associated with reduced ability to modulate inflammation following M. pneumoniae infection. We propose that this dysfunction occurs via dysregulation of TLR2-mediated proinflammatory pathways. We demonstrate that M. pneumoniae significantly induced IL-8 production in asthmatic airway epithelial cells compared with non-asthmatic cells and that SHP-1 is critical in the regulation of this process. Defective activation of SHP-1 in asthma resulted in increased Akt and NF-κB activity, which dramatically increased IL-8 production. Thus, SHP-1 is a critical regulator of M. pneumoniae-induced inflammation in human asthmatic airway epithelial cells.
Materials and Methods
Subjects
Subjects were recruited via newspaper and radio advertisements from the general Research Triangle, North Carolina community. The asthmatic subjects fulfilled criteria for asthma (38), exhibiting a provocative concentration of methacholine resulting in a 20% decrease in the forced expiratory volume in 1 s (FEV1) < 8 mg/ml and reversibility, as demonstrated by ≥12% increase in FEV1 or the forced vital capacity (FVC) with inhaled albuterol. Asthmatic subjects recruited used short-acting β-2 agonists, as needed, and were not on controller medications. Exclusion criteria included the use of any controller therapy within the previous 4 wk, in-patient status, upper or lower respiratory tract infection within 3 mo of study, smoking history >5 pack-years or any cigarette use within the previous 2 y, significant nonasthma pulmonary disease, or other medical problems. Nonasthmatic subjects had no clinical history of atopy, were skin test negative, and took no medications. All subjects provided consent for this Institutional Review Board-approved protocol.
Bronchoscopy
Subjects underwent bronchoscopy with endobronchial-protected brushing and bronchoalveolar lavage, as previously described (11). The brushing of the proximal airways was performed under direct visualization using a separate protected cytologic brush for each pass, for a total of five passes. Bronchoalveolar lavage was performed via instillation of warm sterile saline in 60-ml aliquots, with return via gentle hand suction, for a total of 300 ml. Subjects were discharged when their FEV1 achieved 90% of their prebronchoscopy, postalbuterol value.
Airway epithelial cell culture
Freshly isolated airway epithelial cells from endobronchial brushing were cultured with BEGM (Lonza, Walkersville, MD), as previously described (11). After reaching confluence, cells were trypsinized and seeded onto collagen-coated polyester Transwell insert membranes of 12-mm diameter, at a concentration of 4 × 104/well. Then the cells were cultured at air– liquid interface and cultured for 2 wk to allow for differentiation.
M. pneumoniae culture and infection of cultured airway epithelial cells
M. pneumoniae strain 15531 (American Type Culture Collection, Manassas, VA) was inoculated in SP4 broth (Remel, Lenexa, KS) at 35°C until adherent. The concentration was determined by plating serial dilutions of M. pneumoniae on pleuropneumonia-like organisms agar plates (Remel). CFU were counted after incubation for 14 d. Differentiated airway epithelial cells were infected on the apical surface by M. pneumoniae with a titer of 50 CFU/cell and incubated for 48 h. The concentration of 50 CFU/cell was chosen based on our previous work, in which a dose-response experiment was performed (11). Supernatant was collected for IL-8 measurement by ELISA (R&D Systems, Minneapolis, MN), and lysates were collected and analyzed as described below.
To determine M. pneumoniae amounts in the cultured airway epithelial cells from nonasthmatic and asthmatic subjects, cells infected or not with M. pneumoniae for 48 h were rinsed with PBS three times, and total RNA was extracted using TRIzol reagent (Sigma, St. Louis, MO). Reverse transcription was performed using 1 μg total RNA and random hexamers in a 50-μl reaction, according to the manufacturer's protocol (Applied Biosystems, Branchburg, NJ). M. pneumoniae was quantified by RT-PCR with TaqMan gene-expression assays (Applied Biosystems) specifically for the mpn372 (Community Acquired Respiratory Distress Syndrome toxin) gene in mycoplasma (GenBank accession number DQ447750; http://www.ncbi.nlm.nih.gov/genbank/). Real-time PCR was performed on the M×3005 sequence-detection system (Stratagene). Relative amounts of mycoplasma mpn372 present in airway epithelial cells from non-asthmatic and asthmatic subjects were determined based on values obtained from a standard curve for mycoplasma mpn372 and was normalized to the housekeeping gene GAPDH that was present in the airway epithelial cells.
Small interfering RNA-mediated SHP-1 gene silencing
The oligonucleotides encoding the specific small interfering RNA (siRNA) for SHP-1 (sense, 5′-CAGTTCATTGAAACCACTATTCAAGAGATAGT-GGTTTCAATGAACTGGG-3′; antisense 5′-CCAGTTCATTGAAACCA-CTATCTCTTGAATAGTGGTTTCAATGAACTG-3′) were delivered into primary cultured human airway epithelial cells by double-strand recombinant adeno-associated virus (rAAV) (39) serotype 6. Basic local alignment search tool searches confirmed that the selected oligonucleotide sequences were not homologous to any other genes. A scramble sequence of 5′-TC-TCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGA-A-3′ was used to construct control rAAV as a negative control. Serotype 6 double-strand rAAVs were produced by the triple plasmid cotransfection method and purified by ammonium sulfate precipitation on cesium chloride gradients (40, 41). For rAAV transduction, airway epithelial cells from asthmatic and nonasthmatic subjects were seeded at a concentration of 4 × 104 cells/well in 12-well Transwell plates. Approximately 18 h after seeding, airway epithelial cells were transduced with rAAV carrying the SHP-1–siRNA–silencing cassette or full-length SHP-1 cDNA with a concentration of 0.3 multiplicity of infection. After reaching 100% confluence, the cells were cultured at an air–liquid interface for 2 wk. The culture medium was changed every other day.
Immunofluorescence staining and confocal laser scanning
Airway epithelial cells, which had been cultured at an air–liquid interface for 2 wk, were fixed with 3% paraformaldehyde, permeabilized with buffer containing 20 mM HEPES (pH 7.4), 0.3 M sucrose, 50 mM NaCl, 3 mM MgCl2, and 0.5% Triton X-100, and then incubated with mouse anti-human SHP-1 Ab (Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with Dylight 488-labeled goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA). Cell nuclei were detected by 7-aminoactinomycin D (Molecular Probes, Eugene, OR). Confocal images were acquired with a Zeiss LSM 510 inverted confocal microscope.
Immunoblotting analysis
Following a time-course treatment using M. pneumoniae infection, airway epithelial cells were lysed in ice-cold RIPA buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 0.5% natrium deoxycholate) supplemented with protease inhibitor mixture (Sigma) and PhosSTOP phosphatase inhibitors (Roche, Indianapolis, IN). Cell lysates were resolved on 10% SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were probed with rabbit anti-human TLR2 Ab (IMGENEX, San Diego, CA), mouse anti-human SHP-1 (Santa Cruz Biotechnology), or a Ser473 phosphospecific Ab to Akt (Cell Signaling, Danvers, MA) overnight at 4°C. After washes, the membranes were incubated with HRP-conjugated anti-IgG and developed by ECL, documented with the Kodak Image Station 4000 MM PRO, and quantified using Carestream Molecular Image software (version 5.0).
SHP-1 immunoprecipitation and Western blot
Airway epithelial cell lysates (500 μg) were immunoprecipitated with 2 μg mouse anti-human SHP-1 Ab (Santa Cruz Biotechnology). Immunoprecipitates were run on a 10% gel, transferred, and probed with HRP-conjugated, mouse anti-phosphotyrosine Ab 4G10 (Millipore, Temecula, CA) overnight to detect tyrosine phosphorylated SHP-1 (P–SHP-1). Immunoblots were stripped and reprobed with anti–SHP-1 Ab to confirm equal protein loading and quantified by densitometry. The ratio of densitometry of P–SHP-1/SHP-1 was determined at each time point (0, 2, and 4 h) following mycoplasma infection.
Coimmunoprecipitation analysis
Following a time-course treatment of mycoplasma, airway epithelial cell were lysed in ice-cold Pierce IP lysis buffer (Thermo Scientific, Rockford, IL). Lysates (500 μg/500 μl) were cleared with 1.0 μg normal rabbit IgG (Santa Cruz Biotechnology), together with 20 μl resuspended volume of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) for 30 min at 4°C. Then the supernatants were incubated with 2 μg rabbit anti-human TLR2 Ab (IMGENEX) for 1 h at 4°C, followed by incubation with 20 μl Protein A/G PLUS-Agarose overnight at 4°C. The beads were precipitated by centrifugation steps (1000 × g for 5 min) and washed four times in PBS buffer. The immunoprecipitates were analyzed by SDS-PAGE and immunoblotting using mouse anti-human SHP-1 Ab to determine the total amount of SHP-1 coimmunoprecipitated with TLR2 in the samples. The PVDF membrane was then stripped and reprobed with mouse anti-human TLR2 Ab (IMGENEX) to determine the amount of TLR2 in the immunoprecipitates and quantified by densitometry.
SHP-1 protein phosphatase activity assay
SHP-1 tyrosine phosphatase activity was measured with the use of the Tyrosine-Phosphatase Assay kit (Promega, Madison, WI), following the manufacturer's protocol. Briefly, SHP-1 in airway epithelial cell lysate was immunoprecipitated from 500 μg protein, as described above. The immunoprecipitates were incubated with tyrosine phosphopeptides as substrate. Total phosphate content was determined by phosphate standards run in parallel. Phosphopeptide-specific activity was calculated by subtracting the nonspecific activity.
NF-κB DNA-binding activity assay and Western blot
NF-κB DNA-binding activity was determined using the TransAMNF-κB p65 transcription factor assay kit (Active Motif, Carlsbad, CA), following the manufacturer's instructions, and as previously described (42). Briefly, nuclear extracts were prepared (43) from nonasthmatic and asthmatic airway epithelial cells that were infected with mycoplasma, as described above. Twenty micrograms of nuclear protein was incubated in a 96-well plate coated with oligonucleotide containing the NF-κB consensus-binding sequence 5′-GGGACTTTCC-3′. Bound NF-κB was then detected by a p65-specific primary Ab. An HRP-conjugated secondary Ab was then applied to detect the bound primary Ab, and colorimetric quantification was performed at 450 nm. Serial dilutions of purified p65 recombinant protein (20–0.16 ng) were used as a standard. To detect the cytoplasmic and nuclear NF-κB by Western blot, the cytoplasmic and nuclear proteins were extracted from airway epithelial cells from nonasthmatic and asthmatic subjects and resolved on 10% SDS-PAGE, followed by transfer to a PVDF membrane. The membranes were probed with a p65-specific primary Ab (Cell Signaling) overnight at 4°C. The immunoblots were then incubated with HRP-conjugated secondary Abs, developed by ECL, and quantified by densitometry, as described above.
Statistical analysis
Statistical evaluation was performed using JMP statistical software (SAS, Cary, NC). Data are expressed as mean ± SE, because data were distributed normally. The Student t test or Wilcoxon test was used to evaluate group differences, depending on distribution of the data. Differences between groups with a p value < 0.05 were considered statistically significant.
Results
Subject characteristics
Subject characteristics are shown in Table I. The asthmatic subjects were considered to have mild disease by the National Asthma Education and Prevention Panel criteria and used short-acting β agonists as needed only (38). The nonasthmatic subjects had no history of atopy, smoking, or significant medical illness and were not on medications.
Table I. Subject characteristics.
| Asthma (n = 12) | Normal (n = 10) | p Value | |
|---|---|---|---|
| Gender (male/female) | 5/7 | 4/6 | 0.12 |
| Age (y) | 31 ± 3 | 28 ± 4 | 0.53 |
| Medication | Albuterol | None | |
| FEV1 (l) | 2.82 ± 0.22 | 3.33 ± 0.2 | 0.21 |
| FEV1 (% predicted) | 88 ± 4.23 | 102 ± 4.81 | 0.01 |
| FVC (l) | 4.12 ± 0.52 | 4.51 ± 0.39 | 0.38 |
| FVC (% predicted) | 92 ± 6 | 105 ± 5 | 0.04 |
| FEV1/FVC | 75 ± 1.98 | 79 ± 2.12 | 0.43 |
| Methacholine PC20a (mg/ml) | 0.87 ± 0.45 | >16 | 0.0001 |
The provocative concentration of methacholine resulting in a 20% decrease in FEV1.
Localization of endogenous SHP-1 in human airway epithelial cells
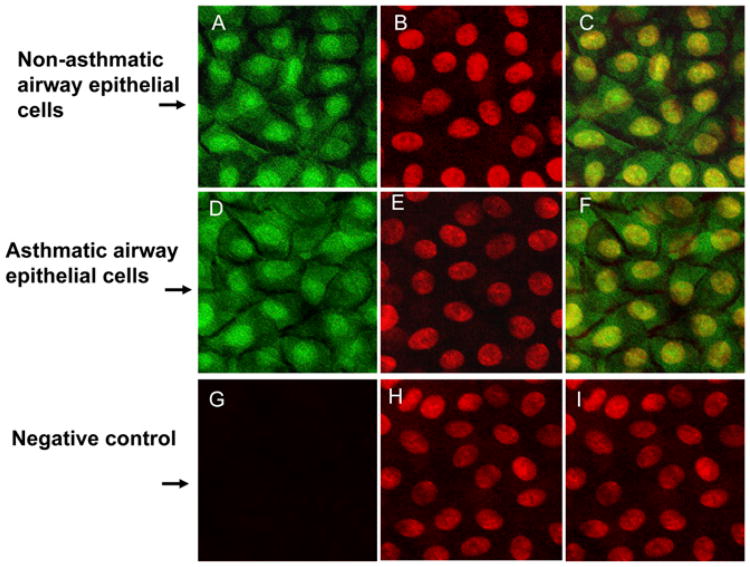
SHP-1 is expressed predominantly in hematopoietic cells, although lower levels of expression have been observed in nonhematopoietic cells, such as HeLa cervical adenocarcinoma, A549 lung carcinoma, and MCF-7 mammary adenocarcinoma cell lines (44, 23). Previous studies demonstrated that SHP-1 localization differs dramatically between the nonhematopoietic and hematopoietic cell lines (45), with the SHP-1 protein being present almost entirely within the cytoplasm of hematopoietic cells and the nucleus of nonhematopoietic cells. To our knowledge, the localization of SHP-1 in human airway epithelial cells has not been reported. Immunostaining using an SHP-1–specific Ab revealed that endogenous SHP-1 in our primary cultured airway epithelial cells from nonasthmatic and asthmatic subjects was distributed mainly within the nucleus, with mild cytoplasm expression also present. There was no difference in the SHP-1 subcellular distribution between nonasthmatic and asthmatic cells (Fig. 1).
Figure 1.
Localization of endogenous SHP-1 protein in nonasthmatic and asthmatic human airway epithelial cells. Airway epithelial cells from nonasthmatic and asthmatic subjects were cultured at an air–liquid interface for 2 wk and immunostained using SHP-1–specific mAb. (A, D, G) SHP-1 (green) immunostaining. (B, E, H) Nuclear (red) immunostaining by 7-aminoactinomycin. (C, F, I) Merge. (G)–(I) are negative controls. SHP-1 was present in both cytoplasm and nucleus (A, D) but predominantly within the nucleus (C, F). Original magnification ×100.
SHP-1 plays a critical role in regulating M. pneumoniae-induced inflammation in human airway epithelial cells
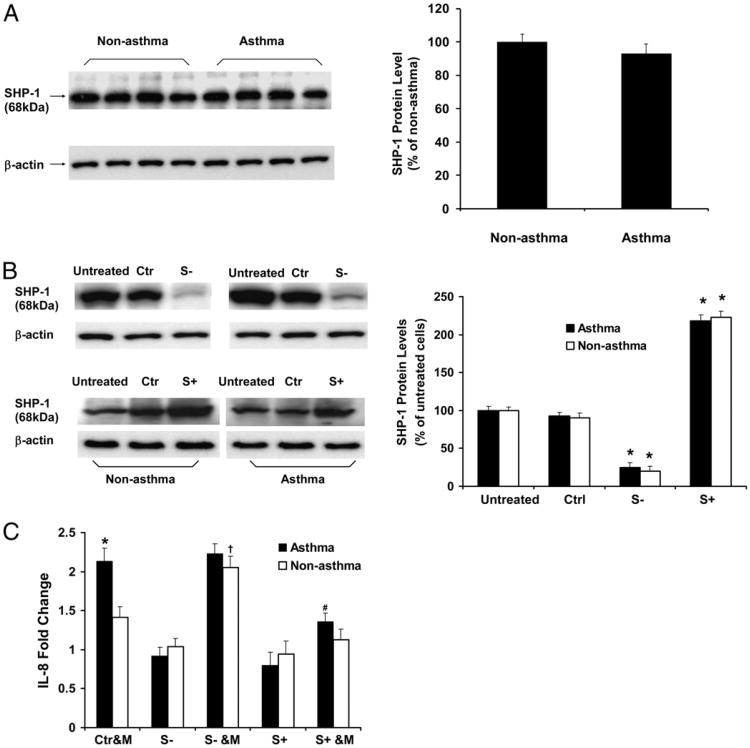
To determine whether SHP-1 plays any role in regulating M. pneumoniae-induced IL-8 production by airway epithelial cells, we performed siRNA-mediated gene silencing experiments to knock down SHP-1. In addition, airway epithelial cells were transduced with rAAV vector that contained full-length SHP-1 cDNA to induce SHP-1 overexpression. The efficacy of knockdown and overexpression were confirmed by immunoblot analyses (Fig. 2B). The level of SHP-2 protein was not affected in the epithelial cells in which SHP-1 was knocked down (data not shown). In addition, rAAV alone did not cause a nonspecific regulatory effect on IL-8 levels in either nonasthmatic or asthmatic airway epithelial cells (Supplemental Fig. 1).
Figure 2.
IL-8 production in nonasthmatic and asthmatic airway epithelial cells infected with M. pneumoniae in the setting of SHP-1 knockdown and overexpression. (A) Immunoblot analysis of baseline SHP-1 expression in airway epithelial cells from nonasthmatic and asthmatic subjects. The data representing the percentage of densitometry values of nonasthmatic cells are expressed as mean ± SEM (right panel). (B) Immunoblot analysis of SHP-1 expression in airway epithelial cells without treatment (untreated), transduced with control rAAV only (Ctr), transduced with SHP-1–specific siRNA carrying rAAV to induce SHP-1 knockdown (S−), and infected with full-length SHP-1 cDNA carrying rAAV to induce SHP-1 overexpression (S+). The data representing the percentage of densitometry values of untreated cells are expressed as mean ± SEM (right panel). *p < 0.01 compared with untreated and control (Ctrl) in the same group. (C) Measurement of IL-8 production induced by M. pneumoniae in nonasthmatic and asthmatic airway epithelial cells with or without SHP-1 knockdown and overexpression. Data representing the fold change of IL-8 from control rAAV-transduced cells from the same group without mycoplasma infection are presented as mean ± SEM (n = 10 for nonasthma; n = 12 for asthma). Baseline SHP-1 expression was not different between asthmatic and nonasthmatic cells, and SHP-1 was knocked down and overexpressed similarly in nonasthmatic and asthmatic cells. IL-8 levels were higher in asthmatic cells infected with M. pneumoniae. SHP-1 overexpression reduced IL-8 production in asthmatic epithelial cells following mycoplasma infection. In the setting of SHP-1 knockdown, mycoplasma infection augmented IL-8 production in nonasthmatic, but not in asthmatic, cells.*,†p < 0.01 compared with Ctr&M of nonasthmatic cells; #p < 0.01 compared with Ctr&M or S− &M of asthmatic cells. Ctr&M, control rAAV-transduced airway epithelial cells treated with M. pneumoniae; S−, airway epithelial cells transduced with SHP-1–specific siRNA carrying rAAV to induce SHP-1 knockdown; S+, airway epithelial cells transduced with full-length SHP-1 cDNA carrying rAAV to induce SHP-1 overexpression; S− &M, airway epithelial cells with SHP-1 knocked down and infected by M. pneumoniae; S+ &M, airway epithelial cells with SHP-1 overexpressed and infected by M. pneumoniae.
To determine the amounts of M. pneumoniae in the airway epithelial cells from nonasthmatic and asthmatic subjects, we performed real-time RT-PCR using primers specific for the mpn 372 gene in mycoplasma, as described in Materials and Methods. Expression of the mpn372 gene was not detectable in either nonasthmatic or asthmatic airway epithelial cells, which had been cultured at an air–liquid interface for 2 wk prior to mycoplasma treatment. Forty-eight hours after M. pneumoniae infection, the mpn372 gene was detected, but there was no significant difference in its expression between nonasthmatic and asthmatic airway epithelial cells (mpn372/GAPDH: 0.61 ± 0.05 versus 0.53 ± 0.04 in the asthmatic and nonasthmatic groups, respectively, p = 0.45; n = 10 for nonasthma, n = 12 for asthma). However, following infection with M. pneumoniae, asthmatic airway epithelial cells produced significantly more IL-8 compared with the cells from nonasthmatic subjects (Fig. 2C), consistent with our previous study (11). SHP-1 overexpression significantly decreased M. pneumoniae-induced IL-8 in asthmatic cells (Fig. 2C). In contrast, SHP-1 knockdown significantly increased IL-8 in the setting of mycoplasma infection in nonasthmatic cells, but it did not increase the IL-8 levels already produced by asthmatic cells following mycoplasma infection (Fig. 2C). These data suggest that SHP-1 expression is decreased or activation is defective in asthmatic airway epithelial cells. There were no significant differences in the baseline SHP-1 mRNA expression (data not shown) and protein levels between nonasthmatic and asthmatic airway epithelial cells (Fig. 2A), suggesting a defect in SHP-1 activation or function in asthmatic cells following infection.
SHP-1 activation induced by M. pneumoniae is defective in asthmatic airway epithelial cells
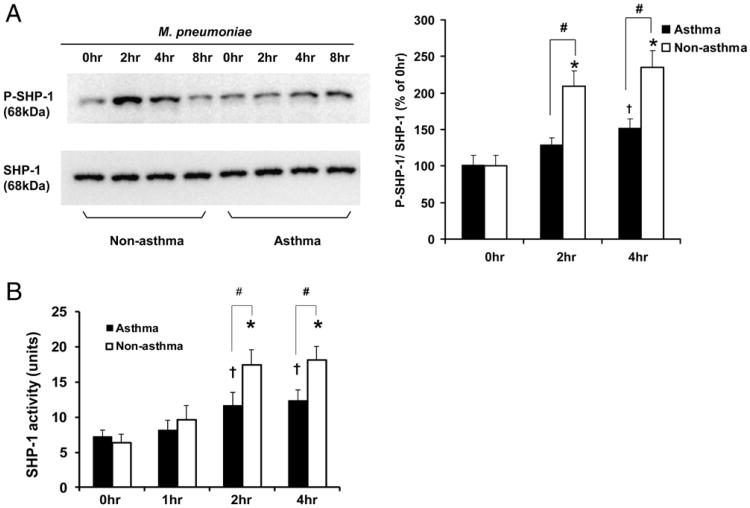
To determine whether SHP-1 activation is defective in asthmatic airway epithelial cells following M. pneumoniae infection, we immunoprecipitated SHP-1 and analyzed its tyrosine phosphorylation by immunoblot using 4G10 Ab. No difference in SHP-1 phosphorylation level under resting or baseline conditions was observed between nonasthmatic and asthmatic cells (Fig. 3A). M. pneumoniae induced significant SHP-1 phosphorylation in nonasthmatic cells at 2 and 4 postinfection (Fig. 3A). However, SHP-1 phosphorylation in asthmatic cells at 2 and 4 after mycoplasma infection was significantly less than that in nonasthmatic cells (Fig. 3A).
Figure 3.
M. pneumoniae infection in airway epithelial cells activates SHP-1. (A) M. pneumoniae infection of airway epithelial cells from non-asthmatic and asthmatic subjects resulted in time-course–dependent SHP-1 tyrosine phosphorylation. Airway epithelial cells without mycoplasma treatment (0 h) or infected with Mycoplasma for 2, 4, and 8 h were immunoprecipitated for SHP-1 and probed with anti-phosphotyrosine Ab 4G10 to detect P–SHP-1. Data representing P–SHP-1/SHP-1 of each time point compared with 0 h in nonasthmatic group are expressed as mean ± SEM, n = 5 (right panel). (B) Tyrosine phosphatase activity of immunoprecipitated SHP-1 from nonasthmatic and asthmatic airway epithelial cells without mycoplasma treatment (0 h) or infected with mycoplasma for 1, 2, or 4 h. *p < 0.01, †p < 0.05, compared with 0 h in the same group; #p < 0.05 between nonasthmatic and asthmatic cells at the same time point. The data are presented as mean ± SEM (n = 10 for nonasthmatic subjects; n = 12 for asthmatic subjects). Although M. pneumoniae infection induced SHP-1 phosphorylation in asthmatic airway epithelial cells, it was significantly less than in nonasthmatic cells at 2 and 4 h following M. pneumoniae infection.
Next, we immunoprecipitated SHP-1 and measured its ability to dephosphorylate tyrosine peptides, a function that directly reflects the SHP-1 activity. We found that M. pneumoniae induced significant time-dependent increases in SHP-1 phosphatase activity in nonasthmatic airway epithelial cells compared with asthmatic cells (Fig. 3B). Both groups demonstrated a similar time course of SHP-1 tyrosine phosphorylation (Fig. 3A). These results collectively indicate that defective SHP-1 activation in asthmatic airway epithelial cells contributes, at least partially, to the increased IL-8 production induced by M. pneumoniae.
M. pneumoniae induces dynamic association between SHP-1 and TLR2 in airway epithelial cells
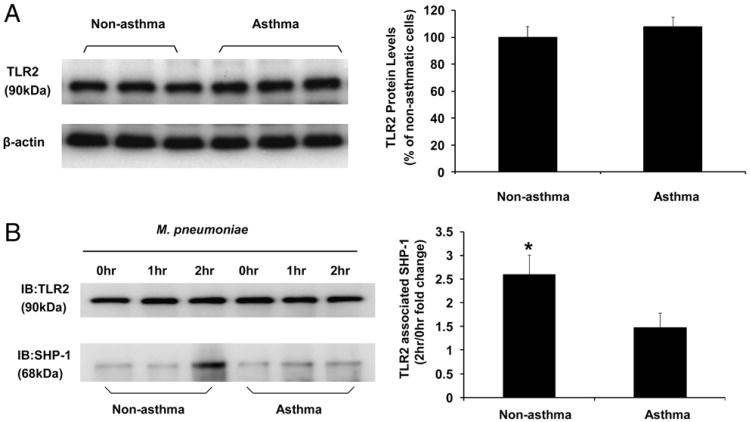
To further explore the mechanism of SHP-1 in regulating TLR2-mediated proinflammatory signaling pathway, we determined the colocalization of SHP-1 and TLR2 in airway epithelial cells by coimmunoprecipitation. M. pneumoniae induced dynamic association between SHP-1 and TLR2 in both nonasthmatic and asthmatic airway epithelial cells (Fig. 4B), which was supported by a previous study by Slevogt et al. (46). Interestingly, the amount of SHP-1 associating with TLR2 at 2 h after M. pneumoniae infection in nonasthmatic airway epithelial cells was significantly higher than that of asthmatic cells (Fig. 4B). No difference was observed in TLR2 protein levels between nonasthmatic and asthmatic cells at baseline (Fig. 4A) and after time-course treatment with mycoplasma (Fig. 4B, left panel). These data support the role of SHP-1 in regulating M. pneumoniae-induced IL-8 production, possibly through interaction with the TLR2-signaling pathway.
Figure 4.
M. pneumoniae infection induced TLR2–SHP-1 dynamic association. (A) Immunoblot analysis of baseline TLR2 expression in airway epithelial cells from nonasthmatic and asthmatic subjects. The data representing the percentage of densitometry values of nonasthmatic cells are expressed as mean ± SEM (right panel). (B) TLR2-associated SHP-1 levels in airway epithelial cells was measured by coimmunoprecipitation. TLR2 was immunoprecipitated from airway epithelial cell lysates of nonasthmatic and asthmatic subjects without mycoplasma infection (0 h), infected with mycoplasma for 1 and 2 h. TLR2 immunoprecipitates were blotted with anti-TLR2 (top left panel) and anti–SHP-1 (bottom left panel) Ab. TLR2-associated SHP-1 at 2 h after M. pneumoniae infection is expressed as fold change of densitometry values from nonasthmatic cells without mycoplasma treatment (0 h) and was increased in nonasthmatic cells compared with asthmatic cells. The data are presented as mean ± SEM (n = 5). *p < 0.01 compared with asthmatic group.
SHP-1 inhibits M. pneumoniae-induced IL-8 production in airway epithelial cells through PI3K and Akt
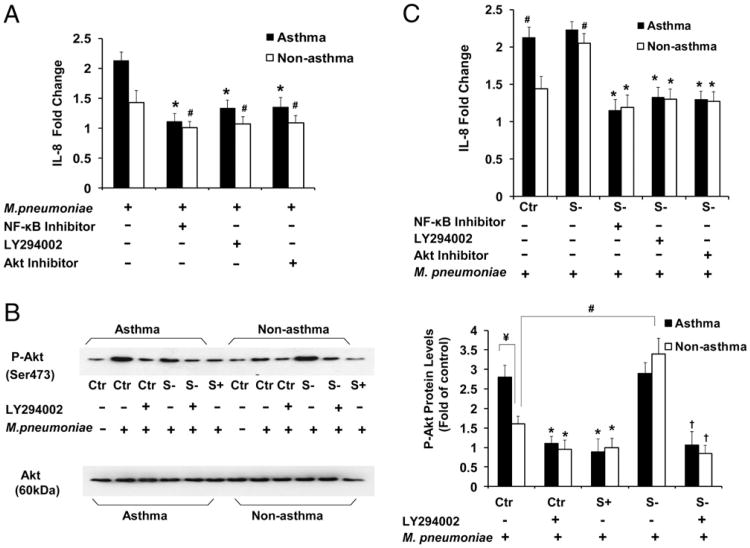
Phosphorylation of the kinase Akt at the serine residue of position 473 (Ser473) is absolutely dependent on PI3K activity (47). Thus, evaluation of phosphorylation of Akt Ser473 provides a surrogate measure of PI3K activity in intact cells. It is also well established that Akt is integrated into the activation process of NF-κB by multiple mechanisms (48–50). To further explore the molecular mechanism of SHP-1 in regulating M. pneumoniae-activated TLR2-signaling pathway in airway epithelial cells, we pretreated the asthmatic airway epithelial cells with an NF-κB inhibitor, a PI3K inhibitor (LY294002), or an Akt inhibitor for 30 min and then infected the cells with M. pneumoniae. PI3K/Akt and NF-κB inhibitors significantly decreased M. pneumoniae-induced IL-8 production in nonasthmatic and asthmatic airway epithelial cells with or without SHP-1 knockdown (Fig. 5A, 5C), suggesting that activation of the PI3K/Akt pathway contributes to M. pneumoniae-induced IL-8 production.
Figure 5.
SHP-1 regulates M. pneumoniae-induced IL-8 production through PI3K/Akt in airway epithelial cells from nonasthmatic and asthmatic subjects. (A) Fold change in IL-8 production in nonasthmatic and asthmatic airway epithelial cells with or without pretreatment with NF-κB inhibitor II (30 μM), LY294002 (25 μM), or Akt inhibitor (50 nM) for 30 min, followed by mycoplasma infection for 48 h. IL-8 levels are expressed as fold change from cells in the same group without mycoplasma infection. *p < 0.01, #p < 0.05, compared with mycoplasma-infected cells without inhibitor pretreatment in the same group. The data are presented as mean ± SEM (n = 10 for nonasthma; n = 12 for asthma). (B) Immunoblot analysis of Akt phosphorylation (P-Akt) in total cell lysates of nonasthmatic and asthmatic cells with or without mycoplasma infection for 2 h (left panel). The levels of P-Akt 2 h after M. pneumoniae infection in asthmatic and nonasthmatic cells, with or without SHP-1 knockdown or overexpression, are expressed as fold change of densitometry from cells in the same group transduced with control rAAV only (right panel). *p < 0.01, compared with control rAAV-transduced cells treated with mycoplasma in the same group; ¥,#p < 0.01, compared with control rAAV-transduced nonasthmatic cells treated with mycoplasma only; †p < 0.01, compared with SHP-1 knockdown cells with mycoplasma infection only in the same group. Data are presented as mean ± SEM (n = 5). Mycoplasma-induced phosphorylation of Akt in asthmatic cells, which was significantly higher than that in nonasthmatic cells, was reversed by pretreatment with LY294002 and attenuated by SHP-1 overexpression. SHP-1 knockdown dramatically increased phosphorylation of Akt in nonasthmatic cells, but not in asthmatic cells, which was reversed by pretreatment with LY294002. (C) IL-8 fold change in SHP-1 knocked down nonasthmatic and asthmatic airway epithelial cells that were pretreated or not with NF-κB inhibitor II (30 μM), LY294002 (25 μM), or Akt inhibitor (50 nM) for 30 min and then infected with mycoplasma for 48 h. SHP-1 knockdown dramatically accentuated IL-8 production in nonasthmatic cells, but not in asthmatic cells, which were all significantly inhibited by these three inhibitors. *p < 0.01 compared with mycoplasma-infected “S−” cells in the same group, without pretreatment of inhibitors; #p < 0.01, compared with “Ctr” cells of nonasthma, without pretreatment of inhibitors. The data are presented as mean ± SEM (n = 10 for nonasthma; n = 12 for asthma). Ctr, control rAAV-transduced airway epithelial cells; S−, airway epithelial cells with SHP-1 knockdown; S+, airway epithelial cells with SHP-1 overexpression.
To further determine the role of SHP-1 in the regulation of mycoplasma-induced PI3K/Akt activation in airway epithelial cells, phosphorylated Akt was measured by Western blot. There was no difference in the baseline phosphorylated Akt (Fig. 5B, left panel) between nonasthmatic and asthmatic airway epithelial cells prior to mycoplasma infection. However, significant phosphorylation of Akt Ser473 after exposure to M. pneumoniae for 2 h was detected in asthmatic airway epithelial cells, which was reversed by pretreatment with LY294002 or by SHP-1 overexpression (Fig. 5B). M. pneumoniae-induced phosphorylation of Akt Ser473 was also detected in nonasthmatic cells, but it was less than that in asthmatic cells, and phosphorylation was enhanced by SHP-1 knockdown and reversed by pretreatment with LY294002 (Fig. 5B). rAAValone did not cause nonspecific effects upon Akt phosphorylation (Supplemental Fig. 2). In addition, SHP-1 knockdown enhanced IL-8 production in the setting of mycoplasma infection in nonasthmatic cells and was significantly inhibited by NF-κB and PI3K/Akt inhibitors (Fig. 5C). Taken together, these data demonstrate that SHP-1 inhibits mycoplasma-induced IL-8 production through PI3K/Akt-signaling pathways in airway epithelial cells.
SHP-1 inhibits NF-κB activation induced by M. pneumoniae in airway epithelial cells
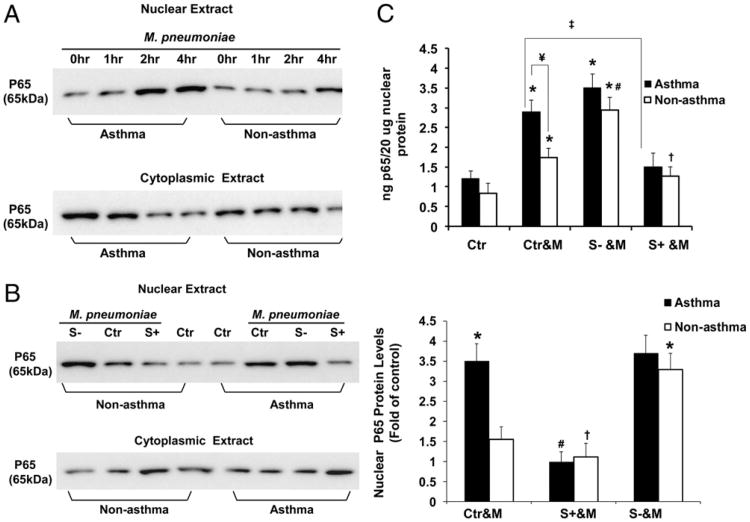
It was reported that M. pneumoniae induces IL-8 (19) and MUC5AC production through activation of NF-κB (11). SHP-1 also negatively regulates NF-κB activation (51–53). Consistent with these previous studies, our results demonstrate that inhibition of NF-κB significantly attenuated M. pneumoniae-induced IL-8 production in asthmatic and nonasthmatic airway epithelial cells with or without SHP-1 knockdown (Fig. 5A, 5C). To further determine the molecular mechanism of SHP-1 in regulating M. pneumoniae-induced IL-8 production by asthmatic airway epithelial cells, we assessed the effect of SHP-1 on M. pneumoniae-induced NF-κB nuclear translocation and activation by immunoblot and p65 DNA binding assay (Fig. 6). Immunoblot analysis showed that M. pneumoniae induced significant NF-κB nuclear translocation in asthmatic airway epithelial cells 2 h after infection (Fig. 6A). However, M. pneumoniae infection induced less NF-κB nuclear translocation in nonasthmatic airway epithelial cells compared with asthmatic cells (Fig. 6A, 6B). SHP-1 knockdown dramatically accentuated M. pneumoniae-induced NF-κB nuclear translocation in nonasthmatic cells (Fig. 6B). In asthmatic cells, NF-κB nuclear translocation following mycoplasma infection was unchanged with SHP-1 knockdown (Fig. 6B). However, SHP-1 overexpression significantly inhibited M. pneumoniae-induced NF-κB nuclear translocation in asthmatic and nonasthmatic airway epithelial cells (Fig. 6B).
Figure 6.
NF-κB activation by M. pneumoniae and inhibition by SHP-1 in airway epithelial cells from nonasthmatic and asthmatic subjects. (A) Immunoblot of cytoplasmic and nuclear protein lysates against p65 in nonasthmatic and asthmatic airway epithelial cells without mycoplasma infection (0 h) or challenged with mycoplasma for 1, 2, or 4 h. (B) Immunoblot of cytoplasmic and nuclear protein lysates against p65 in nonasthmatic and asthmatic airway epithelial cells with or without SHP-1 knockdown or overexpression and infected with mycoplasma for 2 h (left panel). The amount of nuclear p65 NF-κB at 2 h after M. pneumoniae infection is expressed as fold change of densitometry values from cells in the same group transduced with control rAAV only (right panel). *p < 0.01, compared with Ctr&M of nonasthmatic cells; #p < 0.01, †p < 0.05, compared with Ctr&M in the same group. Data are presented as mean ± SEM (n = 5). (C) NF-κB activation was quantified in nonasthmatic and asthmatic airway epithelial cells, with or without SHP-1 knockdown or overexpression, 2 h after mycoplasma infection. The NF-κB DNA-binding activity is reported as nanograms of bound p65 protein/20 μg of nuclear extracts. *p < 0.01, compared with Ctr in the same group; #p < 0.01, S− &M versus Ctr&M of nonasthmatic cells; †p < 0.05, S+ &M versus Ctr&M of nonasthmatic cells; ‡p < 0.01, S+ &M versus Ctr&M of asthma; ¥p < 0.01, nonasthma versus asthma. Data are presented as mean ± SEM values (n = 10 for nonasthma; n = 12 for asthma). Mycoplasma induced NF-κB activation in asthmatic cells, which was significantly higher than that in nonasthmatic cells and was attenuated by SHP-1 overexpression. SHP-1 knockdown dramatically increased NF-κB activation in nonasthmatic cells but not in asthmatic cells. Ctr, control rAAV-transduced airway epithelial cells; Ctr&M, control rAAV-transduced cells with mycoplasma treatment; S−, airway epithelial cells with SHP-1 knockdown; S+, airway epithelial cells with SHP-1 overexpression; S− &M, airway epithelial cells with SHP-1 knocked down and infected by M. pneumoniae; S+ &M, airway epithelial cells with SHP-1 overexpressed and infected by M. pneumoniae.
To further confirm NF-κB nuclear translocation results, we performed a binding activity assay of NF-κB DNA. Nuclear extracts from freshly isolated airway epithelial cells were allowed to bind to an NF-κB consensus oligonucleotide sequence. Bound NF-κB was then detected by a p65 (RelA)-specific Ab and quantified based on a standard curve using purified p65 recombinant protein. NF-κB DNA binding activity was significantly increased in asthmatic airway epithelial cells 2 h after M. pneumoniae infection compared with nonasthmatic cells (Fig. 6C). Baseline NF-κB DNA binding activity was not significantly different between the asthmatic and nonasthmatic groups. In the cells in which SHP-1 was knocked down, M. pneumoniae induced further increased NF-κB activity in nonasthmatic cells, without a significant change in the asthmatic cells. SHP-1 overexpression significantly attenuated M. pneumoniae-induced NF-κB activation in asthmatic and nonasthmatic airway epithelial cells (Fig. 6C). rAAV alone did not induce nonspecific regulatory effects on NF-κB activity (Supplemental Fig. 3). These results collectively demonstrate that SHP-1 plays an important role in inhibiting M. pneumoniae-induced NF-κB activation, which was significantly higher in asthmatic airway epithelial cells compared with nonasthmatic cells (Fig. 7).
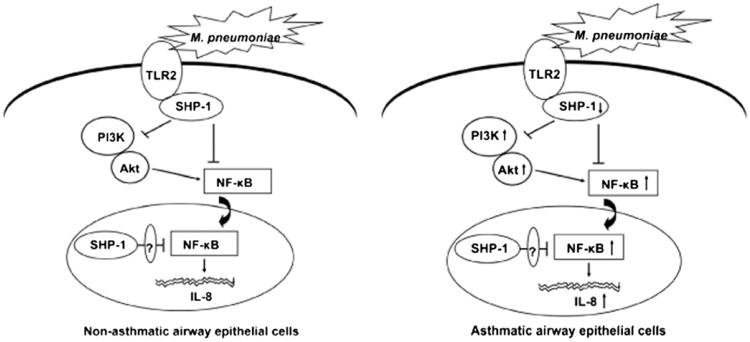
Figure 7.
Proposed model for the mechanism of SHP-1–mediated inhibition of M. pneumoniae-activated TLR2 signaling in normal and asthmatic airway epithelial cells. The ligation of TLR2 by M. pneumoniae initiates TLR2-mediated proinflammatory signaling pathway, resulting in the production of IL-8. M. pneumoniae binding to TLR2 activates and recruits SHP-1, which inhibits the nuclear translocation of NF-κB directly or through inhibition of PI3K/Akt abrogating NF-κB activation and subsequently prevents IL-8 production. In addition, the nuclear SHP-1 may also inhibit NF-κB function by certain nuclear mediators (left panel). In asthmatic airway epithelial cells, M. pneumoniae-induced SHP-1 activation is defective, which contributes to the increased activation of PI3K/Akt and NF-κB, as well as abundant IL-8 production (right panel).
Discussion
M. pneumoniae is an atypical bacterium that causes asthma exacerbations through increased airway inflammation and mucus hypersecretion. Clinical investigation and our ex vivo study show that asthmatic patients are more susceptible to M. pneumoniae infection (8), and asthmatic airway epithelial cells produced significantly higher levels of mucin (11) and IL-8 (54) compared with normal epithelial cells. The mechanisms by which SHP-1 regulates the TLR2-mediated proinflammatory signaling pathway activated by M. pneumoniae in asthmatic airway epithelial cells have not been defined. In this study, the role of SHP-1 in regulating M. pneumoniae-induced inflammation in asthmatic airway epithelial cells, the molecular mechanisms, and the relevant signaling pathways were investigated. Our study demonstrates that SHP-1 plays a critical role in inhibiting M. pneumoniae-induced IL-8 production in human airway epithelial cells. SHP-1 activation was defective in asthmatic cells, which subsequently resulted in overactivation of PI3K/Akt and NF-κB, leading to significantly increased IL-8 production in the setting of M. pneumoniae infection.
In the current study, we showed that M. pneumoniae induced significantly increased IL-8 production in asthmatic airway epithelial cells compared with nonasthmatic cells, which indicates that defective innate immune responses exist in asthmatic cells. Previous studies showed that asthmatic patients are more susceptible to mycoplasma infection (8), but this is not due to increased M. pneumoniae amounts, because we demonstrated that there were no differences in mpn372 gene expression between ex vivo-cultured nonasthmatic and asthmatic airway epithelial cells before or following mycoplasma infection.
Furthermore, SHP-1 overexpression significantly decreased M. pneumoniae-induced IL-8 in asthmatic cells. Conversely, SHP-1 knockdown significantly increased IL-8 production in the setting of mycoplasma infection in nonasthmatic cells, but it did not change the increased IL-8 already produced by asthmatic cells following mycoplasma infection (Fig. 2C). Although one possible explanation for the difference in response to M. pneumoniae between asthmatic and nonasthmatic airway epithelial cells is significantly lower expression levels of baseline SHP-1 in asthmatic cells, we demonstrated that the baseline SHP-1 protein levels were not significantly different between nonasthmatic and asthmatic airway epithelial cells (Fig. 2A). An alternative explanation is that the function or activity of SHP-1 is defective in asthmatic airway epithelial cells, because our data demonstrate significantly decreased SHP-1 tyrosine phosphorylation and phosphatase activity in asthmatic airway epithelial cells after challenge with M. pneumoniae infection.
Reduced SHP-1 phosphorylation levels and phosphatase activity in asthmatic airway epithelial cells might arise from the genetic mutation of the SHP-1 gene itself or from the lower expression or phosphorylation of SHP-1 upstream partners in asthma. Defective SHP-1 activation in asthmatic airway epithelial cells induced by M. pneumoniae may account for the observed enhanced IL-8 production that was not observed in nonasthmatic airway epithelial cells. This observation is further supported by the inhibitory effect of SHP-1 overexpression, which reduced M. pneumoniae-induced IL-8 production in asthmatic cells. To our knowledge, defective SHP-1 activity in asthmatic airway epithelial cells after an infectious challenge has not been previously described.
Activation of the TLR2-signaling pathway is critical in M. pneumoniae-induced mucin and proinflammatory cytokine production (12, 13). After binding to TLR2, M. pneumoniae induces IL-8 production in human epithelial cells through activating MAPKs, ERK1/2, JNK, as well as NF-κB (19). Our study shows that M. pneumoniae induced significant time-dependent nuclear translocation of NF-κB and phosphorylation of PI3K/Akt in asthmatic airway epithelial cells (Figs. 5B, 6A) compared with non-asthmatic cells, resulting in significantly higher IL-8 production in asthmatic cells. Furthermore, inhibition of PI3K, Akt, and NF-κB significantly attenuated M. pneumoniae-induced IL-8 production in asthmatic airway epithelial cells (Fig. 5A, 5C). To our knowledge, we demonstrate for the first time that PI3K and Akt play important roles in the signaling pathway of M. pneumoniae-induced IL-8 production. NF-κB activation could be PI3K/Akt independent or dependent; the latter is supported by a number of studies (55, 56). In addition, SHP-1 knockdown significantly enhanced mycoplasma-induced Akt phosphorylation (Fig. 5B), NF-κB nuclear translocation (Fig. 6B), and IL-8 production in non-asthmatic airway epithelial cells; the latter was dramatically inhibited by PI3K/Akt or NF-κB inhibitor (Fig. 5C). SHP-1 over-expression obviously inhibited mycoplasma-induced Akt phosphorylation and NF-κB nuclear translocation in nonasthmatic and asthmatic cells (Figs. 5B, 6B). Taken together, these results show that SHP-1 plays a critical role in attenuating mycoplasma-induced IL-8 production through the inhibition of Akt phosphorylation and NF-κB nuclear translocation in airway epithelial cells.
The cross-talk between SHP-1 and TLR-mediated proinflammatory signaling pathway has been well demonstrated in hematopoietic cells (57–59) but not in epithelial cells. SHP-1 acts at various receptors through recruitment to phosphorylated ITAM on inhibitory receptors, which deactivates certain critical proinflammatory mediators and terminates the downstream signaling pathways. Similar to hematopoietic cells, we demonstrated in airway epithelial cells that SHP-1 negatively regulates TLR-mediated production of proinflammatory cytokines by inhibiting activation of NF-κB and deactivating PI3K/Akt signaling through dephosphorylation of PI3K, leading to subsequent abrogation of the phosphorylation of Akt. SHP-1 can also inhibit ubiquitin ligase TNFR-associated factor 6-dependent signal transduction activated by the ligand for the cytokine receptor RANK (52), which is also important in TLR-mediated production of proinflammatory cytokines. Furthermore, our present study demonstrates an M. pneumoniae-induced dynamic association between SHP-1 and TLR2 in both nonasthmatic and asthmatic airway epithelial cells. However, the dynamic recruitment of SHP-1 to TLR2 is defective in asthmatic airway epithelial cells in the setting of M. pneumoniae infection (Fig. 4), which may contribute to the defective inhibitory effect of SHP-1–signaling pathway. It is possible that defective structure and function of TLR2 and/or SHP-1 lead to defective recruitment of SHP-1 to TLR2. Alternatively, the IL-4– and IL-13–signaling pathways, which are critical for the asthma phenotype, might sequester SHP-1 from TLR2 and induce overactivation of TLR2-mediated proinflammatory signal transduction. To test this possibility, we performed Western blot of phosphorylated Stat3/Stat6 and coimmunoprecipitation of SHP-1/Stat3 and SHP-1/Stat6. Western blot analysis showed that mycoplasma infection did not induce significant Stat3 and Stat6 phosphorylation (data not shown) in either nonasthmatic or asthmatic airway epithelial cells. There were no significant differences in the amounts of SHP-1 associated with Stat3 or Stat6 between nonasthmatic and asthmatic airway epithelial cells prior to mycoplasma infection (Supplemental Fig. 4). In addition, time-course treatment with mycoplasma did not induce dynamic association between SHP-1 and Stat3/Stat6 in asthmatic and nonasthmatic airway epithelial cells (Supplemental Fig. 4), which indicates that IL-4/IL-13–related signaling pathways may not sequester more SHP-1 and are unlikely to contribute to the defective SHP-1 activity in asthmatic cells under the challenge of mycoplasma infection. Elucidation of the mechanisms that drive reduced SHP-1 phosphatase activity in asthmatic airway epithelial cells requires further investigation. The defect in SHP-1 in asthmatic subjects may increase the susceptibility to other TLR ligands that are known to induce asthma exacerbations, such as rhinovirus, influenza virus, and respiratory syncytial virus (60). It is not known whether M. pneumoniae infection induces SHP-1 redistribution and nuclear translocation, and the importance of cytoplasmic versus nuclear SHP-1 in regulating M. pneumoniae-induced inflammation also requires further investigation.
In conclusion, we identified SHP-1 as a critical regulator of M. pneumoniae-induced inflammation in primary human airway epithelial cells cultured directly from well phenotyped subjects with asthma and nonasthma controls. Defective SHP-1 activation in asthmatic airway epithelial cells contributes to the increased activation of PI3K/Akt and NF-κB after M. pneumoniae infection, which further increases TLR2-mediated IL-8 production (Fig. 7). Deficiency of SHP-1 function in human asthmatic airway epithelial cells in the setting of exogenous insults, such as M. pneumoniae infection, may enhance the inflammatory response in human asthma following insults, such as infection, and lead to exacerbation of the disease. Our findings establish a link between SHP-1 and asthmatic airway epithelial cell inflammation. Elucidating the role of SHP-1–modulated signaling pathways in M. pneumoniae-induced exacerbation of airway inflammation in asthma will shed light on the innate immune regulation and dysfunction in asthma and offer new therapeutic avenues through which to decrease asthma exacerbations.
Supplementary Material
Acknowledgments
We thank Dr. Samulski R. Jude, University of North Carolina Gene Therapy Center, University of North Carolina at Chapel Hill, for providing the plasmids of rAAVs.
Abbreviations used in this article
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- P–SHP-1
tyrosine phosphorylated Src homology protein 1
- PVDF
polyvinylidene difluoride
- rAAV
recombinant adeno-associated virus
- SHP
Src homology protein
- siRNA
small interfering RNA
Footnotes
The online version of this article contains supplemental material.
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Djukanović R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, Howarth PH, Holgate ST. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis. 1992;145:669–674. doi: 10.1164/ajrccm/145.3.669. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 3.Allegra L, Blasi F, Centanni S, Cosentini R, Denti F, Raccanelli R, Tarsia P, Valenti V. Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection. Eur Respir J. 1994;7:2165–2168. doi: 10.1183/09031936.94.07122165. [DOI] [PubMed] [Google Scholar]
- 4.Berkovich S, Millian SJ, Snyder RD. The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child. Ann Allergy. 1970;28:43–49. [PubMed] [Google Scholar]
- 5.Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266:225–230. [PubMed] [Google Scholar]
- 6.Huhti E, Mokka T, Nikoskelainen J, Halonen P. Association of viral and mycoplasma infections with exacerbations of asthma. Ann Allergy. 1974;33:145–149. [PubMed] [Google Scholar]
- 7.Pelaia G, Vatrella A, Gallelli L, Renda T, Cazzola M, Maselli R, Marsico SA. Respiratory infections and asthma. Respir Med. 2006;100:775–784. doi: 10.1016/j.rmed.2005.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, Heilbronner C, Iniguez JL, Chaussain M, Nicand E, Raymond J, Gendrel D. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–1346. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 9.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraft M, Adler KB, Ingram JL, Crews AL, Atkinson TP, Cairns CB, Krause DC, Chu HW. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. Eur Respir J. 2008;31:43–46. doi: 10.1183/09031936.00103307. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, ühlradt PFM, Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 13.Chu HW, Jeyaseelan S, Rino JG, Voelker DR, Wexler RB, Campbell K, Harbeck RJ, Martin RJ. TLR2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J Immunol. 2005;174:5713–5719. doi: 10.4049/jimmunol.174.9.5713. [DOI] [PubMed] [Google Scholar]
- 14.Chambers MA, Whelan AO, Spallek R, Singh M, Coddeville B, Guerardel Y, Elass E. Non-acylated Mycobacterium bovis glycoprotein MPB83 binds to TLR1/2 and stimulates production of matrix metalloproteinase 9. Biochem Biophys Res Commun. 2010;400:403–408. doi: 10.1016/j.bbrc.2010.08.085. [DOI] [PubMed] [Google Scholar]
- 15.Kurokawa K, Lee H, Roh KB, Asanuma M, Kim YS, Nakayama H, Shiratsuchi A, Choi Y, Takeuchi O, Kang HJ, et al. The Triacylated ATP Binding Cluster Transporter Substrate-binding Lipoprotein of Staphylococcus aureus Functions as a Native Ligand for the Toll-like Receptor 2. J Biol Chem. 2009;284:8406–8411. doi: 10.1074/jbc.M809618200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiguchi M, Matsumoto M, Takao T, Hoshino M, Shimonishi Y, Tsuji S, Begum NA, Takeuchi O, Akira S, Toyoshima K, Seya T. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J Immunol. 2001;166:2610–2616. doi: 10.4049/jimmunol.166.4.2610. [DOI] [PubMed] [Google Scholar]
- 17.Seya T, Matsumoto M. A lipoprotein family from Mycoplasma fermentans confers host immune activation through Toll-like receptor 2. Int J Biochem Cell Biol. 2002;34:901–906. doi: 10.1016/s1357-2725(01)00164-9. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu T, Kida Y, Kuwano K. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-kappaB through toll-like receptors 1 and 2. Immunology. 2007;121:473–483. doi: 10.1111/j.1365-2567.2007.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chmura K, Bai X, Nakamura M, Kandasamy P, McGibney M, Kuronuma K, Mitsuzawa H, Voelker DR, Chan ED. Induction of IL-8 by Mycoplasma pneumoniae membrane in BEAS-2B cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L220–L230. doi: 10.1152/ajplung.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen SH, Bastien L, Posner BI, Chrétien P. A protein-tyrosine phosphatase with sequence similarity to the SH2 domain of the protein-tyrosine kinases. Nature. 1991;352:736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- 21.Matthews RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992;12:2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman RM, Jr, Plutzky J, Neel BG. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci USA. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi TL, Cleveland JL, Ihle JN. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992;12:836–846. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashiwada M, Giallourakis CC, Pan PY, Rothman PB. Immunoreceptor tyrosine-based inhibitory motif of the IL-4 receptor associates with SH2-containing phosphatases and regulates IL-4-induced proliferation. J Immunol. 2001;167:6382–6387. doi: 10.4049/jimmunol.167.11.6382. [DOI] [PubMed] [Google Scholar]
- 25.Hanson EM, Dickensheets H, Qu CK, Donnelly RP, Keegan AD. Regulation of the dephosphorylation of Stat6. Participation of Tyr-713 in the interleukin-4 receptor alpha, the tyrosine phosphatase SHP-1, and the proteasome. J Biol Chem. 2003;278:3903–3911. doi: 10.1074/jbc.M211747200. [DOI] [PubMed] [Google Scholar]
- 26.Cyster JG, Goodnow CC. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz U, Ravichandran KS, Burakoff SJ, Neel BG. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc Natl Acad Sci USA. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Somani AK, Yuen D, Yang Y, Love PE, Siminovitch KA. Involvement of the SHP-1 tyrosine phosphatase in regulation of T cell selection. J Immunol. 1999;163:3012–3021. [PubMed] [Google Scholar]
- 29.Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR. Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998;273:33893–33896. doi: 10.1074/jbc.273.51.33893. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Raghunath PN, Vonderheid E, Odum N, Wasik MA. Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter. Am J Pathol. 2000;157:1137–1146. doi: 10.1016/S0002-9440(10)64629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoury JD, Rassidakis GZ, Medeiros LJ, Amin HM, Lai R. Methylation of SHP1 gene and loss of SHP1 protein expression are frequent in systemic anaplastic large cell lymphoma. Blood. 2004;104:1580–1581. doi: 10.1182/blood-2004-03-1151. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Wang HY, Marzec M, Raghunath PN, Nagasawa T, Wasik MA. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc Natl Acad Sci USA. 2005;102:6948–6953. doi: 10.1073/pnas.0501959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christophi GP, Hudson CA, Gruber RC, Christophi CP, Mihai C, Mejico LJ, Jubelt B, Massa PT. SHP-1 deficiency and increased inflammatory gene expression in PBMCs of multiple sclerosis patients. Lab Invest. 2008;88:243–255. doi: 10.1038/labinvest.3700720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christophi GP, Hudson CA, Panos M, Gruber RC, Massa PT. Modulation of macrophage infiltration and inflammatory activity by the phosphatase SHP-1 in virus-induced demyelinating disease. J Virol. 2009;83:522–539. doi: 10.1128/JVI.01210-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamata T, Yamashita M, Kimura M, Murata K, Inami M, Shimizu C, Sugaya K, Wang CR, Taniguchi M, Nakayama T. src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin Invest. 2003;111:109–119. doi: 10.1172/JCI15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh SY, Zheng T, Kim YK, Cohn L, Homer RJ, McKenzie AN, Zhu Z. A critical role of SHP-1 in regulation of type 2 inflammation in the lung. Am J Respir Cell Mol Biol. 2009;40:568–574. doi: 10.1165/rcmb.2008-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho YS, Oh SY, Zhu Z. Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation. Am J Respir Cell Mol Biol. 2008;39:412–419. doi: 10.1165/rcmb.2007-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5, Suppl.):S94–S138. doi: 10.1016/j.jaci.2007.09.043. Published erratum appears in 2008 J. Allergy Clin. Immunol. 121: 1330. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Xu L, Duan H, Pasek DA, Eu JP, Meissner G. Knocking down type 2 but not type 1 calsequestrin reduces calcium sequestration and release in C2C12 skeletal muscle myotubes. J Biol Chem. 2006;281:15572–15581. doi: 10.1074/jbc.M600090200. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Samulski RJ, Xiao X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massa PT, Wu C. Increased inducible activation of NF-kappaB and responsive genes in astrocytes deficient in the protein tyrosine phosphatase SHP-1. J Interferon Cytokine Res. 1998;18:499–507. doi: 10.1089/jir.1998.18.499. [DOI] [PubMed] [Google Scholar]
- 44.Plutzky J, Neel BG, Rosenberg RD. Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci USA. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craggs G, Kellie S. A functional nuclear localization sequence in the C-terminal domain of SHP-1. J Biol Chem. 2001;276:23719–23725. doi: 10.1074/jbc.M102846200. [DOI] [PubMed] [Google Scholar]
- 46.Slevogt H, Zabel S, Opitz B, Hocke A, Eitel J, N'guessan PD, Lucka L, Riesbeck K, Zimmermann W, Zweigner J, et al. CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells. Nat Immunol. 2008;9:1270–1278. doi: 10.1038/ni.1661. [DOI] [PubMed] [Google Scholar]
- 47.Cuevas B, Lu Y, Watt S, Kumar R, Zhang J, Siminovitch KA, Mills GB. SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J Biol Chem. 1999;274:27583–27589. doi: 10.1074/jbc.274.39.27583. [DOI] [PubMed] [Google Scholar]
- 48.Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates Cot induction of NF-kappa B-dependent transcription. Mol Cell Biol. 2002;22:5962–5974. doi: 10.1128/MCB.22.16.5962-5974.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 50.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 51.Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauer J. Functional consequences of the SHP-1 defect in motheaten viable mice: role of NF-kappa B. Cell Immunol. 1998;185:49–58. doi: 10.1006/cimm.1998.1272. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Jimi E, Bothwell AL. Receptor activator of NF-kappa B ligand stimulates recruitment of SHP-1 to the complex containing TNFR-associated factor 6 that regulates osteoclastogenesis. J Immunol. 2003;171:3620–3626. doi: 10.4049/jimmunol.171.7.3620. [DOI] [PubMed] [Google Scholar]
- 53.Zhao J, Brooks DM, Lurie DI. Lipopolysaccharide-activated SHP-1-deficient motheaten microglia release increased nitric oxide, TNF-alpha, and IL-1beta. Glia. 2006;53:304–312. doi: 10.1002/glia.20283. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Voelker DR, Lugogo NL, Wang G, Floros J, Ingram JL, Chu HW, Church TD, Kandasamy P, Fertel D, et al. Surfactant protein A is defective in abrogating inflammation in asthma. Am J Physiol Lung Cell Mol Physiol. 2011;301:L598–L606. doi: 10.1152/ajplung.00381.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Yoshida Y, Yamashita U. DNA-binding activity of NF-kappaB and phosphorylation of p65 are induced by N-acetylcysteine through phosphatidylinositol (PI) 3-kinase. Mol Immunol. 2008;45:3984–3989. doi: 10.1016/j.molimm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nandan D, Lo R, Reiner NE. Activation of phosphotyrosine phosphatase activity attenuates mitogen-activated protein kinase signaling and inhibits c-FOS and nitric oxide synthase expression in macrophages infected with Leishmania donovani. Infect Immun. 1999;67:4055–4063. doi: 10.1128/iai.67.8.4055-4063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyake A, Murata Y, Okazawa H, Ikeda H, Niwayama Y, Ohnishi H, Hirata Y, Matozaki T. Negative regulation by SHPS-1 of Toll-like receptor-dependent proinflammatory cytokine production in macrophages. Genes Cells. 2008;13:209–219. doi: 10.1111/j.1365-2443.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 59.An H, Hou J, Zhou J, Zhao W, Xu H, Zheng Y, Yu Y, Liu S, Cao X. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat Immunol. 2008;9:542–550. doi: 10.1038/ni.1604. [DOI] [PubMed] [Google Scholar]
- 60.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. quiz 1188–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.