Abstract
BACKGROUND
During starvation, individuals with Anorexia Nervosa (AN) experience poor awareness of personal emotions (alexithymia), difficulty understanding others’ mental states (cognitive empathy), and poor regulation of personal emotions (self-regulation). Despite its important role in social interaction and interpersonal relationships, emotional empathy has not been measured in AN. Furthermore, how weight affects relationships among alexithymia, empathy, and self-regulation has not been investigated.
METHODS
Women with AN were tested longitudinally during starvation (N=26) and after weight restoration (N=20) and compared to 16 age-matched healthy women at comparable time-points. The Toronto Alexithymia Scale 20 (TAS-20) assessed alexithymia and the Interpersonal Reactivity Index (IRI) measured empathy. Self-regulation was assessed by a subset of 37 items from the MMPI 2.
RESULTS
Relative to comparison participants, AN participants at starvation and weight restoration reported greater alexithymia and personal distress (a domain of emotional empathy measuring vicarious negative arousal to others’ suffering). Among AN participants, personal distress was positively correlated with alexithymia and negatively correlated with self-regulation, when accounting for depression.
CONCLUSIONS
This study provides evidence that alexithymia and personal distress may represent vulnerability features of AN. Higher levels of personal distress in AN may be related to poor self-regulation and emotional awareness.
Keywords: Anorexia Nervosa, social cognition, alexithymia, empathy, self-regulation, weight effects
Introduction
Numerous studies have suggested that people who develop Anorexia Nervosa (AN) are likely to have poor awareness of personal emotions during starvation and after weight restoration (1-4), a psychological process termed alexithymia (5, 6), which may be a heritable vulnerability factor for AN (7). Furthermore, individuals with AN also have impaired cognitive empathy, or the understanding of others’ emotions (8-11), but the extent to which cognitive empathy impairments persist upon weight restoration has not been characterized longitudinally. Individuals with AN typically also have difficulties regulating their emotions (12, 13) which may influence their responses to others’ emotions.
Whereas cognitive empathy has been investigated in AN, the emotional domain of empathy (i.e., vicariously experiencing the emotions of others or feeling sympathy) largely has been ignored despite its critical role in interpersonal relationships which are known to be deficient in AN. Moreover, associations between empathy, alexithymia and self-regulation have not been fully characterized in AN individuals across the starvation and weight restoration phases. The present study addresses this gap in the literature by investigating alexithymia, empathy, and self-regulation longitudinally in order to examine effects of both starvation and weight restoration and relationships among these psychological processes in AN.
It is well-established that alexithymia is greater in individuals with AN during the acute starvation/malnutrition phase than healthy comparison participants (1, 2, 4, 14-16). There is also growing evidence that even when weight is restored, individuals with AN still report higher alexithymia than healthy comparison participants (3, 4, 17). Depression is often high during the starvation period of AN and may contribute to greater alexithymia during this phase (14, 16, 17). Studies of AN individuals in the starvation phase have found mixed results regarding the effect of depression on alexithymia in AN, with one study showing that alexithymia in the AN group was not significantly different from the control group after accounting for depression (14) and another study demonstrating that higher alexithymia was found in AN individuals for the Difficulty Describing Feelings factor of the TAS-20 when depression was considered (16). However, the first step in resolving this debate may be the Speranza and colleagues study that investigated alexithymia longitudinally over 3 years in AN individuals and found that even after accounting for eating disorder symptom severity and depression severity, the Difficulty Identifying Feelings factor of the TAS-20 predicted treatment outcome (17). An additional consideration may be effects of antidepressant medication usage on alexithymia scores as it is known that antidepressants may lead to emotional blunting (18, 19), and some individuals with AN may be treated with antidepressant medication for their depression during the starvation phase.
There are a number of studies that have found that people who are in the acute, starvation phase of AN have reduced cognitive empathy capacity (8-11). Cognitive empathy refers to adopting others’ mental states, through imagination and perspective taking (20-23), in order to detect their thoughts, feelings and motivations (24). Cognitive empathy studies have found that during starvation, individuals with AN perform poorly on a variety of mental state detection tasks employing differing types of stimuli including emotional expressions of the eye region of the face, voice, films, and written vignettes (8-11).
There is mixed evidence for the degree to which difficulties in mental state detection improve in AN individuals upon weight restoration (9, 10). A cross-sectional study comparing individuals with AN in the starvation phase to a group of individuals who previously had AN but had been weight-restored for at least one year found that difficulties detecting the mental state of others through voices or films were greater in the AN starvation group, and the restored AN group performed more similarly to that of the healthy comparison group (10). In contrast, another study investigating individuals who had been fully recovered from AN and were 18 years post-AN diagnosis had poorer performance detecting others’ thoughts and feelings from cartoons (Happé’s mental and nonmental cartoons task) than healthy matched comparison participants (9). Therefore, further research is needed to elucidate the degree to which these cognitive empathy difficulties persist in AN individuals upon weight restoration.
The study of emotional empathy in AN would add critically to this body of knowledge because it is currently unclear whether it is abnormal and fluctuates with weight changes. The capacity for emotional empathy is an important factor in social interaction because it has been consistently and directly linked to affiliative behaviors in healthy adults, such as helping others in need (20, 25, 26). Higher levels of empathy in healthy adults have also been linked to greater relationship satisfaction (27). Emotional empathy is thought to be made up of two components: 1) empathic concern- experiencing compassion and sympathy for others in need, and 2) personal distress- experiencing negative arousal that involves feelings of unease and upset in response to viewing another person suffer (21, 28). Previous research in healthy adults has shown that individuals with high levels of alexithymia (scores >60 on the TAS-20) have low Empathic Concern but high Personal Distress, as measured by the Interpersonal Reactivity Index (IRI; (21) (29). This suggests that there may be a relationship between alexithymia and emotional empathy, but the directionality of the association may depend on the domain of emotional empathy. While normal or high levels of empathic concern are important for relationship satisfaction (27), it is likely that personal distress may contribute to withdrawal from relationships. Typically, in healthy adults, emotion regulation strategies can be used to reduce personal distress levels in order to allow for sympathy and helping others in need (30).
Poor self-regulation is known to be pervasive in individuals with AN, and this may have potential effects on emotional empathy. Approximately twenty-five percent of individuals with AN meet criteria for an Avoidant Personality Disorder (31-33) which includes an avoidance or suppression of negative emotion. Self-regulation measures the degree to which an individual controls personal thoughts, behaviors, and feelings (34). Previous self-regulation studies have found that individuals suffering with AN show poor reappraisal (i.e., reframing thoughts and emotions in a more positive direction) and high suppression (i.e., modifying one’s behavioral response to an event) (12, 13).
Some studies suggest that social and emotional impairments found in the starvation phase of AN may recover upon weight restoration (10, 35). The first evidence for the effect of starvation on social and emotional processing was reported from the Keyes study in the 1950’s which provided evidence that starvation (and low BMI) among men following a severely restricted diet of six months duration produced social withdrawal which was not observed prior to starvation (36). A more recent study of starved AN patients found that they too had poorer accuracy in determining the mental states of others from voices and films than a separate sample of participants with AN who had recovered their weight (10). However, there is also evidence that a subset of individuals with AN may demonstrate difficulties with social interaction similar to that of Asperger’s syndrome and Obsessive Compulsive Disorder that are still present at 10 year follow-up (37). Many individuals who go on to develop AN have shown poor social functioning prior to illness onset, for example, the majority of AN individuals in this study reported that prior to 10 years of age they had either no friendships or only superficial acquaintances (38). Thus, social and emotional impairment in AN may be a vulnerability factor that precedes illness onset and continue after recovery (37, 38).
In the present study, the effect of weight changes on self-reported alexithymia and emotional empathy were assessed among patients with AN involved in a longitudinal study. Furthermore, relationships among alexithymia, emotional empathy and self-regulation were examined. Because individuals with AN during the starvation phase have low BMI and high levels of depression relative to healthy comparison participants, these variables were accounted for when investigating effects of weight restoration on alexithymia and emotional empathy.
It was hypothesized that participants with AN would show: (1) greater alexithymia, lower emotional empathy, and lower self-regulation than healthy comparison participants during the starvation phase and, (2) upon weight restoration, alexithymia would be lower and emotional empathy and self-regulation would be higher in the AN group than during the starvation phase (thus showing a tendency to return towards baseline), but still would significantly differ from the healthy comparison group. If the predicted results are found, AN participants’ scores on alexithymia, emotional empathy, and self-regulation would reflect both a state effect (due to starvation in the acute phase of the illness) and a trait effect of AN (potentially a vulnerability to develop the illness). Furthermore, based upon previous research in healthy adults it was predicted that alexithymia would be negatively related to the empathic concern domain of emotional empathy and positively related to the personal distress domain in participants with AN (29). Lastly, based upon a study in healthy adults (30), it was hypothesized that self-regulation would be positively associated with the other-oriented domain of emotional empathy (i.e., empathic concern) and negatively associated with the self-oriented domain (i.e., personal distress).
Methods
Participants
As part of a longitudinal study of women with AN approved by the Institutional Review Board at the University of Iowa, participants were assessed with a comprehensive battery of clinical, cognitive, and personality measures during starvation shortly after being hospitalized for treatment. Participants completed the informed consent process prior to testing. Repeated testing was carried out after weight restoration during the hospitalization period or during care in an outpatient day program following hospitalization. The inpatient eating disorder program at our university consists of daily cognitive behavioral therapy groups and individual therapy, supervised eating, occupational and recreational therapy as well as physician supervision.
Participants included 26 individuals with AN who were severely starved and were hospitalized with the main goal of weight restoration (Time 1). Twenty of these participants were re-assessed after weight-restoration (Time 2, see Table 1). Testing at Time 2 (after weight restoration) in AN participants typically occurred after body weight was restored to a BMI of at least 18.5 (M=20.16, SD=1.24). This testing took place approximately 1.35 days (SD=10.22) after the patients’ last day of either their hospitalized or shortly after discharge from our partial hospitalization program. Thus, patients had not been weight restored for an extended period of time before testing.1 These participants are part of a longitudinal study that also involves a 1 year follow-up visit. Testing for this third time-point is still on-going and is not included in the present study.
Table 1.
Demographic, Cognitive, and Clinical Assessments in Participants with AN and Healthy Comparison Participants
| Category | Assessment |
HC
M (SD) |
AN Time
1 M (SD) |
HC vs AN
Time 1 (p) |
AN
Time 2 M (SD) |
AN Time 1
vs 2 (p) |
|---|---|---|---|---|---|---|
| Demographics | Age, yrs. | 24.8 (5.4) |
24.4 (5.5) |
NS | 23.6 (5) |
- |
| Education, yrs. | 14.8 (1.9) |
14.1 (2.1) |
NS | - | - | |
| Body Mass Index |
25 (4.7) |
15.7 (2) |
<.001* | 20.2 (1.2) |
<.001* | |
| AN Subtype | - | R=57.7%, BP=42.3% |
- | - | - | |
| Comorbid Depressive Disorder |
- | 19.2% | - | - | - | |
| Antidepressant Use |
- | 46.2% | - | 60% | - | |
| Comorbid Borderline Personality Disorder |
- | 15.4% | - | - | - | |
| Cognitive Assessments |
WAIS-Full Scale IQ |
112.3 (11.9) |
109.7 (10.9) |
NS | 116.8 (10.3) |
<.001* |
| WAIS-PRI-IQ | 110.2 (15.3) |
105 (11.3) |
NS | 110.2 (8.8) |
<.001* | |
| WAIS-VCI-IQ | 110.5 (8.2) |
111 (15.4) |
NS | 113.5 (14) |
NS | |
| Stroop Interference T- score |
55 (6.7) |
55.7 (7.1) |
NS | 56.8 (4.7) |
NS | |
| CPT Perseveration Score |
50 (6.8) |
49.3 (5.7) |
NS | 51.1 (11.7) |
NS | |
| WCST Perseverative Errors |
5.2 (1.7) |
6.2 (3) |
NS | 4.5 (1.4) |
<.05 | |
| Clinical Assessments |
Eating Attitude Test |
2.9 (3.6) |
44.1 (13.9) |
<.001* | 12.1 (11.3) |
<.001* |
| EDI – Risk Composite Score |
109.7 (11) |
152 (19.5) |
<.001* | 125.8 (19) |
<.001* | |
| YBC-EDS | 0.3 (1) |
23.4 (5.6) |
<.001* | 7.8 (5.1) |
<.001* | |
| HDRS | 1.3 (1.5) |
15.5 (7.3) |
<.001* | 4.2 (3) |
<.001* | |
| HARS | 1.3 (1.7) |
17.3 (10) |
<.001* | 4.6 (3.5) |
<.001* |
Note. HC= Healthy comparison participants. AN= participants with Anorexia Nervosa. Time 1=first assessment time-point during the starvation phase of AN. Time 2=second assessment when weight has been restored in AN. Independent t-tests were used to compare AN participants and healthy comparison participants at Time 1. Paired t-tests were used to compare AN individuals at Time 1 (starvation phase) versus Time 2 (weight restoration phase). NS=non-significant p-value. P-values represented in the table reflect raw, uncorrected values. To account for multiple comparison tests, a corrected p-value threshold was set at p<.004 (p=.05 /number of comparisons; Time 1: AN vs. healthy comparison participant, 14 comparison tests; AN: Time 1 vs. 2, 12 comparison tests). Tests that surpassed this corrected threshold are starred. AN Subtype: R= restricting subtype, BP=binge-purge subtype. Comorbid Depressive Disorder=Presence of comorbid major depression diagnosis. Antidepressant Use= percentage of participants currently using antidepressants. Comorbid Borderline Personality Disorder=percentage of participants with comorbid borderline personality disorder. WAIS-PRI-IQ: Perceptual Reasoning Index. WAIS-VCI-IQ=Verbal Comprehension Index. CPT= Continuous Performance Test. WCST= Wisconsin Card Sort Task. EDI= Eating Disorder Inventory. YBC-EDS=Yale-Brown-Cornell Eating Disorder Scale. HDRS=Hamilton Depression Rating Scale. HARS= Hamilton Anxiety Rating Scale.
The participants with AN were compared to 16 sex- and age-matched healthy comparison women who were tested at the same time intervals as the patients with AN in order to control for differences that could be attributed to the length of time between sessions. Fifteen of the healthy comparison participants returned for testing at Time 2. AN participants were also characterized by subtype: either restricting or binge-purge subtype (see Table 1). Concurrent major depressive and borderline personality disorder diagnoses were recorded as well as anti-depressant treatment. Participants were interviewed regarding psychotropic medication use, and their responses were subsequently corroborated via chart review.
Clinical assessments
Eating disorder symptoms were assessed by the Eating Attitudes Test (EAT) (39, 40) and the Eating Disorder Inventory (EDI-3) (41), while obsessions and compulsions related to eating disorder symptoms were measured by the Yale-Brown-Cornell Eating Disorder Scale (YBC-EDS) (42, 43). Current levels of depression and anxiety were assessed by the Hamilton Depression Rating Scale (HDRS) (44) and the Hamilton Anxiety Rating Scale (HARS) (45) respectively. At Time 1, one AN individual and one healthy comparison participant were not available to complete the YBC-EDS and at Time 2, a second AN individual did not complete this scale. For the HDRS and HARS, at Time 1 one healthy comparison participant did not fill out these questionnaires and at Time 2 one AN individual did not fill out these questionnaires.
Cognitive assessments
The WAIS-IV measured full-scale IQ, FSIQ (46, 47). Attentional ability (sustained attention) was tested by the Conners’ Continuous Performance Test-II (CPT II) (48). To assess cognitive flexibility, or the ability of individuals to disengage from one process or task and engage effectively in another task, the Stroop Color and Word Test (49) and the Wisconsin Card Sorting Test Version 4—WCST (50) were used.2
Alexithymia, empathy, and self-regulation assessments
Alexithymia was measured by the Toronto Alexithymia Scale 20 (TAS-20) (51, 52), a standard and reliable measure of an individual’s difficulties identifying and describing personal emotions. This scale (51, 52) includes 3 factors: (Factor 1) Difficulty Identifying Feelings—DIF, (Factor 2) Difficulty Describing Feelings—DDF, and (Factor 3) Externally Oriented Thinking—EOT. Empathy was assessed through the Interpersonal Reactivity Index (IRI), a valid, multi-dimensional self-report measure (21)3. This questionnaire assesses cognitive empathy through two subscales (Perspective Taking, and Fantasy), measuring one’s ability to mentally adopt others’ thoughts and feelings, and in the case of the Fantasy subscale that of fictional characters in books/movies (21). Emotional empathy is assessed in the IRI through the Empathic Concern subscale (IRI-EC), measuring the degree to which individuals feel sympathy and compassion for others in need, and the Personal Distress subscale (IRI-PD) assesses vicarious negative emotions and arousal due to viewing others’ suffering (21). Self-regulation was assessed by 37 items from the Minnesota Multiphasic Personality Inventory-2 (MMPI-2) (53) which measure the regulation of feelings, thoughts, and behavior (34). This subset of items from the MMPI-2 has been previously used to measure self-regulation in a longitudinal sample of older adults (34). It has been shown that this self-regulation scale has good construct validity because the items are comparable to those of previously validated measures of self-regulation (e.g. Values in Action Inventory of Strengths, and Self-Control Scale) and has adequate internal consistency reliability (Cronbach’s α=0.75) (34).
Statistical analysis
Differences in descriptive demographic, cognitive, clinical, and personality characteristics between participants with AN and healthy comparison participants were assessed using independent sample t-tests, while paired t-tests were used to investigate differences between the starvation and weight restoration phases within the AN group.
Primary hypotheses were examined using repeated measures ANOVAs on alexithymia and emotional empathy scores using time of assessment (starvation phase—Time 1, and weight restoration phase—Time 2) as the within-subjects’ variable and group membership (AN participants and healthy comparison participants) as the between-subjects’ independent variables. Significant results from the ANOVA analysis were followed up with Bonferroni-corrected t-tests. Because previous literature has suggested a relationship between depression and alexithymia in AN, the repeated measures ANOVA examining alexithymia over time was repeated with the inclusion of the covariate depression change score (Time 2 –Time 1) on the HDRS. In addition, Pearson Product Moment correlations were computed to examine relationships between alexithymia, emotional empathy, and self-regulation. Partial correlations were used to investigate relationships between these variables controlling for depression and/or BMI when relevant.
Results
Demographic, cognitive, and clinical characteristics of AN and healthy participants
Demographic, cognitive, and clinical characteristics were compared between participants with AN in the starvation period and healthy comparison participants at Time 1 and between the starvation phase and the weight restoration phase within the AN group (see Table 1).
Alexithymia in AN during starvation and after weight restoration
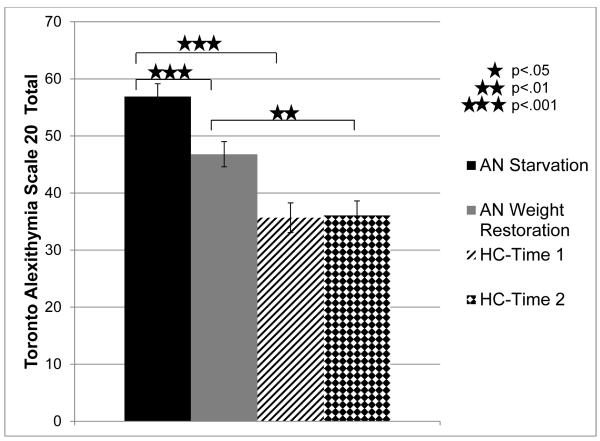
The effect of weight restoration on alexithymia was assessed using an omnibus repeated measures ANOVA comparing TAS-20 total scores as a function of time-point (Time 1: starvation period, Time 2: weight restoration period) and group (AN, healthy comparison participants), see Figure 1. Participants with AN reported greater TAS-20 total scores than healthy comparison participants overall (F(1,33)=30.21, p<.001, ηp2 =.48; AN: M=51.85, SE=1.9, Comparison: M=35.87, SE=2.2) and participants’ scores at Time 1 were higher than at Time 2 (F(1,33)=7.41, p<.05, ηp2 =.18; Time 1: M=46.28, SE=1.72, Time 2: M=41.43, SE=1.69). Investigating the primary research question, whether the restoration of weight affects alexithymia scores, it was found that the time-point by group interaction was significant (F(1,33)=8.68, p<.01, ηp2 =.21). Bonferroni-corrected follow-up t-tests indicated that participants with AN showed a significant decrease in alexithymia from the starvation period to the weight restoration period (p<.001), while the healthy volunteers showed no difference (p>.88). Participants with AN reported significantly greater alexithymia scores relative to healthy comparison participants at both Time 1 (p<.001) and Time 2 (p<.01).
Figure 1. Alexithymia in Participants with AN during Starvation and Weight Restoration Relative to Comparison Participants.
Note. Alexithymia was measured by the Toronto Alexithymia Scale 20 (TAS-20) at Time 1 (the starvation phase of AN) and Time 2 (the weight restoration phase of AN) in participants with AN and healthy comparison participants. AN= participants with Anorexia Nervosa. HC= healthy comparison participants. Error bars reflect standard error.
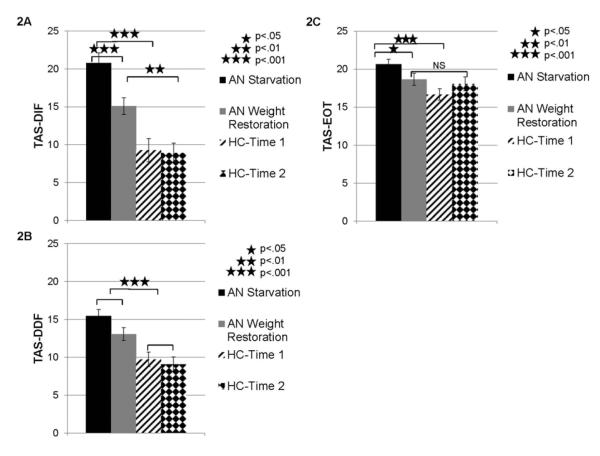
Next, because the omnibus ANOVA revealed that weight restoration did have an effect on TAS-20 total scores in AN, the effect of weight restoration on each of the TAS-20 factors (Difficulty Identifying Feelings-DIF, Difficulty Describing Feelings-DDF, and Externally Oriented Thinking-EOT) was examined through a series of planned comparisons which included repeated measures ANOVAs to investigate the effect of group and time-point on each TAS-20 factor (see Figure 2).
Figure 2. Toronto Alexithymia Scale 20 Factor Scores in Participants with AN during Starvation and Weight Restoration Relative to Comparison Participants.
Note. Alexithymia in AN during starvation (Time 1) and weight restoration (Time 2) relative to healthy comparison participants was measured by three factors from the Toronto Alexithymia Scale 20 (TAS-20) which are represented in the 3 panels: (2A) Factor 1: Difficulty Identifying Feelings—DIF, (2B) Factor 2: Difficulty Describing Feelings—DDF, and (2C) Factor 3: Externally Oriented Thinking—EOT. AN= participants with Anorexia Nervosa. HC= healthy comparison participants. Error bars reflect standard error.
These analyses revealed that for the Difficulty Identifying Feelings factor (DIF—Factor 1), there were significant effects of time-point (F(1,33)= 11.15, p<.01, ηp2 =.25), group (F(1,33)=29.32, p<.001, ηp2 =.47), and a time-point x group effect (F(1,33)=8.82, p<.01, ηp2 =.21). Follow-up Bonferroni-corrected t-tests indicated that AN individuals reported higher scores than healthy comparison participants at Time 1 (p<.001; see Figure 2) and Time 2 (p<.01). Within the AN group, participants reported higher scores at starvation than at weight restoration on the DIF factor (p<.001), but there was no effect of time-point within the healthy comparison group (p>.81). For the Difficulty Describing Feelings (DDF—Factor 2) there was no group by time-point interaction (F(1,33)=1.84, p>.18, ηp2 =.05), but there were significant main effects of time-point (F(1,33)=5.76, p<.05, ηp2 =.15) and group (F(1,33)=18.88, p<.001, ηp2 =.36.) Follow-up tests indicated that participants with AN had higher scores on the DDF factor than healthy comparison subjects overall (p<.001; AN: M=14.25, SE=.73, Comparison: M=9.4, SE=.84) and the sample as a whole reported higher scores on this factor at Time 1 than Time 2 (p<.05; Time 1: M=12.59, SE=.64, Time 2: 11.06, SE=.64). For the Externally Orienting Thinking factor (EOT—Factor 3), there was also a significant interaction between time-point and group (F(1,33)=5.23, p<.05, ηp2 =.14). In addition, there was a significant main effect of group (F(1,33)=7.48, p<.05, ηp2 =.19), but no main effect of time-point (F(1,33)=.16, p>.69, ηp2 =.01). Follow-up tests revealed that participants with AN had greater scores on the EOT factor than healthy comparison participants at Time 1 (p<.001), but not Time 2 (p>.63). Within the AN group, participants reported higher scores at starvation than weight restoration (p<.05).
In summary, in the starvation phase, AN individuals had higher scores than healthy comparison participants on the first and third TAS-20 factors, Difficulty Identifying Feelings (DIF) and Externally Oriented Thinking (EOT). On the DIF factor, AN individuals also demonstrated higher scores than healthy comparison participants upon weight restoration. Within the AN group, there was a decrease in DIF factor scores upon weight restoration (relative to starvation). For the EOT factor, AN individuals showed decreased scores upon weight restoration (in comparison to starvation) and did not differ from healthy comparison participants during the weight restoration phase. Finally, for the second TAS-20 factor, Difficulty Describing Feelings (DDF), AN individuals had higher scores than healthy comparison participants overall, but there was no difference between the starvation and weight restoration phases.
Relationships between alexithymia, depression, and treatment
The extent to which depression affected alexithymia in AN and healthy comparison participants was considered. At Time 1 among AN participants, there was a moderate, positive relationship between TAS-20 and HDRS depression scores (r(26)=.57, p<.01). Thus, the covariate depression change score between Time 1 and Time 2 (HDRS score) was included in a repeated measures ANOVA comparing AN participants to the healthy comparison participants on TAS-20 total scores at Time 1 and 2. The significant interaction between time of assessment and group reported in the preceding section was no longer significant (F(1,31)=0, p>1, ηp2 =0). However, participants with AN still showed greater TAS-20 total scores than healthy participants at the group level when depression was accounted for (F(1,31)=11.6, p<.01, ηp2 =.27; AN=50.45 (SE=2.27), Comparison=37.1 (SE=2.64)).
Next, effects of depression on TAS-20 factors in the AN and healthy comparison participant groups were considered. Similar to the finding for the TAS-20 total score, the inclusion of the depression change score as a covariate in the analysis of the DIF TAS-20 factor resulted in the time-point by group interaction no longer reaching significance (F(1,31)=.11, p>.74, ηp2 =.004). The main effect of group remained significant, with AN participants reporting higher scores on this factor than healthy comparison participants (F(1,31)=9.98, p<.01, ηp2 =.24; AN: M=16.97, SE=1.26; Comparison: M=10.07, SE=1.47). For the Externally Oriented Thinking factor, including the depression change score also resulted in the time-point x group interaction being no longer significant (F(1,31)=.28, p=.6, ηp2 =.009). Similar to the Difficulty Identifying Feelings factor, AN participants showed greater scores on the Externally Oriented Thinking Factor than healthy comparison participants overall (F(1,31)=4.48, p<.05, ηp2 =.13; AN: M=19.76, SE=.66; Comparison: M=17.34, SE=.77). For the Difficulty Describing Feelings factor, the main effect of group remained after considering depression change score as a covariate (F (1,31)=7.56, p<.05, ηp2 =.2; AN: M=13.71, SE=.84; Comparison: M=9.7, SE=.98). Therefore, after accounting for the change in depression scores from the starvation to the weight restoration period, it revealed that AN individuals had higher scores than healthy comparison participants on all three TAS-20 factors, but there was no longer a significant effect of weight restoration.
There is a possibility that alexithymia scores of AN individuals may have been affected by other relevant factors such as taking antidepressant medication which has been linked to reduced emotional awareness (18, 19) or degree of cognitive therapy. In the present sample, there was variability in the number of hospitalizations each AN individual had undergone previously and each of these instances included cognitive therapy. Therefore, exploratory analyses were conducted to assess the degree to which alexithymia (TAS-20 total score) was affected by antidepressant treatment and cognitive therapy (as a proxy, measured by number of previous hospitalizations for AN). These preliminary results indicated that antidepressant usage and hospitalization did not significantly affect alexithymia in the present sample.4
Empathy in AN
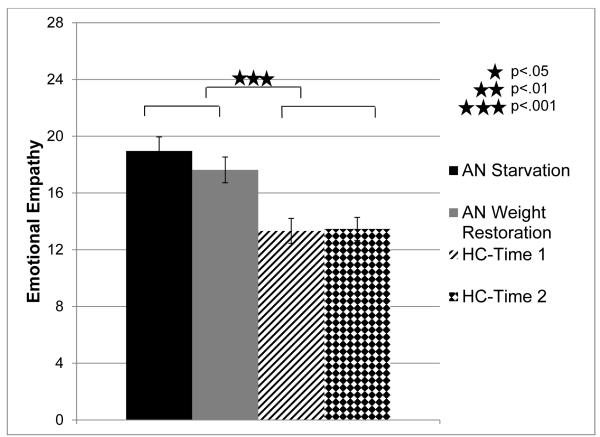
A repeated measures ANOVA was computed using emotional empathy (average of responses to Empathic Concern and Personal Distress subscales) as the dependent variable and time-point and group as independent variables. The main effect of group was found to be significant (F(1,25)=16.88, p<.001, ηp2 =.4), indicating that patients with AN reported greater emotional empathy than comparison participants overall (see Figure 3). There was no significant effect of assessment time-point (F(1,25)=1.83, p>.19, ηp2 =.07) nor a significant time-point by group interaction (F (1,25)=2.74, p>.11, ηp2 =.1).
Figure 3. Emotional Empathy in AN during Starvation and Weight Restoration Relative to Comparison Participants.
Note. Emotional empathy was assessed by taking the average of the Empathic Concern and Personal Distress subscales for each participant on the Interpersonal Reactivity Index (IRI). This was measured at Time 1 (the starvation phase of AN) and Time 2 (the weight restoration phase of AN) in participants with AN and healthy comparison participants. AN= participants with Anorexia Nervosa. HC= healthy comparison participants. Error bars reflect standard error.
To ascertain if specific aspects of emotional empathy were greater in AN relative to comparison participants, planned comparisons were used to investigate AN participants and healthy participants on two aspects of emotional empathy: Empathic Concern (IRI-EC) and Personal Distress (IRI-PD). Because there were no significant effects of time-point on emotional empathy, participants’ scores on the empathy measures were averaged across the two time-points. Participants with AN showed greater Personal Distress than healthy comparison participants (AN: M=14.33, SE=1.54; Comparison: M=7.47, SE=.83; t(25)=4.14, p<.001). The effect of Empathic Concern was marginally significant relative to healthy comparison participants (AN: M=22.25, SE=.87, Comparison: M=19.33, SE=1.09; t(25)=2.02, p>.06)).
For completeness, cognitive empathy (measured by the average of the IRI-Fantasy and Perspective Taking subscales) was also examined. This analysis showed no significant main effects of time-point (F(1,25)=1.38, p>.25, ηp2 =.05), group (F(1,25)=.004, p>.95, ηp2 =.00) nor a time-point by group interaction (F(1,25)=.44, p>.52, ηp2 =.02.)
Self-regulation in AN
AN individuals during the starvation phase reported lower self-regulation scores than healthy adults at this time-point (t(38)=5.93, p<.001; AN: M=22.92, SE=.98; Comparison: M=30.31, SE=.37). Time 2 data was only available for AN individuals and thus a paired t-test was used to compare their self-regulation scores at starvation and upon weight restoration. This analysis revealed that AN individuals did not significantly differ in self-regulation scores at the starvation and weight restoration (t(17)=1.32, p>.21).
Relationships between alexithymia, emotional empathy, and self-regulation in AN
First, the relationship between the Personal Distress domain of emotional empathy (IRI-PD) and alexithymia (TAS-20 total score) during the starvation phase was investigated in AN participants while accounting for depression (HDRS score) and BMI. This analysis revealed a significant, positive relationship between TAS-20 total scores and IRI-PD (r(22)=.46, p<.05). Due to a smaller sample size at the Time 2 weight restoration phase (N=12), an exploratory analysis examined the relationship between IRI-PD and TAS-20 total score at this time-point. Depression and BMI were no longer included as covariates at this time-point because these scores had returned to levels similar to healthy comparison participants. During the weight restoration phase, participants with AN showed a direct, positive correlation between IRI-PD and TAS-20 Total scores (r(12)=.64, p<.05).
Next, relationships between self-regulation, Personal Distress (IRI-PD), and alexithymia (TAS-20 total score) in AN individuals during the starvation phase were examined. Because depression (HDRS score) was higher and BMI was lower in the AN group during the starvation than weight restoration phases, it was assessed whether these variables should be used as covariates in the analysis considering the starvation phase. Pearson correlations indicated that in AN individuals, self-regulation was significantly and negatively correlated with HDRS score (r(24)= −.65, p<.01), but not BMI (r(24)= −.06, p=.78), and thus HDRS score was used as a covariate in the two self-regulation analyses. The p-values underwent Bonferroni correction to account for the two comparisons. The first analysis indicated that TAS-20 total score and self-regulation were not significantly associated (r(21)= −.23, p>.58). However, in the second analysis, IRI-PD was significantly, negatively correlated with self-regulation (r(21)= −.49, p<.05).
Summary of results
AN participants reported higher TAS-20 total alexithymia scores than healthy comparison participants at the starvation and weight restoration phases, even though there was a slight improvement after weight restoration. When the three TAS-20 factors were examined individually, it became evident that weight-related improvements in alexithymia are primarily related to changes in Factors 1 and 3 (Difficulty Identifying Feelings and Externally Oriented Thinking) and that Factor 2 (Difficulty Describing Feelings) didn’t change at all with weight-restoration. While Factor 1 (Difficulty Identifying Feelings) remains abnormally high after weight restoration in AN individuals, Factor 3 (Externally Oriented Thinking) normalized with weight restoration. It is important to note that when depression scores were accounted for, AN participants reported higher total TAS-20 alexithymia scores than comparison participants overall, but the difference between starvation and weight restoration phases was no longer significant. Furthermore, when accounting for depression in the analysis of the TAS-20 factors, this same pattern was upheld, whereby the AN group had higher scores than the healthy participants on all three factors overall, but there was no longer an effect of weight restoration. For emotional empathy, AN participants had higher ratings than comparison participants overall across the starvation and weight restoration phases, but there was no significant effect of weight restoration. Follow-up tests revealed that it was the Personal Distress domain of emotional empathy that was driving this effect. Self-regulation in AN during the starvation phase was found to be lower than in healthy comparison participants and did not significantly change in AN individuals as a function of weight restoration.
There was a significant, positive relationship between TAS-20 total score and the Personal Distress domain of emotional empathy during the starvation phase of AN, when accounting for depression scores and BMI, and a positive association during the weight restoration phase (BMI and depression were no longer covariates because they had returned to normal levels). In contrast, self-regulation was negatively associated with the IRI – Personal Distress domain in AN individuals during the starvation phase (with depression scores being used as a covariate).
Discussion
The present study provides a novel contribution to the literature on emotion and social cognition in AN because of its longitudinal design allowing examination of relationships among alexithymia, empathy, and self-regulation during the acutely ill phase of starvation and again after weight restoration. Furthermore, this is one of the first studies to investigate the emotional component of empathy in AN, as most previous studies have focused on the cognitive component (8-11). Whereas cognitive empathy has been primarily examined through either a cross-sectional analysis (10) or by examining only individuals in the starvation phase (8, 11) or weight recovered phase(9), this study extends previous research by investigating empathy longitudinally in the same individuals with AN. Previous studies of individuals with AN have considered the constructs of alexithymia, empathy and self-regulation separately (1, 9, 11, 13, 54). However, this study is one of the first to directly investigate relationships between these variables within the AN population. This is relevant because it aids in the understanding of how difficulties in various emotional and social abilities in AN may interact with each other throughout the course of the illness and which psychological functions may be vulnerability factors or consequences of starvation. Before discussing the results of the present study in the context of the existing literature, some caveats need to be highlighted.
Limitations
Employing a longitudinal design and self-report questionnaires to measure empathy, the present study did not find a significant difference between AN and healthy comparison participants in reported cognitive empathy. Whereas previous studies have found differences between AN individuals and healthy comparison participants in cognitive empathy, this research has primarily focused on the starvation phase of AN (8, 11). Or in other cases, AN individuals in the starvation phase have been compared to a separate group whose weight had been restored for at least one year (10), rather than using a longitudinal design. In addition, these studies have often used task-based assessment of cognitive empathy (e.g. the Eyes Task) (8-11) rather than a questionnaire, as employed in the present study. When a questionnaire has been employed to assess cognitive empathy (i.e., the Empathy Quotient), no significant differences between AN individuals and healthy women were found (54). Taken together, these findings suggest that the type of measure used (self-report vs. task-based) and the design of the study (cross-sectional vs. longitudinal) may yield differing results when assessing cognitive empathy among participants with AN.
Antidepressant medication use and number of hospitalizations for eating disorder treatment (as a proxy for length of cognitive therapy) did not significantly affect alexithymia and emotional empathy in the present study. However, these variables were dichotomized in the present study (i.e., taking antidepressants versus not taking antidepressants, and previous hospitalizations versus no previous hospitalizations), and thus different results may be found if these variables are considered in a continuous manner in future studies.
Alexithymia in AN
The present study found that participants with AN report higher levels of alexithymia than healthy comparison participants whether alexithymia is measured during the starvation or weight restoration phase. This is consistent with previous literature that has shown that AN participants report greater alexithymia than healthy comparison participants during the starvation phase (1, 2, 15, 16) and the weight restoration phase (3, 4). Despite participants with AN in the present sample showing a slight reduction in alexithymia upon weight restoration, after controlling for depression, the effect of weight restoration on alexithymia was no longer significant. However, participants with AN still reported greater alexithymia than controls overall, even after controlling for depression.
Previous studies have shown that AN may contribute to certain factors of alexithymia over and above that contributed by depression, including the Difficulty Describing Feelings factor (16) and the Difficulty Identifying Feelings factor (17). Similar to these studies, the present study also found that AN individuals had higher alexithymia scores on these two TAS-20 factors, but also on the Externally Oriented Thinking factor. This difference from previous studies may be a function of variation in study design whereby the Sexton and colleagues study (16) compared AN individuals only in the starvation phase to healthy comparison participants (not the weight restoration phase) while Speranza and colleagues employed a longitudinal design investigating individuals in the acute phase and three years later at which time there was variability in the length of time individuals with AN had been recovered (17). Because there was no significant effect of weight restoration after controlling for depression, it suggests that alexithymia may be either a trait feature of AN or a “permanent” change that is a consequence of the illness and may not recover upon weight restoration.
Emotional empathy in AN
Emotional empathy did not differ as a function of weight restoration, and thus increased emotional empathy may be a trait feature in AN. Specifically, it was found that the personal distress domain of emotional empathy was higher in AN individuals than healthy comparison participants. This is in contrast to typical findings involving cognitive empathy which generally demonstrate reduced cognitive empathy in AN individuals in comparison to healthy adults (8-11). In light of this literature, this finding was unexpected because studies of healthy adults suggest that cognitive and emotional empathy levels often show moderate, positive correlations (21). Thus, it was expected that AN participants would show lower emotional empathy in the empathic concern domain in particular than healthy comparison participants. However, this dissociation between emotional and cognitive empathy is, in general, not new (55). For instance, in psychopathy individuals have intact cognitive empathy but show deficits in aspects of emotional empathy that may influence their moral responding to others (55, 56).
Higher levels of personal distress found in individuals with AN may be partially explained by the greater levels of social anxiety and difficulty regulating emotions that individuals with AN experience. Individuals with AN also have been shown to be highly sensitive to facial expressions of social rejection, and may demonstrate an attentional bias towards this type of stimuli (57). When negative emotion is induced through film clips, participants with AN draw their attention away from the stimuli more often than healthy comparison participants (58). Hence, AN participants’ heightened sensitivity to negative emotional expressions may potentially extend to the perception of the suffering of others.
Relationships between emotional empathy, alexithymia, and self-regulation in AN
Empathy is thought to require an understanding of one’s own emotions in order to comprehend the emotions of others. Thus, it was expected that high alexithymia, or poor personal self-awareness of emotions, would be associated with low levels of emotional empathy. Individuals with alexithymia typically show reduced emotional empathy in the domain of empathic concern and increased emotional empathy in the personal distress domain (29). Consistent with this previous study in healthy adults, the present study found that greater alexithymia scores were associated with greater personal distress among AN individuals, but distinct from this previous study no relationship was found between empathic concern and alexithymia. Furthermore, personal distress was negatively associated with self-regulation in the starvation phase (when accounting for depression), suggesting that high levels of personal distress may co-occur with poor self-regulation in AN.
Greater alexithymia may be related to greater personal distress because observing others’ negative emotions (and especially their suffering) may elicit high levels of negative arousal and individuals’ with poor awareness of personal emotions may internalize these feelings without properly regulating them. In fact, a study in healthy adults suggests that emotion regulation is a necessary component for individuals to experience healthy empathic concern because it enables individuals to reduce their feelings of personal distress that they experience as a consequence of others’ suffering and re-appraise their emotional response so that it becomes focused on the other person as compassion (30). This relationship may also extend to other psychiatric disorders, as a study that included patients with borderline personality disorder, AN, their families, and healthy adults reported high levels of personal distress in those individuals who reported high alexithymia (59, 60). Again, this same pattern of association was shown in individuals with OCD (61).
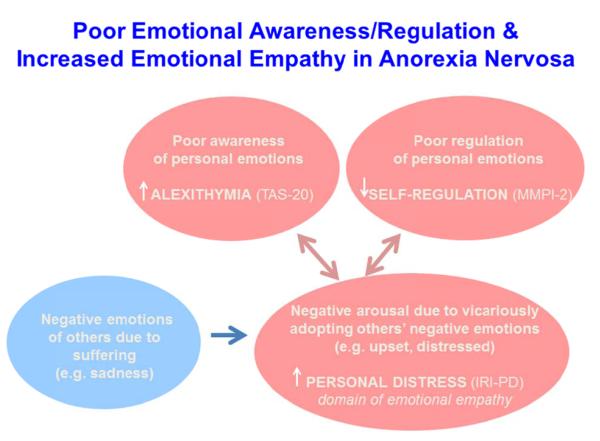
It is known that individuals with AN dislike experiencing strong emotions and often experience a lack of emotional self-control (62). AN individuals often use less psychologically healthy strategies such as thought suppression (12, 13, 63) or engage in unhealthy behaviors (e.g., restricting food or excessive exercising) to control their negative emotions (64-67). Thus, high levels of personal distress in AN individuals may be related to a tendency to engage in poor self-regulation and poor emotional awareness (see Figure 4).
Figure 4. Poor Emotional Awareness/Regulation and Increased Emotional Empathy in Anorexia Nervosa.
Note. TAS-20= Toronto Alexithymia Scale 20. MMPI-2= Minnesota Multiphasic Personality Inventory 2. IRI-PD= Interpersonal Reactivity Index, Personal Distress subscale.
Clinical implications and future directions
The present study adds to the growing literature on emotion and social cognition in AN by examining relationships between alexithymia, emotional empathy, and self-regulation during the starvation phase and after weight has been restored. This study demonstrates that both alexithymia and the personal distress domain of emotional empathy may reflect trait features of AN because high levels of these features are found even upon weight restoration. Whereas the present study focuses on relationships between these psychological processes, future research may consider the degree to which self-regulation and emotional awareness may play a causative role in high levels of personal distress. Furthermore, the present study investigates individuals with AN immediately after weight has been recovered, but it is known that psychological effects due to starvation may not recover immediately upon weight restoration. Thus, future studies may elucidate the degree to which high alexithymia, personal distress and poor self-regulation persist after weight has been recovered for a longer period. The design of our on-going longitudinal study will also be able to speak to this question upon the completion of the 1 year follow-up assessments. Additional research is also needed to investigate these psychological constructs through a more comprehensive battery of tasks including both self-report and performance based measures. Potential therapeutic interventions aimed at reducing personal distress and increasing emotional awareness and regulation may help to facilitate higher quality social interactions in AN individuals.
Acknowledgments
We would like to thank Michael Brumm for all of his assistance involving participant testing and data entry. Grant support was received from NIMH K23 MH083879-01 to LM.
Footnotes
At Time 2, a BMI score was not available for one healthy comparison participant and one individual with AN.
For Time 1, one AN individual was unable to complete the CPT II, and one healthy comparison participant was not available to complete the WAIS-IV, Stroop Color and Word Test, CPT II, and WCST. For Time 2, two AN individuals were not able to complete the Stroop Color and Word Test and three were not available to complete the WCST.
For the Time 2 assessment, only a subsample of participants were able to complete the Interpersonal Reactivity Index, resulting in a sample size of N=12 for the AN group, while the sample size remained the same for the HC group.
AN participants who were or were not taking antidepressants at the time of the study did not differ on their TAS-20 scores either during starvation or weight restoration [starvation: t(24)=1.41, p>.17; weight restoration: t(18)=1.02, p>.32]. The effect of prior hospitalizations was not statistically significant both at Time 1 [t(24)=.27, p>.8] and Time 2 [t(18)=.61, p>.55]. Because these variables did not significantly affect TAS-20 total score, follow-up analyses on the TAS-20 factors were not conducted.
The official published version of this article can be found at: https://www.aacp.com
This version of the article was accepted for publication and the article presently appears in a revised form, subsequent to peer review and/or editorial input by the American Academy of Clinical Psychiatrists.
References
- 1.Corcos M, Guilbaud O, Speranza M, Paterniti S, Loas G, Stephan P, et al. Alexithymia and depression in eating disorders. Psychiatry Res. 2000 Apr 10;93(3):263–6. doi: 10.1016/s0165-1781(00)00109-8. [DOI] [PubMed] [Google Scholar]
- 2.Kessler H, Schwarze M, Filipic S, Traue HC, von Wietersheim J. Alexithymia and facial emotion recognition in patients with eating disorders. Int J Eat Disord. 2006 Apr;39(3):245–51. doi: 10.1002/eat.20228. [DOI] [PubMed] [Google Scholar]
- 3.Parling T, Mortazavi M, Ghaderi A. Alexithymia and emotional awareness in anorexia nervosa: Time for a shift in the measurement of the concept? Eat Behav. 2010 Dec;11(4):205–10. doi: 10.1016/j.eatbeh.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Tchanturia K, Davies H, Harrison A, Fox JR, Treasure J, Schmidt U. Altered social hedonic processing in eating disorders. Int J Eat Disord. 2012 Dec;45(8):962–9. doi: 10.1002/eat.22032. [DOI] [PubMed] [Google Scholar]
- 5.Marty P, De Muzan M. Functional aspects of the dream life. “operative thinking”. Rev Fr Psychanal. 1963;27(SUPPL3):45–56. [PubMed] [Google Scholar]
- 6.Sifneos PE. The prevalence of ‘alexithymic’ characteristics in psychosomatic patients. Psychother Psychosom. 1973;22(2):255–62. doi: 10.1159/000286529. [DOI] [PubMed] [Google Scholar]
- 7.Espina A. Alexithymia in parents of daughters with eating disorders: Its relationships with psychopathological and personality variables. J Psychosom Res. 2003 Dec;55(6):553–60. doi: 10.1016/s0022-3999(03)00016-3. [DOI] [PubMed] [Google Scholar]
- 8.Russell TA, Schmidt U, Doherty L, Young V, Tchanturia K. Aspects of social cognition in anorexia nervosa: Affective and cognitive theory of mind. Psychiatry Res. 2009 Aug 15;168(3):181–5. doi: 10.1016/j.psychres.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Gillberg IC, Billstedt E, Wentz E, Anckarsater H, Rastam M, Gillberg C. Attention, executive functions, and mentalizing in anorexia nervosa eighteen years after onset of eating disorder. J Clin Exp Neuropsychol. 2010 Apr;32(4):358–65. doi: 10.1080/13803390903066857. [DOI] [PubMed] [Google Scholar]
- 10.Oldershaw A, Hambrook D, Tchanturia K, Treasure J, Schmidt U. Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosom Med. 2010 Jan;72(1):73–9. doi: 10.1097/PSY.0b013e3181c6c7ca. [DOI] [PubMed] [Google Scholar]
- 11.Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotion recognition and regulation in anorexia nervosa. Clin Psychol Psychother. 2009 Jul-Aug;16(4):348–56. doi: 10.1002/cpp.628. [DOI] [PubMed] [Google Scholar]
- 12.Davies H, Swan N, Schmidt U, Tchanturia K. An experimental investigation of verbal expression of emotion in anorexia and bulimia nervosa. Eur Eat Disord Rev. 2011 Sep 22; doi: 10.1002/erv.1157. [DOI] [PubMed] [Google Scholar]
- 13.Geller J, Cockell SJ, Hewitt PL, Goldner EM, Flett GL. Inhibited expression of negative emotions and interpersonal orientation in anorexia nervosa. Int J Eat Disord. 2000 Jul;28(1):8–19. doi: 10.1002/1098-108x(200007)28:1<8::aid-eat2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Montebarocci O, Codispoti M, Surcinelli P, Franzoni E, Baldaro B, Rossi N. Alexithymia in female patients with eating disorders. Eat Weight Disord. 2006 Mar;11(1):14–21. doi: 10.1007/BF03327739. [DOI] [PubMed] [Google Scholar]
- 15.Taylor GJ, Parker JD, Bagby RM, Bourke MP. Relationships between alexithymia and psychological characteristics associated with eating disorders. J Psychosom Res. 1996 Dec;41(6):561–8. doi: 10.1016/s0022-3999(96)00224-3. [DOI] [PubMed] [Google Scholar]
- 16.Sexton MC, Sunday SR, Hurt S, Halmi KA. The relationship between alexithymia, depression, and axis II psychopathology in eating disorder inpatients. Int J Eat Disord. 1998 Apr;23(3):277–86. doi: 10.1002/(sici)1098-108x(199804)23:3<277::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Speranza M, Loas G, Wallier J, Corcos M. Predictive value of alexithymia in patients with eating disorders: A 3-year prospective study. J Psychosom Res. 2007 Oct;63(4):365–71. doi: 10.1016/j.jpsychores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 18.McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry. 2010 Mar 1;67(5):439–45. doi: 10.1016/j.biopsych.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sansone RA, Sansone LA. SSRI-induced indifference. Psychiatry (Edgmont) 2010 Oct;7(10):14–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Coke JS, Batson CD, McDavis K. Empathic mediation of helping: A two-stage model. J Pers Soc Psychol. 1978;36(7):752–66. [Google Scholar]
- 21.Davis MH. Individual differences in empathy: A multidimensional approach [dissertation] ProQuest Information & Learning; US: 1980. [Google Scholar]
- 22.Krebs D. Empathy and altruism. J Pers Soc Psychol. 1975;32(6):1134–46. doi: 10.1037//0022-3514.32.6.1134. [DOI] [PubMed] [Google Scholar]
- 23.Stotland E. Exploratory investigations of empathy. In: Berkowitz L, editor. Advances in experimental social psychology. Academic Press; New York: 1969. [Google Scholar]
- 24.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003 Mar 29;358(1431):459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batson CD, Duncan BD, Ackerman P, Buckley T, Birch K. Is empathic emotion a source of altruistic motivation? J Pers Soc Psychol. 1981;40(2):290–302. [Google Scholar]
- 26.Batson CD. The altruism question: Toward a social-psychological answer. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ, England: 1991. [Google Scholar]
- 27.Davis MH, Oathout HA. Maintenance of satisfaction in romantic relationships: Empathy and relational competence. J Pers Soc Psychol. 1987;53(2):397–410. [Google Scholar]
- 28.Batson CD, O’Quin K, Fultz J, Vanderplas M, Isen AM. Influence of self-reported distress and empathy on egoistic versus altruistic motivation to help. J Pers Soc Psychol. 1983;45(3):706–18. [Google Scholar]
- 29.Moriguchi Y, Ohnishi T, Lane RD, Maeda M, Mori T, Nemoto K, et al. Impaired self-awareness and theory of mind: An fMRI study of mentalizing in alexithymia. Neuroimage. 2006 Sep;32(3):1472–82. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- 30.Eisenberg N, Fabes RA, Murphy B, Karbon M, Maszk P, Smith M, et al. The relations of emotionality and regulation to dispositional and situational empathy-related responding. J Pers Soc Psychol. 1994 Apr;66(4):776–97. doi: 10.1037//0022-3514.66.4.776. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt U, Treasure J. Anorexia nervosa: Valued and visible. A cognitive-interpersonal maintenance model and its implications for research and practice. Br J Clin Psychol. 2006 Sep;45(Pt 3):343–66. doi: 10.1348/014466505x53902. [DOI] [PubMed] [Google Scholar]
- 32.Skodol AE, Oldham JM, Hyler SE, Kellman HD, Doidge N, Davies M. Comorbidity of DSM-III-R eating disorders and personality disorders. Int J Eat Disord. 1993 Dec;14(4):403–16. doi: 10.1002/1098-108x(199312)14:4<403::aid-eat2260140403>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Marsa M, Carrasco JL, Saiz J. A study of temperament and personality in anorexia and bulimia nervosa. J Pers Disord. 2000 Winter;14(4):352–9. doi: 10.1521/pedi.2000.14.4.352. [DOI] [PubMed] [Google Scholar]
- 34.Kubzansky LD, Park N, Peterson C, Vokonas P, Sparrow D. Healthy psychological functioning and incident coronary heart disease: The importance of self-regulation. Arch Gen Psychiatry. 2011 Apr;68(4):400–8. doi: 10.1001/archgenpsychiatry.2011.23. [DOI] [PubMed] [Google Scholar]
- 35.Oldershaw A, Dejong H, Hambrook D, Broadbent H, Tchanturia K, Treasure J, et al. Emotional processing following recovery from anorexia nervosa. Eur Eat Disord Rev. 2012 Jan 13; doi: 10.1002/erv.2153. [DOI] [PubMed] [Google Scholar]
- 36.Keys A, Brožek J, Henschel A, Mickelsen O, Taylor HL. The biology of human starvation. 2 vols. Univ. of Minnesota Press; Oxford, England: 1950. [Google Scholar]
- 37.Nilsson EW, Gillberg C, Gillberg IC, Råstam M. Ten-year follow-up of adolescent-onset anorexia nervosa: Personality disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(11):1389–95. doi: 10.1097/00004583-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Gillberg C, Råstam M. Do some cases of anorexia nervosa reflect underlying autistic-like conditions? Behavioural Neurology. 1992;5(1):27–32. doi: 10.3233/BEN-1992-5105. [DOI] [PubMed] [Google Scholar]
- 39.Garner DM, Garfinkel PE. The eating attitudes test: An index of the symptoms of anorexia nervosa. Psychol Med. 1979 May;9(2):273–9. doi: 10.1017/s0033291700030762. [DOI] [PubMed] [Google Scholar]
- 40.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: Psychometric features and clinical correlates. Psychol Med. 1982 Nov;12(4):871–8. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 41.Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int J Eat Disord. 1983;2(2):15–34. [Google Scholar]
- 42.Mazure CM, Halmi KA, Sunday SR, Romano SJ, Einhorn AM. The yale-brown-cornell eating disorder scale: Development, use, reliability and validity. J Psychiatr Res. 1994 Sep-Oct;28(5):425–45. doi: 10.1016/0022-3956(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 43.Sunday SR, Halmi KA, Einhorn A. The yale-brown-cornell eating disorder scale: A new scale to assess eating disorder symptomatology. Int J Eat Disord. 1995 Nov;18(3):237–45. doi: 10.1002/1098-108x(199511)18:3<237::aid-eat2260180305>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960 Feb;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 46.Wechsler D. Wechsler adult intelligence Scale—Fourth edition. Pearson Assessment; San Antonio, TX: 2008. [Google Scholar]
- 47.Wechsler D. Wechsler adult intelligence Scale—Fourth edition: Technical and interpretive manual. Pearson Assessment; San Antonio, TX: 2008. [Google Scholar]
- 48.Conners CK. Conner’s continuous performance test II. Multi-Health Systems Inc.; Canada: 2009. [Google Scholar]
- 49.Golden CJ. Stroop color and word test. Stoelting; Chicago, IL: 1978. [Google Scholar]
- 50.Heaton RK. Wisconsin card sorting test: Computer version 4, research edition (WCST: CV4) 2003.
- 51.Bagby RM, Taylor GJ, Parker JD. The twenty-item toronto alexithymia scale--II. convergent, discriminant, and concurrent validity. J Psychosom Res. 1994 Jan;38(1):33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 52.Bagby RM, Parker JD, Taylor GJ. The twenty-item toronto alexithymia scale--I. item selection and cross-validation of the factor structure. J Psychosom Res. 1994 Jan;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 53.Ben-Porath YS, Tellegen A. MMPI-2RF: Manual for administration,scoring, and interpretation. University of Minnesota Press; Minneapolis, MN: 2008. [Google Scholar]
- 54.Hambrook D, Tchanturia K, Schmidt U, Russell T, Treasure J. Empathy, systemizing, and autistic traits in anorexia nervosa: A pilot study. Br J Clin Psychol. 2008 Sep;47(Pt 3):335–9. doi: 10.1348/014466507X272475. [DOI] [PubMed] [Google Scholar]
- 55.Blair RJ. Fine cuts of empathy and the amygdala: Dissociable deficits in psychopathy and autism. Q J Exp Psychol (Hove) 2008 Jan;61(1):157–70. doi: 10.1080/17470210701508855. [DOI] [PubMed] [Google Scholar]
- 56.Blair RJ. A cognitive developmental approach to mortality: Investigating the psychopath. Cognition. 1995 Oct;57(1):1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- 57.Cardi V, Matteo RD, Corfield F, Treasure J. Social reward and rejection sensitivity in eating disorders: An investigation of attentional bias and early experiences. World J Biol Psychiatry. 2012 Mar 16; doi: 10.3109/15622975.2012.665479. [DOI] [PubMed] [Google Scholar]
- 58.Davies H, Schmidt U, Stahl D, Tchanturia K. Evoked facial emotional expression and emotional experience in people with anorexia nervosa. Int J Eat Disord. 2011 Sep;44(6):531–9. doi: 10.1002/eat.20852. [DOI] [PubMed] [Google Scholar]
- 59.Guttman HA, Laporte L. Empathy in families of women with borderline personality disorder, anorexia nervosa, and a control group. Fam Process. 2000;39(3):345–58. doi: 10.1111/j.1545-5300.2000.39306.x. [DOI] [PubMed] [Google Scholar]
- 60.Guttman HA, Laporte L. Alexithymia, empathy, and psychological symptoms in a family context. Compr Psychiatry. 2002;43(6):448–55. doi: 10.1053/comp.2002.35905. [DOI] [PubMed] [Google Scholar]
- 61.Kang JI, Namkoong K, Yoo SW, Jhung K, Kim SJ. Abnormalities of emotional awareness and perception in patients with obsessive-compulsive disorder. J Affect Disord. 2012 Apr 27; doi: 10.1016/j.jad.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Bruch H. Anorexia nervosa: Therapy and theory. Am J Psychiatry. 1982 Dec;139(12):1531–8. doi: 10.1176/ajp.139.12.1531. [DOI] [PubMed] [Google Scholar]
- 63.Hambrook D, Oldershaw A, Rimes K, Schmidt U, Tchanturia K, Treasure J, et al. Emotional expression, self-silencing, and distress tolerance in anorexia nervosa and chronic fatigue syndrome. Br J Clin Psychol. 2011 Sep;50(3):310–25. doi: 10.1348/014466510X519215. [DOI] [PubMed] [Google Scholar]
- 64.Fairburn CG, Cooper Z, Shafran R. Cognitive behaviour therapy for eating disorders: A “transdiagnostic” theory and treatment. Behav Res Ther. 2003 May;41(5):509–28. doi: 10.1016/s0005-7967(02)00088-8. [DOI] [PubMed] [Google Scholar]
- 65.Polivy J, Herman CP. Causes of eating disorders. Annu Rev Psychol. 2002;53:187–213. doi: 10.1146/annurev.psych.53.100901.135103. [DOI] [PubMed] [Google Scholar]
- 66.Peñas-Lliedó E, Vaz Leal FJ, Waller G. Excessive exercise in anorexia nervosa and bulimia nervosa: Relation to eating characteristics and general psychopathology. Int J Eat Disord. 2002;31(4):370–5. doi: 10.1002/eat.10042. [DOI] [PubMed] [Google Scholar]
- 67.Fox JR, Smithson E, Baillie S, Ferreira N, Mayr I, Power MJ. Emotion coupling and regulation in anorexia nervosa. Clin Psychol Psychother. 2012 Nov 20; doi: 10.1002/cpp.1823. [DOI] [PubMed] [Google Scholar]