Abstract
Background
Oral naltrexone's effectiveness as an opioid antagonist has been limited due to poor patient adherence. A long-acting naltrexone formulation may be beneficial. This study evaluated the effects of extended-release injectable naltrexone (XR-NTX), targeted for a one-month duration of action, in blocking opioid agonist challenge effects in humans.
Methods
Outpatient non-dependent opioid abusers (N=27) were randomly assigned to a single double-blind IM administration of 75, 150, or 300 mg XR-NTX. To assess the extent of opioid blockade, hydromorphone challenges (0, 3, 4.5, 6 mg IM in ascending order at 1-hr intervals [up to 13.5 mg total]) were given at pretreatment baseline and on days 7, 14, 21, 28, 42, and 56. Opioid blockade was assessed via (1) tolerability of the ascending hydromorphone doses; (2) Visual Analog Scale (VAS) ratings of subjective opioid effects and (3) pupil diameter. Effects on the VAS and pupils were assessed via the slope of the time-action function over ascending hydromorphone doses, with zero slope indicating complete blockade.
Results
Blockade of the VAS “any drug effect” response to 3 mg hydromorphone was complete for 14, 21, and 28 days, respectively, for the XR-NTX doses of 75, 150 and 300 mg. Subjective effects were more readily blocked than was pupil constriction. Higher hydromorphone doses produced only modest increases in agonist effects. With the 300 mg XR-NTX dose the slope of VAS responses remained at or near zero for one month even with maximal cumulative hydromorphone dosing.
Conclusions
These data quantify the month-long opioid blockade underlying XR-NTX's efficacy in opioid dependence treatment.
Keywords: naltrexone, opioid blockade, opioid challenge, extended-release naltrexone, depot naltrexone, injectable naltrexone, Vivitrol, hydromorphone
1. Introduction
Naltrexone is an opioid receptor antagonist that blocks or attenuates the effects of opioids and demonstrates efficacy in trials for eliminating or markedly diminishing opioid self-administration, discrimination, and opioid-induced subjective effects (Gonzales et al., 1988; Mello et al., 1981; Martin et al., 1973; Resnick et al., 1974; Walsh et al., 1996). Oral dosage forms of naltrexone have been approved by the U.S. Food and Drug Administration (FDA) for treatment of opioid dependence since 1984, and alcohol dependence since 1994. In terms of clinical effectiveness, however, the impact of oral naltrexone in opioid dependence treatment has been limited due to low medication adherence rates by patients (Fram et al., 1989; Harris et al., 2004; Mark et al., 2003). The National Institute on Drug Abuse has long encouraged development of an extended-release formulation (Willette, 1976; Willette and Barnett, 1981).
In 2010, the FDA approved an injectable extended-release naltrexone (XR-NTX) administered once-monthly for the prevention of relapse to opioid dependence following opioid detoxification based on the results of a large scale, randomized, double-blind, placebo-controlled, trial (Krupitsky et al., 2011). The efficacy of XR-NTX in the treatment of alcohol dependence had previously been demonstrated (Garbutt et al., 2005; O'Malley et al., 2007). The extended-release technology used in XR-NTX involves the embedding of the drug molecule within a polymeric matrix of microspheres made of poly(d,l-lactide-co-glycolide) (PLG; Shive and Anderson, 1977). PLG is a common biodegradable copolymer that has been used safely in humans for a variety of applications, including sutures, orthopedics, bone plates and other extended-release pharmaceuticals. The biodegradable polymers can be fabricated into small diameter, injectable microspheres (<100 microns) that provide release of drugs for pre-determined durations ranging from days to months. Studies of XR-NTX in animals (Dean, 2005) and humans (Johnson et al., 2004; Dunbar et al., 2006; Turncliff et al., 2005) indicated that the extended-release formulation of naltrexone maintained stable, pharmacologically relevant plasma levels of naltrexone for at least 28 days. Although XR-NTX has shown clinical efficacy in alcohol dependent patients and stable plasma levels for at least 28 days, no studies have examined the ability of XR-NTX to block opioids.
The objective of this study was to evaluate the degree and duration of action of XR-NTX in blocking the effects of an opioid agonist (hydromorphone) challenge in opioid-using adults. This included (1) randomized double blind comparison of three dose levels (75, 150, and 300 mg) of XR-NTX on degree and duration of opioid blockade; (2) dose-effect evaluation of hydromorphone challenge effects to assess surmountability of the XR-NTX opioid blockade; and (3) assessment of time course of plasma naltrexone and 6-beta-naltrexol concentrations from XR-NTX.
Prior reports addressing similar issues (Comer et al., 2002; Comer et al., 2006; Sullivan et al., 2006) have used a different extended-release naltrexone formulation, from a different manufacturer, that has not achieved clinical marketing approval. While those studies have been important proof-of-concept demonstrations they have not characterized the opioid blockade produced by this clinically-available XR-NTX formulation.
2. Methods
2.1. Study Design
This was a two-site randomized, double-blind study to assess the degree and duration of opioid blockade by different doses of extended-release naltrexone (XR-NTX). Outpatient, experienced, opioid-abusing volunteers were randomized to receive a single injection of XR-NTX of either 75 mg (N=9), 150 mg (N=8), or 300 mg (N=10), with repeated assessments, at weekly or bi-weekly intervals over 8 weeks, of response to opioid agonist challenge with hydromorphone to assess the level of mu-opioid blockade.
Challenge sessions consisted of an ascending-dose sequence of hydromorphone injections (0, 3, 4.5, 6 mg, intramuscularly, at 1-hr intervals) to assess agonist dose-effects. Challenge sessions were scheduled at pretreatment and on days 7, 14, 21, 28, 42, and 56; an all-placebo challenge sequence was randomly substituted on one of the post-randomization days for each subject as a control for expectancy effects. Plasma naltrexone and 6-beta-naltrexol levels were obtained at each challenge session, and opioid-sensitive subjective effect measures and physiological measures were assessed repeatedly throughout each challenge session. The Institutional Review Board at each site approved the study.
2.2. Subjects
Participants were non-physically-dependent, opioid-abusing, adult, paid volunteers. Inclusion criteria included: at least a one year recent history of non-medical opioid use, without physical dependence (formal psychiatric diagnosis was not performed); some period of weekly use; opioid use no more than three times per week on average for the 30 days prior to screening; general good health (no clinically significant medical condition or laboratory abnormality, as assessed by physical examination, electrocardiogram, and blood tests); and no participation in another clinical trial within the past 30 days. Exclusionary factors included: opioid physical dependence or seeking treatment for opioid abuse; mood disorder or symptoms of psychosis; pregnant or nursing. Women of childbearing potential were required to use effective birth control. Qualified subjects needed to have a positive, typical opioid-like response to hydromorphone during the baseline challenge session to qualify for randomization; all did. Each subject gave informed written consent prior to participation.
2.3. Study Procedures
A Baseline (BL) hydromorphone challenge session was conducted within 21 days before XR-NTX injection on Day 0. At least 5 days after that BL hydromorphone challenge, a naloxone challenge (0.8 mg i.m.) was performed, followed at least 30 min later by an oral naltrexone (50 mg) tolerability assessment to ensure that subjects had no opioid drugs in their system and safely tolerated naltrexone prior to XR-NTX administration; this preceded the Day 0 administration of the randomized XR-NTX depot dose by at least one day. All subjects passed this naloxone/naltrexone tolerability testing. At Day 0, each subject received a single injection of XR-NTX, in a double-blind, randomized procedure. The duration of action of the single naltrexone dose was expected to be about four weeks; therefore, subjects were followed for 8 weeks after their XR-NTX injection to ensure characterization of the expected time course. Injections were intramuscular to the gluteus maximus, via a 20 gauge needle, in a volume that varied by dose (0.8, 1.6, 3.3 cc), by a nurse who had no further contact with the subject.
Hydromorphone challenge sessions, with 0 mg (placebo), 3 mg, 4.5 mg, and 6 mg of intramuscular injected hydromorphone (10 mg/ml) administered in ascending order at 1 hr intervals during a 4-hour session, were held on the pretreatment BL day and on days 7, 14, 21, 28, 42, and 56 after XR-NTX injection. Subjects were blinded to the doses and the ascending sequencing of the challenge doses. The degree of opioid dose tolerability was determined in the following manner: medical monitors could discontinue challenge dosing within any session if, based on the subject's vital signs, behavior, and physical demeanor, there appeared to be substantial clinical opioid agonist effects such that the monitor was reluctant to administer additional opioid. For blinding purposes and as a control for expectancy effects, for each subject one randomly selected challenge session consisted (double blind) of all placebo injections instead of ascending hydromorphone doses. If a subject was observed to be intoxicated prior to a challenge session the session was postponed to another day. Urine samples were collected prior to each session; positive toxicology results (indicating drug use in recent days) were infrequent and were not alone sufficient to postpone a session.
2.4. Subjective Measures
Six subject-rated computer-presented visual analog scale (VAS) questions were used to measure the extent and duration of opioid blockade following hydromorphone challenge (“Do you feel any drug effect?”, “How high are you right now?”, “Do you like the drug?”, “Does the drug have any good effects?”, “Does the drug have any bad effects?” and “Does the drug make you sick?”). The first question (“Do you feel any drug effect?”) was designated a priori as a primary outcome measure. These VAS questions were selected based on previous demonstration of their sensitivity to opioid agonist and antagonist effects (Preston et al., 1988). For each VAS question, subjects positioned the cursor along a horizontal line marked at either end with “not at all” and “extremely” and responses were scored on a 0-100 point scale. These VAS questions were administered 15 minutes before the first challenge injection and every 15 minutes for 1 hour after each challenge injection.
2.5. Physiological Measures
Pupil diameter, blood pressure (by electronic monitor), respiration rate, heart rate, oxygen saturation, and skin temperature were measured 15 minutes before the first challenge dose and at 15, 30, 45, and 60 minutes after each challenge injection throughout each session. Pupil diameter was designated a priori as a primary outcome measure. Subjects' pupils were photographed in constant ambient illumination and measured from the photographs; each recorded diameter was the average of the horizontal and vertical diameters (in millimeters).
2.6. Pharmacokinetic Assessments
Blood samples were taken 15 minutes prior to each challenge session to measure plasma concentrations of naltrexone and its active metabolite, 6-beta-naltrexol. Standard venipuncture techniques were used to collect blood in two 6 mL polypropylene EDTA blood tubes, and centrifuged at 2 to 8°C, 2000 × g, for 4 minutes. At least 4 mL plasma was transferred into 2 separate tubes and frozen at −20°C. One set of samples remained at the site; the second set was sent to Cedra Corporation (Austin, TX) on dry ice for analysis. Naltrexone and 6-beta-naltrexol concentrations were determined using a validated high performance liquid chromatography method with tandem mass spectrometry detection. The assay was linear over the range 0.200 to 100 ng/mL for naltrexone and 0.500 to 250 ng/mL for 6-beta-naltrexol.
2.7. Data Analysis
The VAS question “Do you feel any drug effect?” and pupil diameter were the two pre-specified primary outcome measures, and these were the focus of data analysis. The other VAS items received the same analysis and are presented as secondary outcome indices.
The pre-specified data analytic plan called for analysis and comparison of the slopes of the time-action (i.e., dose-effect) functions within sessions. Slope analysis was selected because it provides a value for each session representing the degree of drug effect that accommodates the fact that the number of active injections (total dose) per session could vary. For each variable, for each challenge session, a linear regression line over time was calculated for the repeated measures in that session; because of the ascending-dose hydromorphone sequence, the slope of this line over time also represents the slope as a function of hydromorphone dose. Thus, the slopes of these time-action functions represent the degree of opioid agonist effects or opioid blockade within each session, and each slope functions as a surrogate for the hydromorphone dose-effect function. The steeper the slope, the greater the opioid agonist effect from hydromorphone. Opioid agonist effects appear as smaller pupil diameters (negative slope) and as higher VAS drug effect ratings (positive slope). In contrast, a zero or near-zero slope indicates absence of an opioid agonist effect and complete opioid blockade.
Slopes were calculated in two ways: (1) when assessing the presence and duration of opioid blockade, slopes were limited to the regression lines over only the 0 mg and 3 mg hydromorphone doses, and (2) when assessing the degree of surmountability, slopes were for the regression lines over the full sequence of hydromorphone doses administered. In both cases slopes were based on data from all relevant assessment time-points (i.e., 15 min intervals). Slope calculations extended only through the hour following the last active hydromorphone dose given; this provided a useable slope even for sessions when, due to medical monitor concern, hydromorphone dose escalation was stopped.
Because of slower-than-anticipated enrollment and sponsor's corporate time considerations the study was administratively ended before achieving its planned sample size. From a statistical perspective it is a pilot study. Analyses focused on characterizing the extent and duration of opioid agonist effects and opioid blockade by testing the statistical significance of the slopes of the time-action (dose-effect) regression lines. Because of the small N's per group, statistical significance testing used a non-parametric test, the Exact Wilcoxon Sign Rank Test, which tested, for each outcome variable, whether the median slope of each dose group at each time point was significantly different from zero. The small N's and non-parametric methods prevent providing meaningful confidence intervals for the individual slope values. Significant slopes (p<0.05) represent a significant opioid agonist effect. Median slopes of zero or near-zero are interpreted as showing complete or near-complete opioid blockade. Absence of even a trend toward significance of the slope (p>0.20) is interpreted as showing significant opioid blockade. In addition, median peak VAS scores and median peak pupil constriction after hydromorphone challenge are descriptively presented, as are data on respiratory depression, and illicit opioid use.
3. Results
3.1. Subject Characteristics, Retention and Adherence
The sample was predominately male and non-white (Table 1). Subjects had used opioids non-medically for an average of 11.6 years. Their qualifying opioid use histories were primarily with heroin. Alcohol use and cocaine use were also prevalent. Routes of administration were not recorded. There were no significant differences between the treatment groups on baseline characteristics, with the exception of age (the 150 mg group was older than the other two groups; p=0.026).
Table 1. Demographic and Baseline Clinical Characteristics of Participants.
| Characteristic | XR-NTX Group | ||
|---|---|---|---|
|
| |||
| 75 mg N = 9 | 150 mg N = 8 | 300 mg N = 10 | |
| Age, Mean (SD) | 35.2 (9.3) | 42.9 (4.7) | 32.5 (8.0) |
| Sex, % male | 89% | 100% | 80% |
| Race, % white | 33% | 12%) | 50% |
| Years of opioid use Mean (SD) | 11.1 (5.2) | 14.3 (10.0) | 10.0 (6.9) |
| Substance use in past 30 days, (% of subjects) | |||
| Alcohol | 100% | 100% | 100% |
| Heroin | 100% | 100% | 80% |
| Methadone | 11% | 12% | 0% |
| Other Opioids | 22% | 12% | 20% |
| Cocaine | 100% | 88% | 80% |
| Marijuana | 67% | 38% | 40% |
| Amphetamines | 11% | 0% | 0% |
Only age differed significantly between groups.
Of the 27 randomized subjects, 21 completed the study: 8 of 9 in the 75 mg group, 6 of 8 in the 150 mg group, and 7 of 10 in the 300 mg group. Non-completions were for personal or scheduling reasons unrelated to the study, except for one subject who discontinued due to leg pain judged possibly related to the XR-NTX injection procedure (not to the drug itself). During the sponsor's close-out visits to the study sites, it was discovered that 2 subjects had enrolled at both sites (sequentially, not concurrently). One completed the study twice, first in the 300 mg condition and then later in the 75 mg condition; the second, who completed the study in the 75 mg condition, was later randomized again to that same condition and received the XR-NTX injection, but then dropped out due to incarceration, so the second enrollment contributed only the pre-treatment Baseline session data. Due to the study's small samples, and the fact that bias risk was mitigated by no individual's contributing multiply to the outcomes of a single dose condition, it was judged better to include all available data, and these enrollments were treated as independent cases in the analyses.
There was infrequent evidence of illicit opioid use during study participation. Urine samples collected at study days 7, 14, 21, 28 (the days of intended opioid blockade) were opioid positive on 19%, 17%, and 0% of occasions for the 75, 150, and 300 mg XR-NTX dose groups, respectively, with at least one opioid-positive urine provided by 50%, 12%, and 0% of subjects, respectively. No samples were methadone-positive; buprenorphine was not tested.
Session cancellation or rescheduling due to intoxication were rare. Schedule variation due to subject availability was more common. Study results are reported according to their nominal time point (e.g., Days 7, 14, 28, etc.), but actual session times could vary slightly. Of 131 total session 56% were on the nominal day and 95% were within two days of the nominal day number. The means (and standard deviations) for the actual session times were 6.8 (1.0), 14.0 (1.3), 21.0 (1.2), 28.3 (2.4), 42.0 (1.1), and 55.8 (1.1) days.
3.2. Hydromorphone Dose Escalation Tolerability
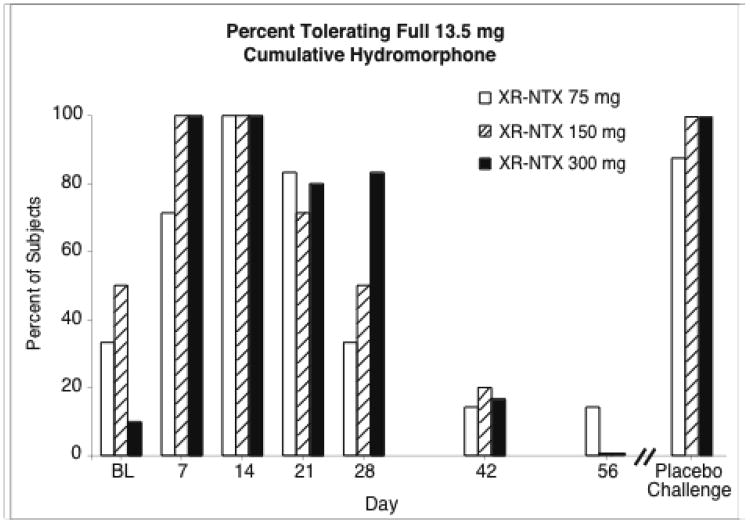
One index of the extent of opioid blockade by XR-NTX is whether subjects received the full sequence of ascending hydromorphone challenge doses without its being interrupted by the on-site medical monitor. Figure 1 shows, for each XR-NTX dose, the percent of subjects who received the full 4-injection sequence, totaling 13.5 mg hydromorphone, at each study time point. In the 22 randomly selected double blind sessions, when all placebo injections were substituted for hydromorphone, the full 4-injection sequence was tolerated without interruption on 95% of sessions. At the BL sessions, prior to XR-NTX administration, only 30% of subjects received the full injection sequence. After XR-NTX administration, this percentage rose to higher levels through day 28 (56-100% for all XR-NTX groups combined), and then declined to low levels (6-17%) at days 42-56. All three XR-NTX doses had time points with percent above 80%; for the 300mg dose the percent was 80% or above at every time point from day 7 through day 28. The data show a clear XR-NTX effect in increasing tolerability of escalating opioid agonist doses, and are suggestive of a dose-effect relationship, but not a pronounced one. At no time-point, including BL, was the difference between XR-NTX dose conditions statistically significant on this measure. Table 2 shows the mean total cumulative hydromorphome dose administered on each test day for each XR-NTX group.
Figure 1.
The percent of subjects tolerating and receiving the maximum available 13.5 mg cumulative hydromorphone in the within-session, 4-injection ascending dose sequence is shown for each XR-NTX dose group for each time point. The Placebo Challenge data show the percent of subjects receiving the full 4-injection sequence when all injections were placebo instead of hydromorphone. Days are number of days after XR-NTX administration. BL is the baseline session preceding XR-NTX administration.
Table 2. Mean Total Hydromorphone Mg Given on Each Test Day.
| XR-NTX | Day | ||||||
|---|---|---|---|---|---|---|---|
| Dose | BL | 7 | 14 | 21 | 28 | 42 | 56 |
| 75 mg | 9.0 | 11.1 | 13.5 | 12.5 | 9.5 | 7.7 | 6.4 |
| 150 mg | 9.9 | 13.5 | 13.5 | 11.8 | 10.5 | 8.7 | 6.6 |
| 300 mg | 6.8 | 13.5 | 13.5 | 12.3 | 12.5 | 7.8 | 4.8 |
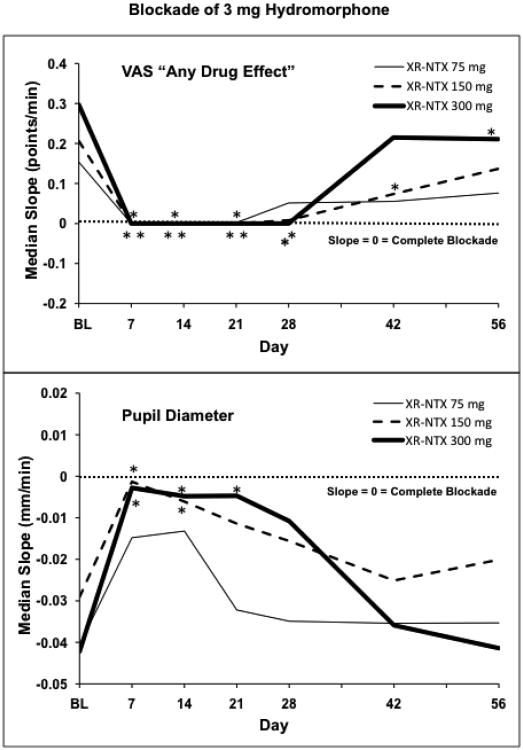
3.3. Blockade of Hydromorphone 3mg Effects
Table 3 and Figure 2 show data for XR-NTX blockade of 3mg hydromorphone. This is the highest hydromorphone dose uniformly present in every challenge session, and so provides a constant metric for evaluating opioid effects and opioid blockade. Figure 2 shows the median slope of the 3mg hydromorphone time-action (dose) functions for each XR-NTX dose at each assessment time point for each of the two pre-specified primary outcome variables, VAS Drug Effect, and pupil diameter. Table 3 shows the median slopes for these two primary measures and also for each of three other measures reflective of opioid agonist effects, VAS High, VAS Good Effects, and VAS Liking – and, in addition, shows the results of the statistical analysis of each slope. Major features in the data are:
Sensitivity to opioid agonist effects: Statistically significant opioid agonist effects in the expected directions are present at the pre-XR-NTX BL assessment for every measure for every XR-NTX dose group.
Opioid blockade by XR-NTX: Throughout the month following XR-NTX administration there is a high prevalence of zero or near-zero slopes, indicating complete or near-complete blockade of hydromorphone's opioid agonist effects.
Duration of blockade: The slopes of the opioid agonist measures were substantially reduced (zero or near zero) for about one month, especially the VAS measures, and returned toward BL values at the later time points (days 42 and 56).
Table 3. Blockade of 3 mg hydromorphone: Entries are slopes of the time-action regression lines.
| XR-NTX Group | ||||
|---|---|---|---|---|
|
|
||||
| 75 mg | 150 mg | 300 mg | ||
|
| ||||
| Measure | Day | Median Slope (N) | Median Slope (N) | Median Slope (N) |
| VAS: Do you feel any drug effect? | ||||
| BL | +0.1535 (9) ↑ | +0.2064 (8) ↑ | +0.2968 (10) ↑ | |
| 7 | 0.0000 (7) * | 0.0000 (6) * | 0.0000 (7) * | |
| 14 | 0.0000 (7) * | 0.0000 (7) * | 0.0000 (6) * | |
| 21 | +0.0018 (6) * | 0.0000 (7) * | 0.0000 (5) * | |
| 28 | +0.0513 (6) | +0.0079 (4) * | 0.0000 (6) * | |
| 42 | +0.0554 (7) | +0.0737 (5) * | +0.2149 (6) | |
| 56 | +0.0761 (7) ↑ | +0.1368 (5) | +0.2108 (5) * | |
| VAS: How high are you right now? | ||||
| BL | +0.1589(9) ↑ | +0.2368 (8) ↑ | +0.3261 (10) ↑ | |
| 7 | 0.0000 (7) * | 0.0000 (6) * | 0.0000 (7) * | |
| 14 | 0.0000 (7) * | 0.0000 (7) * | 0.0000 (6) * | |
| 21 | 0.0000 (6) * | 0.0000 (7) * | 0.0000 (5) * | |
| 28 | +0.0561 (6) | +0.0061 (4) * | 0.0000 (6) * | |
| 42 | +0.0653 (7) | +0.1230 (5) * | +0.2162 (6) | |
| 56 | +0.0754 (7) ↑ | +0.1429 (5) | +0.1923 (5) | |
| VAS: Do you like the drug? | ||||
| BL | +0.1456(9) ↑ | +0.3491 (8) ↑ | +0.1768(10) ↑ | |
| 7 | 0.0000 (7) * | 0.0000 (6) * | 0.0000 (7) * | |
| 14 | 0.0000 (7) * | 0.0000 (7) * | 0.0000 (6) * | |
| 21 | +0.0018 (6) * | 0.0000 (7) * | 0.0000 (5) * | |
| 28 | +0.0533 (6) | +0.0101 (4) * | 0.0000 (6) * | |
| 42 | +0.0631 (7) | +0.1000 (5) * | +0.1654 (6) | |
| 56 | +0.0626 (7) ↑ | +0.0812 (5) | +0.2052 (5) | |
| VAS: Does the drug have any good effects? | ||||
| BL | +0.1615 (9) ↑ | +0.3361 (8) ↑ | +0.2229 (10) ↑ | |
| 7 | 0.0000 (7) * | 0.0000 (6) * | 0.0000 (7) * | |
| 14 | 0.0000 (7) * | 0.0000 (7) * | 0.0000 (6) * | |
| 21 | +0.0012 (6) * | 0.0000 (7) * | 0.0000 (5) * | |
| 28 | +0.0507 (6) | +0.0097 (4) * | 0.0000 (6) * | |
| 42 | +0.0698 (7) | +0.0924 (5) * | +0.1719 (6) | |
| 56 | +0.0638 (7) ↑ | +0.0510 (5) | +0.1889 (5) | |
| Pupil Diameter | ||||
| BL | -0.0406 (9) ↓ | -0.0291 (8) ↓ | -0.0422 (10) ↓ | |
| 7 | -0.0148 (7) ↓ | -0.0013 (6) * | -0.0028 (7) * | |
| 14 | -0.0132 (7) ↓ | -0.0061 (7) * | -0.0048 (6) * | |
| 21 | -0.0322 (6) ↓ | -0.0114(7) ↓ | -0.0047 (5) * | |
| 28 | -0.0349 (6) ↓ | -0.0156 (4) | -0.0108 (6) | |
| 42 | -0.0354 (7) ↓ | -0.0251 (5) | -0.0359 (6) ↓ | |
| 56 | -0.0353 (7) ↓ | -0.0201 (5) | -0.0414 (5) | |
- Arrows indicate a statistically significant opioid agonist effect from hydromorphone as reflected by a statistically significant (p<0.05) slope over time (and ascending dose) within session. Direction of the arrow indicates direction – upward or downward – of that significant slope.
- Asterisks indicate absence of opioid agonist effect from hydromorphone – i.e., not even a trend (p>0.20) for the slope to differ from zero.
- Slopes are expressed as VAS score points per minute or pupil diameter mm per minute.
- Pupil Diameter slopes are in bold if they are attenuated from the BL slope by ≥0.01 mm per minute.
- VAS slopes are in bold if they are attenuated from the BL slope by ≥0.1 points per minute.
Figure 2.
The median slope of the hydromorphone time-action function is shown for each XR-NTX dose group at each challenge session. Slopes are based on the time course of response to 3 mg hydromorphone. Top panel shows response to the VAS questions “Do you feel any drug effect?” Bottom panel shows pupil diameter response. Days are number of days after XR-NTX administration. BL is the baseline session preceding XR-NTX administration. Asterisks indicate points for which there is not even a trend (p>0.20) for the slope of the hydromorphone time-action function to differ from zero.
VAS subjective effect measures were more completely blocked than the pupil diameter measure, though the pupil measure also shows substantial blockade by XR-NTX. Blockade of the VAS subjective “any drug effect” measure was complete (i.e., zero slope) for 28 days with 300 mg XR-NTX, for 21 days with 150 mg, and for 14 days with 75mg. Similar dose x duration of blockade relationships are seen with each of the other VAS opioid agonist effect measures. The data show a clear and sustained blockade of opioid agonist effects by XR-NTX and suggest a dose-effect relationship, with more complete and/or longer duration opioid blockade with higher XR-NTX doses.
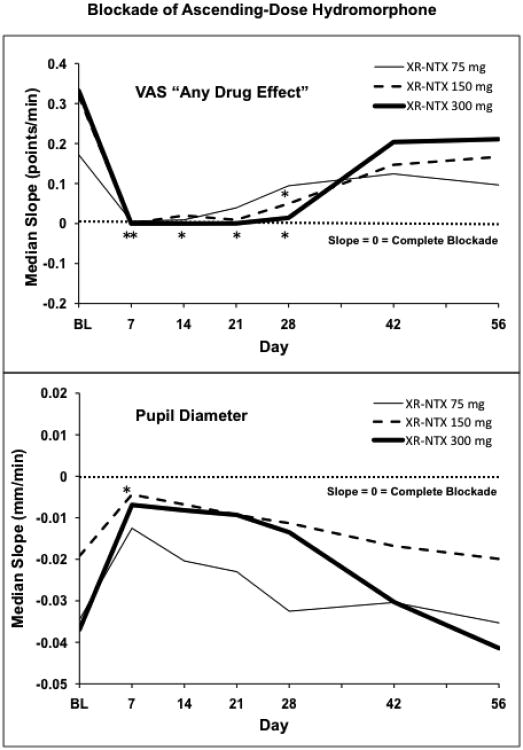
3.4. Blockade of Escalating-Dose Hydromorphone Effects
Table 4 and Figure 3 show data analogous to that above, but with the slopes based on the full cumulative hydromorphone dose sequence actually administered in each session, i.e., with the total hydromorphone dose varying somewhat across subjects and across time points, but often totaling 13.5 mg per session during the month after XR-NTX administration. This provides an assessment of how readily opioid blockade by XR-NTX can be surmounted by higher opioid agonist doses. In general, despite this administration of substantially higher hydromorphone doses, the relationships in the data are similar to those described for 3 mg hydromorphone challenges:
Substantial and often complete blockade of opioid agonist effects (i.e., zero slopes), especially on the VAS subjective effect measures, but also evident on the pupil diameter measure;
Blockade duration of approximately one month; and
An apparent dose-effect relationship, with more complete and/or longer duration opioid blockade with higher XR-NTX doses.
Table 4. Blockade of ascending-dose hydromorphone: Entries are slopes of the time-action regression lines.
| XR-NTX Group | ||||
|---|---|---|---|---|
|
|
||||
| 75 mg | 150 mg | 300 mg | ||
|
| ||||
| Measure | Day | Median Slope (N) | Median Slope (N) | Median Slope (N) |
| VAS: Do you feel any drug effect? | ||||
| BL | +0.1718(9) ↑ | +0.3182(8) ↑ | +0.3317(10) ↑ | |
| 7 | +0.0058 (7) | 0.0000 (6) * | 0.0000(7) * | |
| 14 | +0.0089 (7) ↑ | +0.0199 (7) ↑ | 0.0000 (6) * | |
| 21 | +0.0392 (6) | +0.0092 (7) | +0.0003 (5) * | |
| 28 | +0.0944 (6) ↑ | +0.0498 (4) * | +0.0143 (6) * | |
| 42 | +0.1241 (7) ↑ | +0.1468 (5) | +0.2038 (6) | |
| 56 | +0.0965 (7) ↑ | +0.1667 (5) | +0.2108 (5) | |
| VAS: How high are you right now? | ||||
| BL | +0.1659(9) ↑ | +0.3333 (8) ↑ | +0.3418(10) ↑ | |
| 7 | +0.0020 (7) | 0.0000 (6) * | 0.0000 (7) * | |
| 14 | +0.0174 (7) ↑ | +0.0232 (7) | 0.0000 (6) * | |
| 21 | +0.0398 (6) | +0.0137 (7) | 0.0000 (5) * | |
| 28 | +0.0769 (6) ↑ | +0.0543 (4) * | 0.0000 (6) * | |
| 42 | +0.1989(7) ↑ | +0.2043 (5) | +0.2173 (6) | |
| 56 | +0.1322 (7) ↑ | +0.2042 (5) | +0.1923 (5) | |
| VAS: Do you like the drug? | ||||
| BL | +0.1069(9) ↑ | +0.3084 (8) ↑ | +0.2572 (10) ↑ | |
| 7 | +0.0005 (7) | 0.0000 (6) * | 0.0000 (7) * | |
| 14 | +0.0049 (7) | +0.0296 (7) | 0.0000 (6) * | |
| 21 | +0.0408 (6) | +0.0144 (7) | 0.0000 (5) * | |
| 28 | +0.0742 (6) ↑ | +0.0379 (4) * | +0.0140 (6) * | |
| 42 | +0.0816 (7) ↑ | +0.1649 (5) | +0.1371 (6) | |
| 56 | +0.0560 (7) ↑ | +0.1056 (5) | +0.2052 (5) | |
| VAS: Does the drug have any good effects? | ||||
| BL | +0.1108 (9) ↑ | +0.3060 (8) ↑ | +0.2778 (10) ↑ | |
| 7 | 0.0000 (7) | 0.0000 (6) * | 0.0000 (7) * | |
| 14 | +0.0058 (7) ↑ | +0.0319 (7) | 0.0000 (6) * | |
| 21 | +0.0417 (6) | +0.0146 (7) | 0.0000 (5) * | |
| 28 | +0.0621 (6) ↑ | +0.0378 (4) * | +0.0113 (6) * | |
| 42 | +0.0738 (7) ↑ | +0.1404 (5) | +0.1353 (6) | |
| 56 | +0.0701 (7) ↑ | +0.1092 (5) | +0.1889 (5) | |
| Pupil Diameter | ||||
| BL | -0.0345 (9) ↓ | -0.0192 (8) ↓ | -0.0369 (10) ↓ | |
| 7 | -0.0125 (7) ↓ | -0.0044 (6) * | -0.0069 (7) ↓ | |
| 14 | -0.0204 (7) ↓ | -0.0068 (7) ↓ | -0.0082 (6) ↓ | |
| 21 | -0.0230 (6) ↓ | -0.0093 (7) ↓ | -0.0093 (5) | |
| 28 | -0.0325 (6) ↓ | -0.0113 (4) | -0.0135 (6) ↓ | |
| 42 | -0.0304 (7) ↓ | -0.0168 (5) | -0.0303 (6) ↓ | |
| 56 | -0.0353 (7) ↓ | -0.0199 (5) | -0.0414 (5) | |
- Arrows indicate a statistically significant opioid agonist effect from hydromorphone as reflected by a statistically significant (p<0.05) slope over time (and ascending dose) within session. Direction of the arrow indicates direction – upward or downward – of that significant slope.
- Asterisks indicate absence of opioid agonist effect from hydromorphone – i.e., not even a trend (p>0.20) for the slope to differ from zero.
- Slopes are expressed as VAS score points per minute or pupil diameter mm per minute.
- Pupil Diameter slopes are in bold if they are attenuated from the BL slope by ≥0.01 mm per minute.
- VAS slopes are in bold if they are attenuated from the BL slope by ≥0.1 points per minute.
Figure 3.
The median slope of the hydromorphone time-action function is shown for each XR-NTX dose group at each challenge session. Slopes are based on the time course of response to the full cumulative hydromorphone dose actually received by each volunteer (maximum possible = 13.5 mg). Top panel shows response to the VAS questions “Do you feel any drug effect?” Bottom panel shows pupil diameter response. Days are number of days after XR-NTX administration. BL is the baseline session preceding XR-NTX administration. Asterisks indicate points for which there is not even a trend (p>0.20) for the slope of the hydromorphone time-action function to differ from zero.
As in the 3 mg analysis, there were statistically significant opioid agonist effects for every measure and every XR-NTX dose group at BL assessment, a high-prevalence of zero or near-zero slopes during the month following XR-NTX administration, and return of slopes toward BL values at the later time points (days 42 and 56). In comparison to the 3 mg analysis, there are fewer zero slopes with the escalating-dose hydromorphone challenges, and there are more statistically significant opioid agonist effects, especially at the lowest XR-NTX dose of 75 mg. As before, VAS subjective effect measures were more completely blocked than the pupil diameter measure. The VAS “any drug effect” measure was completely blocked (i.e., zero slope) for 14 days, and was substantially blocked (i.e., not even a trend toward a significant slope) for 28 days after 300 mg XR-NTX. Extent and duration of blockade were somewhat less with the lower XR-NTX doses.
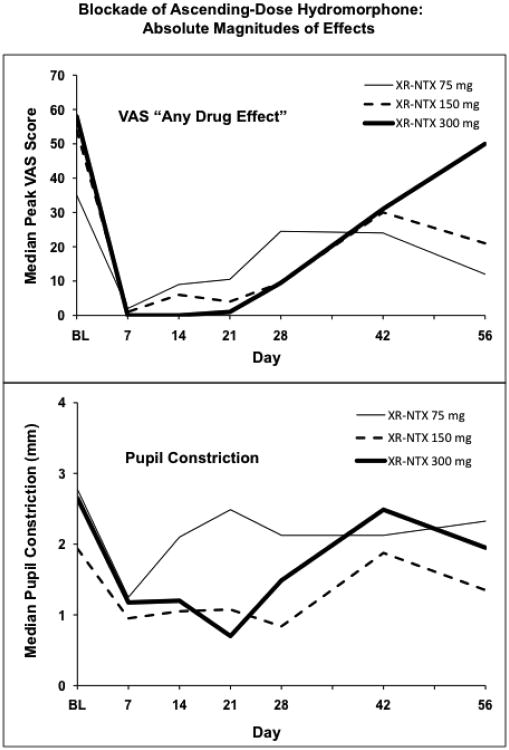
3.5. Absolute Magnitudes of Effects
Two examples illustrate the absolute magnitudes of effects represented by the above slope data and analyses. Peak scores on the VAS “any drug effect” measure at BL, 28 days, and 56 days were summarized for the subset of subjects with data available at all 3 timepoints; the two higher XR-NTX doses (150 and 300 mg), combined, provided an N of 7 such subjects. The summary used peak scores from the full cumulative dose given, which was usually higher on day 28 than on the other days. Mean peak scores were 54.3, 10.4, and 39 for BL, day 28, and day 56, respectively. Six of these 7 subjects also had data from all-placebo challenge sessions, and all had peak scores of 0 in those sessions. In addition, median peak VAS scores and median peak pupil constriction for all available subjects are presented in Figure 4 (sample sizes vary over time from 9 to 7 for the 75 mg group, 8 to 5 for the 150 mg group, and 10 to 5 for the 300 mg group). These data show reduced subjective response and reduced hydromorphone-produced pupil constriction as a function of XR-NTX administration.
Figure 4.
Top panel shows the median peak score for the VAS question “Do you feel any drug effect?” for each dose group for each challenge session. Bottom panel shows median peak pupil constriction for each session (median change from each subject's within-session pre-hydromorphone pupil diameter). BL is the baseline session preceding XR-NTX administration.
3.6. Effects on Respiratory Depression
The opioid effects and opioid blockade effects seen with subjective effects measures and pupil constriction were also seen on physiological parameters related to opioid safety. Respiratory rate and blood oxygen saturation were both significantly depressed during the Baseline (unblocked) hydromorphone challenge sessions. Respiratory rate was reduced by a mean of 4.8 breaths per minute (95% CI: 3.7 to 5.9); oxygen saturation was reduced by a mean of 3.3% (95% CI: 2.1 to 4.6%). Mean respiratory depression (for both these measures) fell well outside these 95% confidence intervals during sessions in which placebo was substituted for hydromorphone (mean respiratory rate change -1.3 bpm; mean oxygen saturation change -0.6%), and for XR-NTX blocked sessions (mean respiratory rate change -1.6 bpm; mean oxygen saturation change -0.5%). These means XR-NTX-blocked sessions are based on the earliest post XR-NTX active hydromorphone challenge session (typically Day 7) for each subject across all XR-NTX doses combined, and are quite similar to the mean changes in placebo challenge sessions.
3.7. Naltrexone Plasma Concentrations
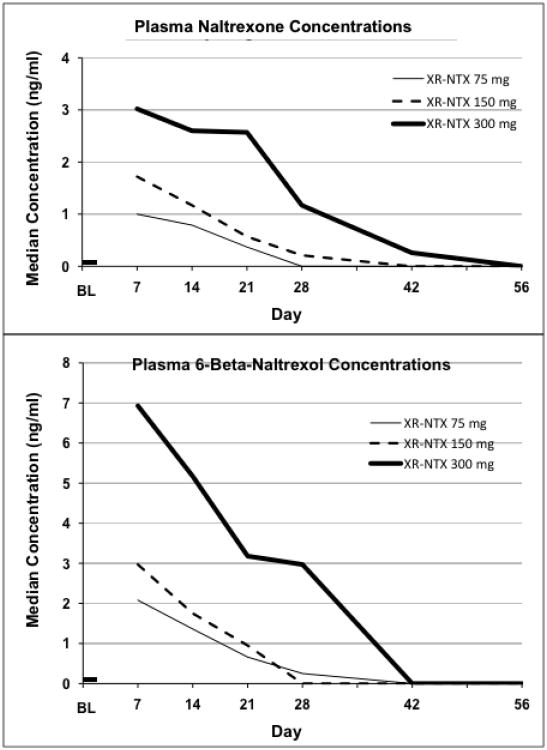
Median plasma concentrations of naltrexone and 6-beta-naltrexol are shown in Figure 5 for each XR-NTX dose at each time point. Concentrations were dose-related and declined over time, reaching levels below the limits of quantification by day 56. Concentrations of naltrexone's major metabolite 6-beta-naltrexol were initially about twice those of naltrexone itself, but fell below detectable levels sooner. Hydromorphone's pharmacodynamic effects were moderately correlated with these plasma concentrations; the critical plasma concentration for achieving opioid blockade was approximately 1 ng/mL for naltrexone and approximately 3 ng/mL for 6-beta-naltrexol.
Figure 5.
Top panel shows the median naltrexone plasma concentrations over time for each XR-NTX dose group. Bottom panel shows the median 6-beta-naltrexol concentrations over time.
3.8. Adverse Events and Injection Site Reactions
One subject in each dose group reported adverse events of mild severity that were considered possibly related to the study drug. These were: increased heart rate, increased sweating, and tremors in the arms (75 mg), myalgia (150 mg), and dizziness and nausea (300 mg). The subject who reported myalgia in the right lower leg (after receiving the study injection in the right buttock) received extensive multi-specialty evaluation without determination of pathology or etiology; the event was rated as possibly related to study procedures and the subject was discontinued from the study.
Twelve of 27 subjects (44%) experienced at least one injection site reaction (4 in the 75 mg group; 5 in the 150 mg group; 3 in the 300 mg group). These included tenderness and induration and there was one instance of slight bruising at the injection site. None of the injection site reactions were considered clinically significant.
4. Discussion
These data document substantial and long-lasting opioid blockade by XR-NTX. The extent and duration of opioid blockade were directly related to the dose of XR-NTX. Following a single intramuscular administration of XR-NTX of 75, 150 or 300 mg, substantial opioid blockade was produced that persisted for durations of 14, 21 and 28 days, respectively. This long duration of sustained opioid blockade suggests that this XR-NTX formulation may offer substantial effectiveness in the treatment of opioid dependence, where adherence and retention with the oral formulations of naltrexone have been a substantial problem.
Naltrexone, the active ingredient in XR-NTX, functions as a competitive opioid antagonist. Thus, the extent of blockade, or extent of “breakthrough” opioid agonist effects following opioid challenge, is related to the dose of the opioid agonist challenge as well as to the dose of naltrexone. Therefore, it is not surprising that the escalating hydromorphone dose sequence, rising to a total of 13.5 mg, produced somewhat more evidence of opioid agonist effects than did the 3 mg hydromorphone challenge. However, the important finding is that with the higher XR-NTX doses the magnitude of agonist effects was modest, and blockade was substantial even at high opioid agonist challenge doses.
The opioid agonist challenge doses used in this study have substantial clinical relevance. The specific agonist used, hydromorphone, is a mu-opioid agonist that is about 3 times as potent as heroin. It is commonly used as a surrogate for heroin in opioid abuse clinical pharmacology studies in the U.S. because of its similarity of effects to heroin and because it is available as a marketed pharmaceutical in the U.S., whereas heroin is not. Similarly, intramuscular administration is commonly used in opioid abuse liability and abuse pharmacotherapy studies (Bickel et al., 1988; Strain et al., 1997), due to its greater convenience and lower risk relative to intravenous administration, despite its being an uncommon route of opioid abuse; nevertheless, opioid blockade relationships likely vary somewhat by route of administration. Both heroin and hydromorphone are typical morphine-like mu-opioid agonists. Morphine is the other pharmaceutical most commonly used in opioid abuse clinical pharmacology studies; it is approximately one half as potent as heroin, with similar pharmacodynamic effects (Jasinski and Preston, 1986). The 3 mg hydromorphone dose used in this study is approximately equivalent to 20-25 mg of morphine, a dose in the range commonly used in opioid abuse liability assessment studies. The cumulative dose of 13.5 mg hydromorphone used in this study is approximately equivalent to 100 mg of morphine, i.e., a large dose, with substantial clinical relevance to concerns about possible overriding of the opioid blockade effect by use of large opioid agonist doses. While such overriding may be possible, we believe the present data illustrate such a substantial degree of blockade that such overriding is not likely to be a common clinical problem. Efforts to override the opioid blockade effect of naltrexone have very rarely been a significant problem in clinical use of the marketed oral formulations of naltrexone. The present data regarding blockade of opioid-produced respiratory depression indicate that XR-NTX treatment may prevent adverse opioid effects.
The XR-NTX formulation used in this study has been found to be efficacious in a multicenter clinical trial (Krupitsky et al., 2011) and is now marketed in the U.S. as a treatment for the prevention of relapse in opioid dependence, post detoxification, in a 380 mg dose. The doses used in this study are all lower than the XR-NTX dose marketed for the prevention of relapse in opioid dependence. Thus, the present data on dose-relatedness suggest that the marketed XR-NTX dose should offer equal or better blockade against opioid effects. Prior clinical studies of injectable sustained release naltrexone in opioid dependence used a similar biodegradable product from Biotek, Inc., that has not achieved regulatory or marketing approval for any indication. Those studies provide important proof-of-concept documentation, but are not necessarily generalizable to the present clinically available XR-NTX formulation. In human laboratory studies (Comer et al., 2002; Sullivan et al., 2006) the Biotek product, at a naltrexone dose of 384 mg, produced substantial blockade or attenuation of the subjective and reinforcing effects of heroin (up to 25 mg, i.v.; equivalent to approximately 50 mg morphine (Jasinski and Preston, 1986) for 4-5 weeks following a single intramuscular administration; heroin is approximately twice as potent as morphine). Those results are comparable to those in the present study with the marketed XR-NTX product. In the one outpatient clinical trial of the Biotek formulation the primary effect of the active naltrexone (compared to placebo) was to enhance patient retention; if missing samples were imputed as opiate-positive there was an apparent benefit on urine toxicology indices (Comer et al., 2006).
Several limitations of this study deserve note. The sample size was relatively small, allowing detection of only large effects. Also, medical monitors varied within and between study sites, discontinuations of dosing were monitors' personal clinical decisions, and we were not able to implement uniform “stop” criteria.
The present human laboratory results document the sustained pharmacological effect of XR-NTX in blocking or attenuating opioid agonist effects. These results document and quantify opioid antagonism as the presumed therapeutic mechanism of XR-NTX in the prevention of relapse to opioid dependence following opioid detoxification.
References
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988;247:47–53. [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RL. The preclinical development of Medisorb® naltrexone, a once a month long-acting injection, for the treatment of alcohol dependence. Front Biosci. 2005;10:643–655. doi: 10.2741/1559. [DOI] [PubMed] [Google Scholar]
- Dunbar JD, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single and multiple dose pharmacokinetics of long-acting naltrexone. Alcohol Clin Exp Res. 2006;30:480–490. doi: 10.1111/j.1530-0277.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- Fram DH, Marmo J, Holden R. Naltrexone treatment—the problem of patient acceptance. J Subst Abuse Treat. 1989;6:119–122. doi: 10.1016/0740-5472(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gonzales JP, Brogden RN. Naltrexone: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35:192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- Harris KM, Devries A, Dimidjian K. Trends in naltrexone use among members of a large private health plan. Psychiatr Serv. 2004;55:221. doi: 10.1176/appi.ps.55.3.221. [DOI] [PubMed] [Google Scholar]
- Jaskinski DR, Preston KL. Comparison of intravenously adminisistered methadone, morphine and heroin. Drug Alcohol Depend. 1986;17:301–310. doi: 10.1016/0376-8716(86)90079-7. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Aubin HJ, van den Brink W, Guzzetta R, Loewy J, Silverman B, Ehrich E. A pilot evaluation of the safety and tolerability of repeat dose administration of long-acting injectable naltrexone (Vivitrex) in patients with alcohol dependence. Alcohol Clin Exp Res. 2004;28:1356–1361. doi: 10.1097/01.alc.0000139823.30096.52. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Song X. Understanding US addiction physicians' low rate of naltrexone prescription. Drug Alcohol Depend. 2003;71:219–228. doi: 10.1016/s0376-8716(03)00134-0. [DOI] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence. Arch Gen Psychiatry. 1973;28:784–791. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther. 1981;216:45–54. [PubMed] [Google Scholar]
- O'Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. J Clin Psychopharmacol. 2007;27:507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Butorphanol-precipitated withdrawal in opioid dependent human volunteers. J Pharmacol Exp Ther. 1988;2:441–448. [PubMed] [Google Scholar]
- Resnick R, Volavka J, Freedman AM, Thomas M. Studies of EN-1639 A (naltrexone): a new narcotic antagonist. Am J Psychiatry. 1974;131:646–650. doi: 10.1176/ajp.131.6.646. [DOI] [PubMed] [Google Scholar]
- Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Strain EC, Walsh SL, Preston KL, Liebson LA, Bigelow GE. The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacology. 1997;129:329–338. doi: 10.1007/s002130050199. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- Turncliff RZ, Dunbar JL, Dong Q, Silverman BL, Ehrich EW, Dilzer SC. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45:1259–1267. doi: 10.1177/0091270005280199. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther. 1996;279:524–538. [PubMed] [Google Scholar]
- Willette R. Narcotic Antagonists: The Search for Long-Acting Preparations Research Monograph Series #4 DHEW Publication No (ADM) 76-296. National Institute on Drug Abuse; Rockville, Maryland: 1976. [PubMed] [Google Scholar]
- Willette RE, Barnett G. Narcotic Antagonists: Naltrexone Pharmacochemistry and Sustained-Release Preparations NIDA Research Monograph #28 DHEW Publication No (ADM) 81-902. National Institute on Drug Abuse; Rockville, Maryland: 1981. [PubMed] [Google Scholar]