Abstract
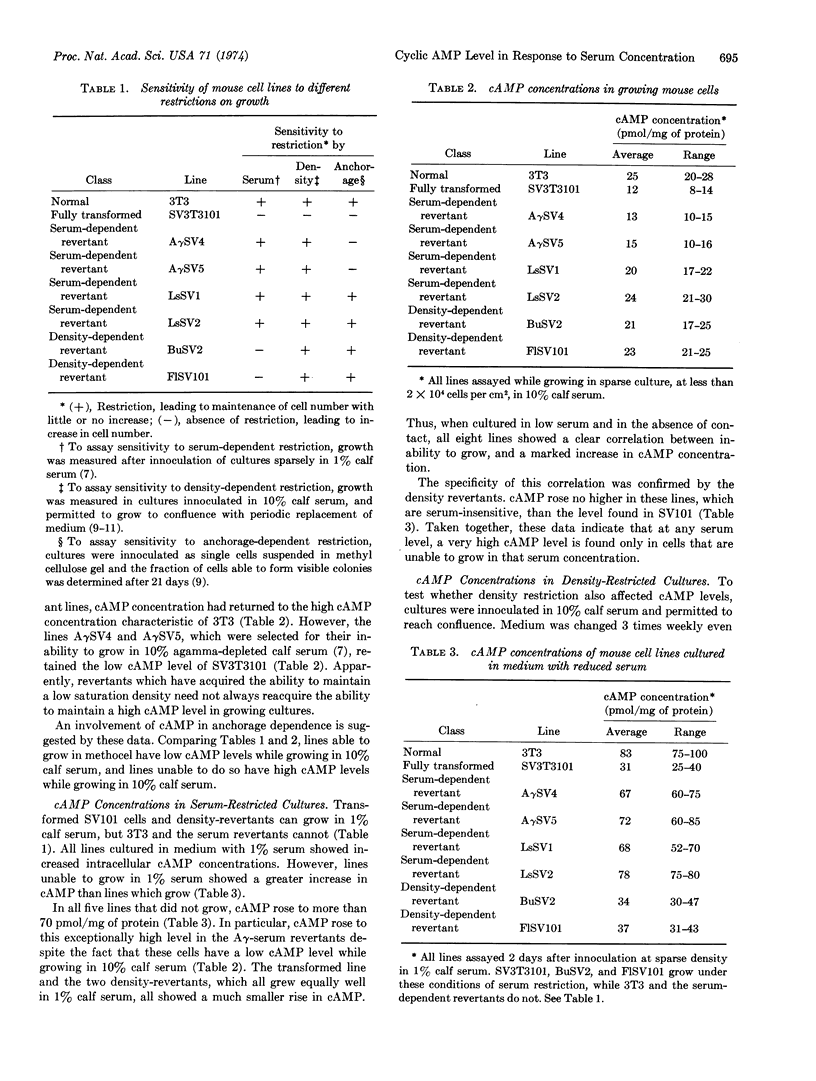
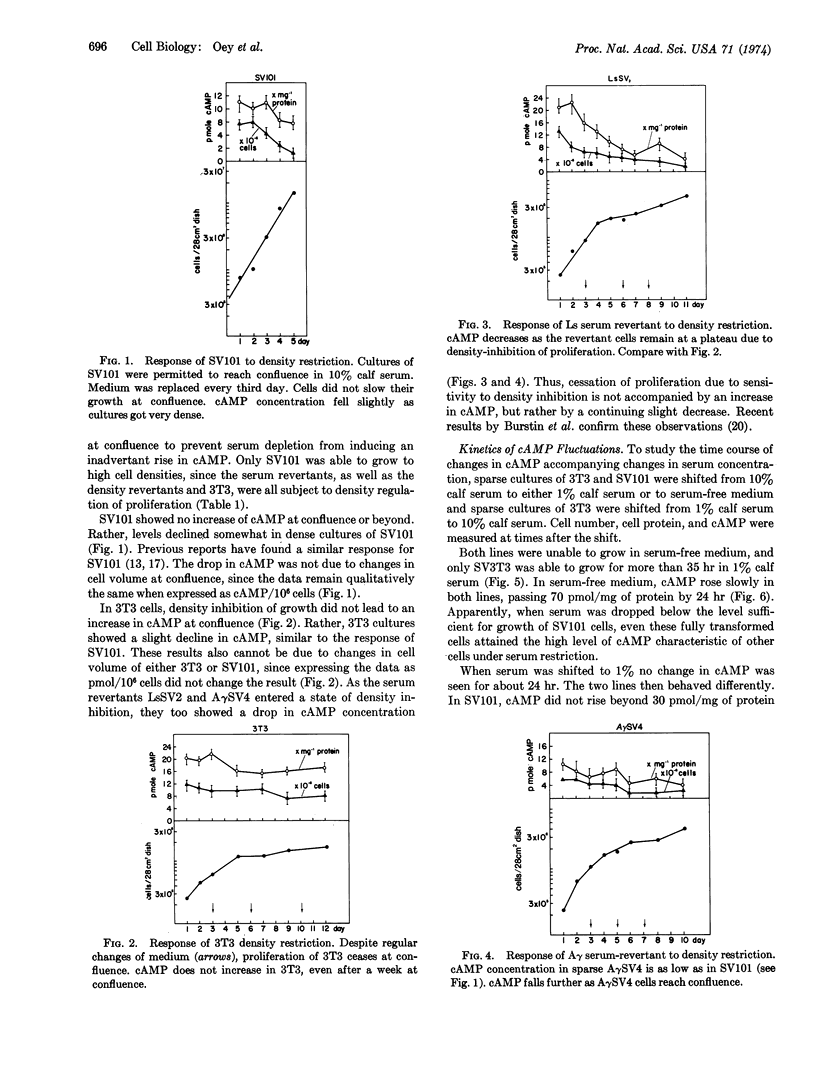
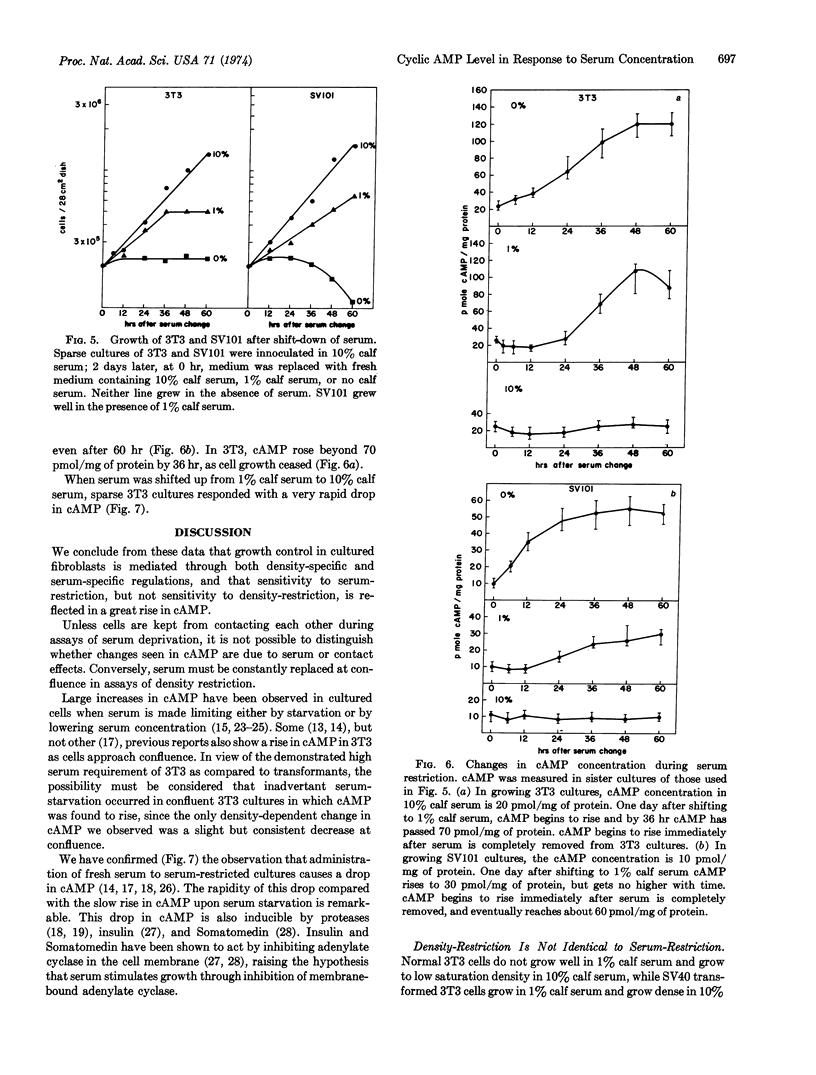
Intracellular concentrations of cyclic AMP increase when cells are deprived of serum. Studies with the mouse fibroblast line 3T3, an SV40-transformed subline of 3T3, and six different revertant lines derived from this clone show that a marked increase in cyclic AMP occurs only when the serum concentration is reduced below the minimum necessary for growth of a given line. Conversely, density-dependent inhibition of growth is not accompanied by an increase in cyclic AMP concentration in any line.
Keywords: transformation, reversion, mouse fibroblasts, serum requirement, density-dependent growth inhibition
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Johnson G. S., Pastan I. Transformation of chick-embryo fibroblasts by wild-type and temperature-sensitive Rous sarcoma virus alters adenylate cyclase activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1055–1059. doi: 10.1073/pnas.70.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombik B. M., Burger M. M. c-AMP and the cell cycle: inhibition of growth stimulation. Exp Cell Res. 1973 Jul;80(1):88–94. doi: 10.1016/0014-4827(73)90278-4. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Froehlich J. E., Rachmeler M. Effect of adenosine 3'-5'-cyclic monophosphate on cell proliferation. J Cell Biol. 1972 Oct;55(1):19–31. doi: 10.1083/jcb.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrick M. L., Ryan W. L. Adenosine 3',5'-cyclic monophosphate and contact inhibition. Cancer Res. 1971 Sep;31(9):1313–1315. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez de Asúa L., Surian E. S., Flawia M. M., Torres H. N. Effect of insulin on the growth pattern and adenylate cyclase activity of BHK fibroblasts. Proc Natl Acad Sci U S A. 1973 May;70(5):1388–1392. doi: 10.1073/pnas.70.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Mamont P., Tomkins G. M. Pleiotypic control by adenosine 3':5'-cyclic monophosphate: a model for growth control in animal cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1432–1436. doi: 10.1073/pnas.70.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert W., Paul D. Levels of cyclic AMP in sparse and dense cultures of growing and quiescent 3T3 cells. Nat New Biol. 1972 Dec 27;240(104):281–283. doi: 10.1038/newbio240281a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Todaro G., Matsuya Y., Bloom S., Robbins A., Green H. Stimulation of RNA synthesis and cell division in resting cells by a factor present in serum. Wistar Inst Symp Monogr. 1967;7:87–101. [PubMed] [Google Scholar]
- Tordaro G. J., Green H. An assay for cellular transformation by SV40. Virology. 1964 May;23(1):117–119. doi: 10.1016/s0042-6822(64)80018-0. [DOI] [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Oct;82(2):189–198. doi: 10.1002/jcp.1040820207. [DOI] [PubMed] [Google Scholar]
- Vogel A., Risser R., Pollack R. Isolation and characterization of revertant cell lines. 3. Isolation of density-revertants of SV40-transformed 3T3 cells using colchicine. J Cell Physiol. 1973 Oct;82(2):181–188. doi: 10.1002/jcp.1040820206. [DOI] [PubMed] [Google Scholar]



