Abstract
TNF-related apoptosis-inducing ligand, or TRAIL, is a promising anti-cancer agent as it can induce apoptosis in a wide range of cancers whilst generally sparing non-malignant cells. However, the translation of TRAIL into the clinic has been confounded by its short half-life, inadequate delivery methods and TRAIL-resistant cancer cell populations. In this review we discuss how TRAIL has been functionalized to diversify its traditional tumor-killing role and novel strategies to facilitate its effective deployment in preclinical cancer models. The successes and failures of the most recent clinical trials using TRAIL agonists are discussed and we provide a perspective for improving its clinical implementation.
Keywords: TRAIL, apoptosis, cancer, targeted therapy, stem cells
1. Cancer and TRAIL
Cancer is the leading cause of premature death in humans and despite improvements in detection methods, clinical intervention and increased public awareness of risk factors, the prevalence of cancer in economically developed countries continues to rise[1]. Essentially cancer is a disease resulting from unregulated cell growth. Genes involved in balancing cell proliferation and cell death are mutated such that tissue homeostasis goes awry culminating in cancerous cells that rapidly divide and escape inherent cell death induction[2]. Typically the current standard of care to treat solid cancers includes surgery to remove the bulk of the tumor and subsequent radiotherapy and/or chemotherapy to kill residual cancerous cells. The downside of using these conventional adjuvant therapies is their unspecific mode of action, often causing substantial death of healthy cells. Ideally, cancer therapies should specifically and robustly target cancerous cells whilst leaving normal healthy cells untouched. One strategy is to enhance cell death-related signaling pathways in cancers using pro-apoptotic proteins[3]. In the mid-90s, a new member of the Tumor Necrosis Factor (TNF) family was discovered and named TNF-related apoptosis-inducing ligand (TRAIL)[4, 5] (Figure 1). TRAIL was shown to possess the ability to induce apoptosis in a wide range of human cancer cell lines without significant cytotoxicity towards normal cells[6–8]. Nearly twenty years on the focus is to understand how to optimize the therapeutic efficacy of TRAIL in pre-clinical models in an effort to translate this promising agent into the clinic[9].
Figure 1.
Organization of human TRAIL
2. TRAIL signaling and apoptosis
Apoptosis is an essential event in maintaining tissue homeostasis by eliminating harmful cells from the body[10]. There are two pathways through which apoptosis may occur. The first is the extrinsic pathway which acts independently of the transcription factor p53 and is mediated by death receptors belonging to the TNF receptor superfamily such as TRAIL-R1/R2[11]. The intrinsic, or mitochondrial pathway is triggered in response to cellular stress and DNA damage and involves activation of p53 and the release of pro-apoptotic factors from the mitochondria[12] (Figure 2). TRAIL-induced apoptosis is mediated by the extrinsic pathway, but in certain circumstances, when a cell is additionally stressed, it can be enhanced by the intrinsic pathway resulting in expedition of apoptosis[13]. Crosstalk occurs at many points between the extrinsic and intrinsic apoptotic signaling pathways creating a complex and intricately balanced system[14]. One such node is caspase-8, required to cleave differential substrates in both pathways[15] (Figure 2). In order to maintain control of the apoptotic machinery both the extrinsic and intrinsic pathways are highly regulated at multiple levels by pro- and anti-apoptotic modulators. For example at the TRAIL death-inducing signaling complex (DISC), variants of a protease-deficient caspase homolog called cFLIP (cellular FLICE-inhibitory protein) inhibits the activation of caspase-8 and thus the propagation of apoptotic signaling. It is worth noting that TRAIL signaling does not always result in apoptosis of cancer cells. Studies have shown that TRAIL may induce pro-survival response via signaling factors that include NF-κB, mitogen-activated protein kinase (MAPK) and Akt (Protein kinase B)[16]. These pro-apoptotic and pro-survival signals compete with each other to determine survival outcome.
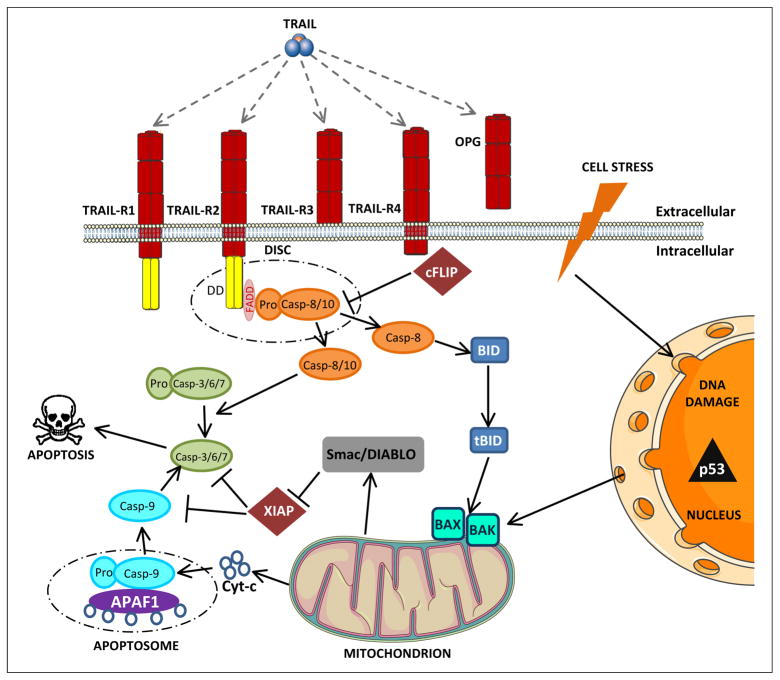
Figure 2. Apoptosis overview.
Apoptosis occurs through two pathways. The extrinsic apoptosis pathway occurs independently of p53 and requires the binding of pro-apoptotic ligands such as TRAIL. TRAIL can bind to four membrane-bound receptors (TRAIL-R1-4) and one soluble receptor (OPG). TRAIL-R1 and TRAIL-R2 contain a cytoplasmic death domain (DD) through which TRAIL can transmit an apoptotic signal. TRAIL-R3 and TRAIL-R4 also bind TRAIL, but because of the absence of a DD they are unable to initiate signaling. For this reason they are called decoy receptors. Binding of TRAIL to TRAIL-R1/2 leads to recruitment of the adaptor FADD and initiator procaspase-8 and 10 to rapidly form the DISC. Procaspase-8 and 10 are cleaved to form caspase-8 and 10 which activate effector caspase-3, 6 and 7 committing the cell to apoptose. At the DISC, variants of a protease-deficient caspase homolog called cFLIP inhibit the activation of caspase-8 and thus the propagation of apoptotic signaling. The intrinsic apoptosis pathway is initiated in response to cell stress such as chemotherapy and radiotherapy. This results in DNA damage causing the p53 oncogene to activate the pro-apoptotic Bcl-2 family proteins BAK and BAX. Pro-apoptotic proteins cytochrome c and Smac/DIABLO are released from mitochondria. Cytochrome c forms a protein complex, the apoptosome, with APAF-1 and activates caspase-9 which in turn activates effector caspase-3, 6 and 7 resulting in apoptosis. Smac/DIABLO inhibits apoptosis-inhibiting proteins such as XIAP, thus amplifying apoptotic signaling. APAF-1, Apoptotic protease activating factor-1; Bcl-2, B cell chronic lymphocytic leukaemia/lymphoma 2; BAK, Bcl-2 homologous antagonist/killer; BAX, Bcl-2-associated protein; FADD, Fas-associated death domain; cFLIP, cellular FLICE- inhibitory protein; DISC, Death-inducing signaling complex; SMAC, second mitochondria-derived activator of caspase; XIAP, X-linked inhibitor of apoptosis protein.
3. TRAIL Therapy
3.1 Systemic delivery of TRAIL
To date, many recombinant versions of human TRAIL have been created to augment its tumor-killing potential (Table 1). Untagged soluble human TRAIL (also called dulanermin), has a short serum half-life of approximately 30 minutes in non-human primates, shown to have comparable receptor binding to humans[17]. Owing to its small size, systemically delivered TRAIL is rapidly cleared from the body via the kidneys[17, 18]. This necessitates the need for repeated administration or more complex delivery methods to maintain therapeutically efficacious levels in the circulation [3]. One approach has been to facilitate its oligomerization by addition of peptide ‘tags’ which non-covalently interact. Increasing the total size of the oligomer has a twofold effect, firstly by retarding in vivo clearance from the circulation and also by reducing the proportion of inactive aggregates. Examples of this strategy include addition of a FLAG tag (FLAG-TRAIL[19]), leucine or isoleucine zipper (LZ-TRAIL[8]; iz-TRAIL[20, 21]) or a tanascin-C (TNC) oligomerization domain (TNC-TRAIL[22]). These modifications increase the stability of TRAIL and often enhance its activity (Table 1). Another strategy to improve the pharmacokinetic profile of a recombinant protein is to covalently link it to a molecule with more favorable properties[23]. For example, adding human serum albumin (HAS), which has a superior plasma half-life compared to TRAIL, to its N-terminus significantly improves its circulating half-life in vivo whilst maintaining its anti-tumor activity[24]. A similar strategy has been to attach PEG at different molecular weights to make PEGylated TRAIL derivatives which demonstrate protracted anti-tumor activity compared to untagged TRAIL[25, 26]. Instead of chemically modifying TRAIL, Kim et al., used a nanocomplex system for its long-term delivery[27]. In an in vivo xenograft tumor model these TRAIL-loaded microspheres were shown to inhibit tumor growth and displayed sustained TRAIL release over 10 days[27]. These approaches have helped to improve the therapeutic efficacy of systemically delivered TRAIL.
Table 1.
TRAIL modifications that enable detection and/or improve therapeutic performance.
| Name | Modification | Improvement | Application | Refs |
|---|---|---|---|---|
| LZ-TRAIL | Addition of a leucine zipper | Efficacy | Facilitated trimerization, activity and stability in vitro and in vivo | [8] |
| [125I]rhTRAIL | Radioiodinated recombinant human TRAIL | Detection | In vivo biodistribution and clearance | [18] |
| FLAG-TRAIL | Addition of FLAG tag to TRAIL | Efficacy | FLAG tag allows cross linking of TRAIL by using an anti-FLAG antibody | [19] |
| S-TRAIL-GFP | Fusion of the extracellular domain of Flt3L and an isoleucine zipper to the N-terminus of TRAIL. GFP fused to C-terminus | Efficacy + Detection | Enhanced apoptosis via bystander effect and stabilized oligomerization of TRAIL. GFP expression allows visualization of TRAIL-expressing cells | [20] |
| iz-TRAIL | Addition of an isoleucine zipper | Efficacy | Facilitated oligomerization resulting in improved cytotoxicity | [21] |
| scFv-sTRAIL | scFv-sTRAIL bifunctional fusion proteins | Efficacy | Increased specificity and strength of TRAIL response. Permits paracrine signaling | [23, 69] |
| HSA-Flag-TNC-TRAIL | Human serum albumin genetically fused to N-terminus of secretable TRAIL | Efficacy | Increased serum halflife to improve bioavailability | [24] |
| Transferrin-PEG-TRAIL (Tf-PEG10K-TRAIL) | PEGylated TRAIL attached to transferrin | Efficacy + Targeting | Combined tumor targeting/killing properties | [25] |
| PEG-TRAIL | PEGylation of TRAIL with PEGa of different molecular weights | Efficacy + Longevity | Increased serum half-life and protracted activity in vivo | [26] |
| TRAIL-loaded PLGAb microspheres | Nanocomplex system | Efficacy + Longevity | Sustained TRAIL release and tumor killing properties in vivo | [27] |
| SRL0L2TR | Luciferase fused to the N-terminus of secretable TRAIL | Detection | Direct extracellular visualization and monitoring of levels, time of delivery and localization of stem cell-delivered proteins by bioluminescent imaging | [38] |
| pORF-hTRAIL | Multifunctional nanoparticle comprising doxorubicin (DOX) and pORF-hTRAIL | Efficacy | Anti-glioma efficacy in vivo. Low pH conditions, such as those found in tumors, result in fast DOX release via cleavage of a pH-sensitive hydrazine bond | [49] |
| TRAIL-UCA | Ultrasound contrast agents chemically conjugated to TRAIL | Detection | Tracked by ultrasound imaging and fragmented into nanoparticles by focused ultrasound | [71] |
| Nanoparticle-TRAIL | TRAIL conjugated to nanoparticles | Efficacy | Enhances anti-tumor activity in glioma and glioma stem cells in vitro and in vivo | [72] |
PEG, Polyethylene glycol.
PLGA, poly(lactic-co-glycolic acid).
Although many soluble forms of TRAIL are well tolerated and therapeutically efficacious against TRAIL-sensitive tumors, cells expressing high levels of decoy receptors (TRAIL-R3/R4) might be protected from TRAIL-induced apoptosis. To overcome this problem, several groups have shown that tumor cell apoptosis can be enhanced through the use of monoclonal antibodies (mAbs) that target specific TRAIL receptors[28, 29]. In vivo, therapeutic antibodies have a longer half-life than recombinant soluble TRAIL, and as the antibodies specifically target death-inducing TRAIL receptors any problems concerning decoy receptors are circumvented. An arsenal of humanized and fully human agonistic mAbs have been developed against human death receptors and tested in cancer lines to assess their efficacy. One such example is TRA-8, a humanized mouse mAb specific for TRAIL-R2 that has been shown to induce apoptosis in many cancers [30]. Furthermore, treatment of primary human hepatocytes with TRA-8 did not initiate an apoptotic response indicating the tumor-specific action of this mAb[31]. Although encouraging preclinical results have been obtained using mAbs targeted towards death receptors, the recruitment of immune cells is a potential concern. In addition, as with systemic TRAIL delivery, mAbs do not cross the blood brain barrier which confounds their use in intracranial tumors. Nevertheless, a number of monoclonal antibodies that have proven pre-clinically successful have entered clinical trials.
3.2 Viral delivery
Viral vectors are essential to develop novel and efficient gene and cell-based therapies. TRAIL has been delivered via a variety of vector types that vary in their mode of action, and therefore their suitability for different applications. Adeno-associated viruses (AAVs) are able to stably integrate into dividing and non-dividing cells and display low immunogenicity. Secretable TRAIL has been incorporated into AAVs and used to treat a variety of carcinomas[32, 33]. The disadvantage of AAVs is their limited cloning capacity, preventing the use of large therapeutic genes. Similarly, adenoviruses (AVs) are able to infect dividing and non-dividing cells. In addition, large fragments of DNA (up to 7.5 kb) can be integrated into the genome making them more amenable to express recombinant DNA. Several groups have expressed TRAIL in AV vectors and used them to treat malignancies including non-small lung cancer, renal cell carcinoma and GBMs[34–36]. The drawbacks of using AVs are they are relatively immunogenic and display instability of transgene expression which can limit their therapeutic potential.
Lentiviruses (LVs) are a subclass of the retrovirus family and are a highly efficient gene delivery vector owing to their ability to infect dividing and non-dividing cells and deliver a large amount of viral RNA into the host DNA. The copy number of target cells is also relatively predictable and LVs have been effectively used to deliver TRAIL in conjunction with other therapeutic and diagnostic proteins. Examples include the co-infection of secretable TRAIL (sTRAIL) and Bcl-2 shRNA to treat lymphoma[37] and sTRAIL in conjunction with fluorescent and bioluminescent proteins to infect stem cells that track and treat GBM[38]. As with AVs, LVs can also cause an immune response and care must be taken when introducing foreign DNA into the LV genome that unwanted viral side effects are avoided. In the field of cancer biology, oncolytic viruses appear to be the most promising viral vectors to date. Oncolytic viruses are genetically modified viruses that, upon infection, preferentially replicate and lyse cancer cells whilst sparing normal cells[39]. Of the oncolytic viruses being developed, oncolytic herpes simplex virus (oHSV)-1 is being extensively used to treat many types of cancer[40]. We have shown that oHSV bearing sTRAIL (oHSV-TRAIL) can be used to treat a number of TRAIL-resistant GBM lines[41]. oHSV-TRAIL can modulate specific signaling pathways, ‘priming’ TRAIL-resistant GBM lines to apoptose by activation of caspase 8, 9 and 3, leading to an inhibition of tumor growth and prolonged survival of tumor-bearing mice. This study demonstrated that oHSV and TRAIL can function in concert to overcome TRAIL resistance[41].
To summarize, viral delivery has proven to be an effective means to express soluble TRAIL in a pre-clinical context. Now the challenge is to ensure absolute safety of the viral system to avoid complications in its clinical translation. The first step is to minimize the immune response that is elicited; this can not only impede delivery to target cells but also cause severe reactions to the patient. Secondly it is essential that the integration of DNA into the genome of the cell is targeted, not random, to avoid disruption of necessary cellular processes which might themselves form cancer. If both of these concerns are addressed then viral delivery could be a highly effective strategy to succeed in the clinic.
3.3 Stem cell delivery of TRAIL
Many types of stem cell have been shown to exhibit inherent tropism towards tumors, malignant lesions and other sites of pathology[42]. In addition, adult stem cells in their unmodified state have been demonstrated to have anti-tumor effects owing to factors that are released and physical interactions that are established between the stem cell and the tumor cell[43]. These attributes make stem cells an ideal candidate to treat cancer, acting as effective vehicles for delivering therapies to isolated tumors. The degree of stem cell migration towards a tumor depends on the nature of the stem cell (heterogeneity of population, culture conditions, expression of migratory factors) and the tumor microenvironment. Evidence suggests that migration of stem cells can be facilitated by locally irradiating the tumor[44, 45]. This strategy could be combined with radiotherapy regimes implemented in the clinic to enhance the effectiveness of tumor-specific stem cell targeting.
In the last decade, many studies have attempted to complement the inherent pathotropic properties of stem cells by modifying them to express antitumor agents which can then be locally delivered to the tumor [46]. We and others have engineered a variety of adult stem cell types to express soluble TRAIL which are then transplanted into mice bearing tumors (Table 2). The TRAIL protein is modified to make it secretable, enabling the stem cell to elicit a specific and sustained TRAIL response in the vicinity of the tumor. Stem cell-based delivery of TRAIL has been particularly well studied in mouse models of GBM. Primary or established GBM cell lines are orthotopically implanted into the brain and allowed to establish before engraftment of therapeutic stem cells that subsequently track the tumor, inhibit its growth and prolong survival[47–51]. Viral infection is mainly used to introduce TRAIL into stem cells, although a small number of studies have used non-viral methods to alleviate any safety concerns[48, 52]. Stem cell delivery of TRAIL can be combined with other compounds to potentiate its effect. For example we used PI-103 (PI3-kinase/mTOR inhibitor) in combination with murine neural stem cells (NSC)-TRAIL to augment its in vivo response towards established GBM[53]. The combination of murine neural precursor cells with anti-miR-21 oligonucleotides also resulted in synergism with TRAIL in vivo, leading to the eradication of highly proliferative GBM[54]. TRAIL-expressing stem cells have been successfully applied to other cancer models. Loebinger and colleagues engineered human MSCs to conditionally express TRAIL using the Tet promoter[55]. In co-culture experiments, the activation of TRAIL in MSCs was sufficient to selectively kill lung, breast, squamous and cervical cancer cells. Furthermore, when applied to a pulmonary metastasis model, tumor growth was significantly reduced and lung metastases were targeted and completely cleared[55]. Renal cell carcinoma, malignant fibrous histiocytoma and lung metastases have also been successfully treated using stem cell expression of TRAIL demonstrating that this approach has much potential in treating a variety of bulk tumors and micrometastatic lesions[52, 56].
Table 2.
Stem cell delivery of TRAIL.a
| Stem cell type | Form of TRAIL | Tumor type | Combination therapy | Principle findings | Refs |
|---|---|---|---|---|---|
| Human UCB-MSCs | Adenoviral infection of secretable trimeric TRAIL | U87-MG | Irradiation of tumor | Irradiation enhances tumor tropism and therapeutic potential of SCs | [45] |
| Human BM-MSCs | Lentiviral infection of secretable TRAIL | Primary + Established human GBM | - | Use of real-time imaging to follow migration and therapeutic effect of MSCs on tumor volume in vivo | [47] |
| Human A-MSCs | Non-viral nucleofection of TRAIL | Rat glioma model | - | Reduction of tumor volume and significant survival benefit in vivo | [48] |
| Rat BM-MSCs | Adenoviral transduction of dodecomeric TRAIL | Renal cell carcinoma (RCC) | Co-expression of herpes simplex virus thymidine kinase | Complete elimination of established RCC in vivo | [50] |
| Human MSC/Mouse MSC | Secretable TRAIL | Established GBMs | Stem cells express herpes simplex virus thymidine kinase | Stem cells are eliminated after therapeutic effect by addition of the prodrug gancyclovir | [51] |
| MSCs | Secretable TRAIL introduced using non-viral PEI(600)-Cyd | Lung metastasis | - | Lung tumor homing and reduction in metastasis | [52] |
| Mouse NSCs | Lentiviral infection of secretable TRAIL | GBM6/8/12 in vitro, Gli36-EGFRVIII in vivo | PI-103 (PI3-kinase/mTOR inhibitor | PI-103 augments in vivo response of gliomas to TRAIL | [53] |
| Mouse NPCs | Lentiviral infection of secretable TRAIL | Human U87 established glioma model | Locked nucleic acid (LNA) anti-miR-21 oligonucleotides | Synergism with TRAIL resulting in eradication of tumor in vivo | [54] |
| Human MSCs | LV-TRAIL under tet promoter | Pulmonary metastasis model | Conditional expression of TRAIL using Dox | Cleared metastatic disease in lung metastasis model | [55] |
| Human A-MSCs | Secretable TRAIL | Malignant fibrous histiocytoma | - | Decrease in metastasis | [56] |
| Mouse NSC/Human MSC | Secretable TRAIL | Established + Primary GBMs | Stem cells encapsulated in sECM | Increased retention of stem cells within resection cavity | [64] |
| Human MSC | Inducible TRAIL | Breast + bone metastasis model | Stem cells encapsulated in silk scaffold | Halts breast cancer growth and decreases degree of bone and lung metastasis | [65] |
| Mouse NSC | LV-EGFR-Nanobody TRAIL (ENb2-TRAIL) | Established GBMs | - | Target EGFR and TRAIL signaling pathways simultaneously | [70] |
Abbreviations: UCB-MSCs, Umbilical cord blood-derived mesenchymal stem cells; BM-MSCs, Bone marrow-derived mesenchymal stem cells; A-MSCs, Adipose-derived mesenchymal stem cells; NSCs, Neural stem cells; NPCs, Neural progenitor cells; sECM, synthetic extracellular matrix; EGFR, Epidermal growth factor receptor.
4. Tackling TRAIL resistance using combination therapy
Tumor cells have a mixed response to TRAIL-mediated killing. In an in vitro study looking at the efficacy of untagged TRAIL on multiple cancer cell lines the majority displayed some degree of cytostatic or cytotoxic effects, although 20% were refractory to its action[7]. Furthermore, cancer cells can acquire TRAIL resistance during the evolution of the tumor[57]. Both intrinsic and acquired resistance to TRAIL poses a huge problem in establishing clinically efficacious TRAIL therapies. Evidence suggests that resistance can occur through defects at every level of the TRAIL-signaling pathway from ligand binding to cleavage of the effector caspases[13, 58]. Considering the proportion of cancer cells with some degree of intrinsic or acquired resistance towards TRAIL, one might predict the success of using TRAIL as a therapeutic agent modest at best. However, many groups have shown that radiation and/or various classes of drugs can synergize with TRAIL when used in combination[28]. The signaling mechanisms responsible for this synergy are still being defined, and differ depending on the drugs’ specific mode of action[58]. From an apoptotic standpoint, because TRAIL initiates cell death in a p53-independent manner[13] (Figure 2), as opposed to chemotherapy or radiation which are often p53-dependant, a combination of the two treatments augment the total apoptotic signal produced by the cell[28].
Many studies have combined chemotherapeutic drugs and TRAIL as a means to treat tumors. For example the use of gemcitabine, oxaliplatin and irinotecan in combination with TRAIL, treated gastroenterological tumors more effectively than any of these agents alone[21]. Another genotoxic drug, cisplatin, in combination with TRAIL-encoding retrovirus resulted in higher anticancer activity in ovarian carcinoma cells in vitro and in xenografts[59]. Inhibiting the proteasome using bortezomib synergizes with TRAIL to sensitize tumor cell lines and primary tumor cells[60], a phenomenon also seen by inhibiting histone deacetylases (HDAC)[61–63]. The molecular mechanism of TRAIL-sensitization is starting to become apparent and we have shown that upon HDAC inhibition TRAIL death receptors are upregulated on the cell surface[61]. Using a TRAIL-R1/2 reporter system the upregulation of death receptors in response to HDAC inhibition was followed in vitro and in vivo in real time and correlated with increased TRAIL sensitivity[61]. Furthermore, treatment of GBM cells with the HDAC inhibitor MS275 prime these cells for TRAIL-induced apoptosis by increasing the binding of c-myc to the cFLIP promoter thereby reducing its activity[62]. These findings demonstrate that combinatorial therapy can elicit enhanced TRAIL-mediated apoptosis in a variety of tumor cell lines and in some cases reverse resistance to TRAIL. Though it might risk sensitizing normal cells, combinatorial therapy represents a promising strategy for treating cancers that are resistant to TRAIL.
5. Promising Preclinical studies
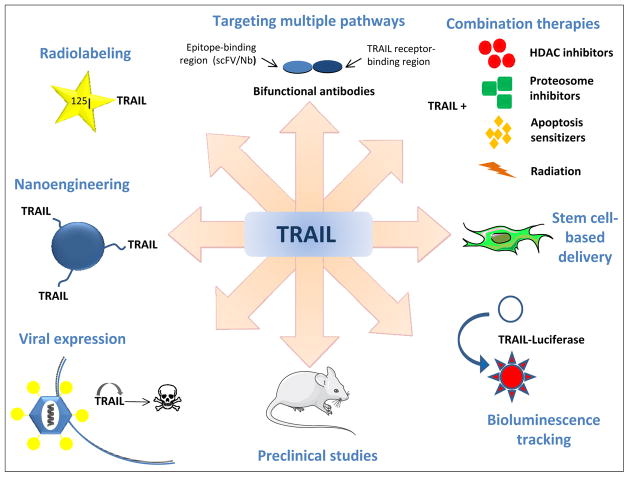
The use of TRAIL to treat malignancies in a preclinical setting has entered an exciting phase with groups around the world finding innovative ways to modify and deploy TRAIL to maximize its tumor-killing potential (Figure 3). Outlined below are a number of recent studies which show significant clinical promise.
Figure 3. Applications of TRAIL.
TRAIL has been modified in a variety of ways to enhance its efficacy and diversify its applications
5.1 Encapsulating TRAIL-expressing stem cells
TRAIL displays a short half-life making it difficult to maintain therapeutically efficacious levels in the vicinity of the tumor[17, 18]. One strategy to overcome this challenge is to encapsulate TRAIL-expressing stem cells in a synthetic scaffold, allowing them to be retained in a localized manner. Two recent papers have utilized this approach in glioma, bone and lung metastasis mouse models[64, 65]. In the first example we encapsulated stem cells expressing TRAIL in a synthetic extracellular matrix (sECM) which were then introduced into the resection cavity after GBM surgical debulking[64]. The mouse model of tumor resection attempts to recapitulate the clinical situation in humans, whilst the encapsulated cells are prevented from being washed out of the cavity by cerebrospinal fluid, enabling them to act on residual GBM cells found in the resection margins[64]. This approach delayed regrowth of malignant and invasive brain tumors and significantly increased survival of mice[64]. In the second study, Reagan et al., seeded biocompatible silk scaffolds with TRAIL-expressing hMSCs which were implanted subcutaneously into mice bearing bone or lung metastases[65]. TRAIL expression was regulated using an inducible promoter and when expressed, mice showed a decrease in tumor burden compared to no TRAIL controls[65]. These two papers demonstrate the effectiveness of encapsulating therapeutic stem cells and insight into how this technology could be translated into the clinic.
5.2 Two birds, one stone: targeting multiple signaling pathways
An emerging branch of cancer therapies is the use of smaller antibody fragments such as single-chain variable fragments (scFv) and nanobodies (consisting of just the VHH domain) that bind to epitopes overexpressed on tumor cells to perturb specific signaling pathways[66]. These molecules are significantly smaller in size than mAbs allowing increased tissue dispersal and improved stability compared to their full length counterparts [67, 68]. These proteins can be additionally fused to TRAIL resulting in a bifunctional fusion protein that can activate the TRAIL signaling pathway and modulate an additional signaling pathway in parallel. This strategy has been applied to make an arsenal of scFv-sTRAIL fusion proteins that bind to various targets[23]. For example, a scFvCD19-sTRAIL bispecific antibody fragment was able to selectively induce apoptosis in leukemic B-lymphoid cells[69]. Interestingly, strong induction of apoptosis was also observed in CD19-negative B-lymphoid cells via potent bystander effect, exemplifying the potential of this approach at tackling a heterogeneous tumor population through paracrine receptor activation[69]. In another recent study we fused sTRAIL to an EGFR-specific nanobody to make a pro-apoptotic immunoconjugate (ENb2-TRAIL) which concurrently targets cell proliferation and death pathways[70] (Figure 4). Stem cells were engineered to express ENb2-TRAIL, which were then applied to tumor models of malignant and primary invasive GBMs[70]. EGFR signaling was efficiently inhibited in tumor cells which were then killed by TRAIL-mediated apoptosis. In vivo, when mice bearing intracranial GBM were treated with stem cell-delivered ENb2-TRAIL, tumor burden was significantly decreased and mice survival was prolonged[70]. Taken together, bifunctional antibody fragments possess many attributes that makes this a novel and potentially effective means to treat malignancies.
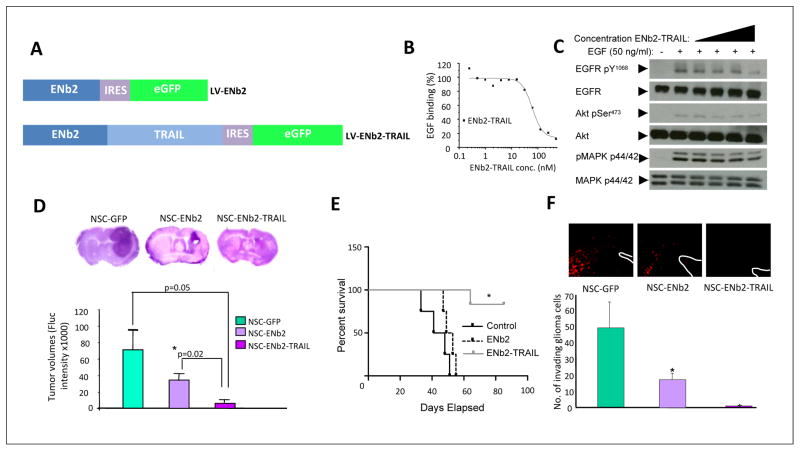
Figure 4. Mouse neural stem cells engineered to secrete nanobody-TRAIL prolong the life of mice bearing GBM tumors.
(A) Schematic representation of lentiviral transfer vectors comprising eGFP, anti-EGFR nanobodies (LV-ENb2) and cytotoxic TRAIL (LV-ENb2-TRAIL). (B) ELISA showing EGFR competition by ENb2-TRAIL. (C) Western blot analysis showing inhibition of EGFR and downstream signaling via the AKT and MAPK pathways on serum-starved Her14 cells incubated with ENb2-TRAIL. (D) Photomicrographs of H&E stained sections and tumor volumes from the brains of GBM-bearing mice treated with NSC-GFP, NSC-ENb2, and NSC-ENb2-TRAIL. (E) Kaplan-Meier survival curves of mice bearing established tumors and implanted with NSC expressing GFP, ENb2 or ENb2-TRAIL intratumorally (n=5 per group). (F) Representative fluorescent images and plot showing the number of GBM8-mCherry invading cells 7 days after NSC-GFP, NSC-ENb2, and NSC-ENb2-TRAIL treatment. Data represented as mean ± SEM, * denotes P< 0.05, Student’s t test. White lines indicate the wall of the lateral ventricle. eGFP, Enhanced green fluorescent protein. Adapted with permission from[70].
5.3 Tracking TRAIL
TRAIL has been modified in various ways to directly visualize and/or detect the protein in vitro and in pre-clinical tumor models (Table 1). A common method is to create fusion proteins of TRAIL whereby DNA encoding fluorescent proteins such as GFP are placed in-frame with TRAIL cDNA in a suitable expression vector allowing one to visualize the expression of TRAIL-expressing cells [20]. This approach was extended by combining fluorescent proteins with bioluminescent proteins, such as firefly luciferase, to allow non-invasive visualization using bioluminescent imaging (BLI). One example is SRL0L2TR, a fusion protein we engineered to contain luciferase linked to the N-terminus of secretable TRAIL[38]. This protein allows the direct visualization and monitoring of extracellular TRAIL levels by bioluminescent imaging[38]. Other groups have modified TRAIL by directly conjugating moieties onto the protein. Recently Duiker et al., radioiodinated recombinant human TRAIL enabling them to measure the biodistribution and clearance of TRAIL in vivo[18]. In addition the monoclonal antibody mapatumumab, which binds to TRAIL-R1, was conjugated to 111I enabling one to predict clinical efficacy depending on the tumor type and its TRAIL-R1 status[18]. In another approach, human recombinant TRAIL was conjugated to a polymeric ultrasound contrast agent (UCA)[71]. Ligation of TRAIL to the UCA facilitates its targeting to extravascular sites and once there, the microcapsule can be fragmented into nanoparticles by focused ultrasound allowing dispersal throughout the tumor[71]. TRAIL was also conjugated to magnetic ferric oxide nanoparticles and shown to have superior anti-tumor activity towards glioma cells and glioma stem cells in vitro and in vivo as compared to unconjugated TRAIL[72]. These examples demonstrate that TRAIL can be additionally functionalized to offer additional desirable diagnostic characteristics on top of its inherent pro-apoptotic mode of action.
6. TRAIL in clinical trials
A number of different compounds targeting TRAIL receptors have proven sufficiently efficacious in preclinical studies to warrant progression to clinical trials[28, 58]. Phase I trials of single agents have largely been undertaken on patients with advanced solid tumors and include soluble rhTRAIL (dulanermin)[73], the TRAIL-R1 mAb agonist mapatumumab[74] and TRAIL-R2 mAb agonists tigatuzumab, lexatumumab and Apomab[75–77]. Whilst these compounds were largely well tolerated their anti-cancer response was poor with the vast majority of patients showing no remission. To date, the most promising monotherapy has been mapatumumab which entered a phase II clinical trial in patients with non-Hodgkin lymphoma with almost one-third of patients responding and one showing complete recovery[78].
Combination therapies that pass through phase Ib trials, in which dosage range and side effects are assessed, enter phase II where patient cohorts are increased in number. Genentech, Amgen, GlaxoSmithKline and The National Cancer Institute have all sponsored trials that have reached phase II. When looking for details regarding specific trials on http://www.clinicaltrials.gov it is apparent that several have been terminated or withdrawn (NCT01017822, NCT01017822, NCT00819169 and NCT00400764). Another portion have been completed but results have not been updated on the site making it difficult to assess their success (NCT00630552, NCT00583830, NCT00626704, NCT00534027, NCT00583830, NCT00508625 and NCT00480831). The remaining active trials comprise conatumumab (formally AMG 655), a fully human mAb against TRAIL-R2 in combination with additional chemotherapeutic agents. Trial NCT00625651 uses conatumumab in combination with FOLFOX6 (folinic acid, 5-fluorouracil and oxaliplatin) and the angiogenesis inhibitor bevacizumab (Avastin) to treat patients with colorectal cancer (CRC), whilst trial NCT01327612 uses the same combination therapy to treat patients with CRC, lymphoma and non-small cell lung cancer. The rationale is that genotoxic FOLFOX6 will increase p53-dependant apoptosis and sensitize TRAIL-resistant cancer cells, whilst bevacizumab will inhibit the formation of nascent blood vessels in the tumor. Conatumumab is also being used in combination with FOLFOX6 or FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) to treat CRC in trials NCT00625651 and NCT00813605 respectively. These trials are ongoing and the relative success of these combination therapies on patients remains to be seen.
7. Concluding Remarks and Future Perspectives
A dichotomy seems to exist in TRAIL research. Preclinical studies on understanding TRAIL-induced apoptosis and its anti-tumor mode of action are going from strength to strength and a panoply of TRAIL derivatives have been developed to improve its pharmacokinetic, therapeutic and diagnostic properties (Table 1). However the translation of encouraging preclinical studies into the clinic seems to have stalled, with the majority of clinical trials faltering at phase I. It is important to consider that none of the trials preselect patients on the basis of degree of TRAIL sensitivity. Indeed, for a tumor to flourish it is likely to have developed ways to bypass endogenous TRAIL-killing mechanisms, rendering the tumor TRAIL-resistant[57]. To improve the effectiveness of TRAIL therapy efforts should be spent in understanding how the TRAIL pathway is disrupted in individual cancers and which combination therapies can be deployed most effectively. To tackle this challenge the cancer field would benefit from detailed characterization of the genetic and epigenetic makeup of patient-derived tumors to identify defects in cell proliferation and death pathways[79]. Computational modeling could prove invaluable to help collate and interpret huge datasets, finding novel targets that might enhance apoptosis in TRAIL-resistant populations[80]. To complement this approach, preclinical studies should focus on using primary patient-derived cell lines as opposed to established cancer cell lines. Testing combination therapies on improved preclinical models which more faithfully reflect the clinical situation will help identify relevant therapies and possibly improve their success rate. Perhaps in the future, a biopsied tumor from a patient could be molecularly screened, assigned an individual TRAIL-sensitivity signature and matched to similar tumors in a cancer bank. Using this information should enable a more tailored, theranostic approach, permitting better informed therapeutic intervention.
Acknowledgments
We apologize to all colleagues whose work could not be cited owing to space limitations. This work was supported by RO1CA138922, R01CA173077 and James McDonald Foundation.
Glossary
- Apoptosis
a type of programmed cell death that leads to characteristic cellular changes and death. These events include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation and chromosomal fragmentation. There are two apoptosis pathways: the extrinsic pathway acts independently of the transcription factor p53 and is mediated by death receptors such as the tumor necrosis factor (TNF) receptor superfamily. The intrinsic, or mitochondrial pathway is triggered in response to cellular stress and DNA damage and involves activation of p53 and the release of pro-apoptotic factors from the mitochondria
- Caspases
or cysteine-aspartic proteases are a family of enzymes that are essential in apoptosis, necrosis and inflammation. There are two types: Initiator caspases cleave inactive pro-forms of effector caspases, therefore activating them. Effector caspases, once activated, cleave other protein substrates to trigger the apoptotic cascade
- Combination therapy
the use of more than one medication or therapy to treat a disease
- Death domain (DD)
a protein interaction module composed of six alpha-helices. DDs are found in the cytoplasmic tails of death receptors and mediate their self-association which initiates apoptosis signal transduction events
- Death-inducing signaling complex (DISC)
a multi-protein complex including members of the apoptosis-inducing cellular receptors such as TRAIL-R1 and caspase-8. Upon ligand binding, the DISC transduces a signaling cascade resulting in apoptosis
- Death receptors
a trimeric cytokine receptor that binds tumor necrosis factors
- Decoy receptor
in contrast to the apoptosis-inducing death receptors, decoy receptors are unable to transduce an apoptotic signal. For example TRAIL-R3 lacks a DD and TRAIL-R4 has a truncated DD enabling TRAIL to bind but rendering them unable to initiate apoptosis
- Glioblastoma multiforme (GBM)
the most common and aggressive malignant primary brain tumor in humans
- Mitochondrial outer membrane permeabilization (MOMP)
leads to the release of pro-death factors from the mitochondrial intermembrane space, which engage caspases to propagate the apoptotic signaling cascade
- Nanobody
an antibody fragment consisting of a single monomeric variable antibody domain. Nanobodies are able to bind to specific antigens and have a molecular weight of 12–15 kDa compared to the 150–160 kDa of conventional antibodies. This allows them to display better permeability in tissues, possess short plasma half-lives and do not trigger the complement system because of the absence of an Fc region
- Tumor Necrosis Factor (TNF)
a group of cytokines whose family can cause apoptosis. All members form trimeric complexes that are recognized by their cognate receptor
- TNF-related apoptosis-inducing ligand (TRAIL)
a cytokine that is produced and secreted by the majority of mammalian cells. TRAIL elicits apoptosis primarily in tumor cells by binding to death receptors expressed at the cell surface
- TRAIL resistance
cancer cells that are resistant to apoptosis induction by TRAIL. Resistance can be intrinsic (cells were always resistant to TRAIL), or acquired (cells that were once sensitive to TRAIL have become resistant after repeated exposure). Understanding the mechanisms underlying resistance and developing strategies to overcome it is vital for the successful use of TRAIL for cancer therapy
References
- 1.Jemal A, et al. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, et al. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 4.Wiley SR, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 5.Pitti RM, et al. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. The Journal of biological chemistry. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 6.Allen JE, El-Deiry WS. Regulation of the human TRAIL gene. Cancer biology & therapy. 2012;13:1143–1151. doi: 10.4161/cbt.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashkenazi A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. The Journal of clinical investigation. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walczak H, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature medicine. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone RW, et al. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nature reviews. Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorburn A. Death receptor-induced cell killing. Cellular signalling. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Galluzzi L, et al. Mitochondria: master regulators of danger signalling. Nature reviews. Molecular cell biology. 2012;13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 13.Johnstone RW, et al. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 14.Spencer SL, Sorger PK. Measuring and modeling apoptosis in single cells. Cell. 2011;144:926–939. doi: 10.1016/j.cell.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochimica et biophysica acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Falschlehner C, et al. TRAIL signalling: decisions between life and death. The international journal of biochemistry & cell biology. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Kelley SK, et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. The Journal of pharmacology and experimental therapeutics. 2001;299:31–38. [PubMed] [Google Scholar]
- 18.Duiker EW, et al. Development of a radioiodinated apoptosis-inducing ligand, rhTRAIL, and a radiolabelled agonist TRAIL receptor antibody for clinical imaging studies. British journal of pharmacology. 2012;165:2203–2212. doi: 10.1111/j.1476-5381.2011.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider P. Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods in enzymology. 2000;322:325–345. doi: 10.1016/s0076-6879(00)22031-4. [DOI] [PubMed] [Google Scholar]
- 20.Shah K, et al. Inducible release of TRAIL fusion proteins from a proapoptotic form for tumor therapy. Cancer research. 2004;64:3236–3242. doi: 10.1158/0008-5472.can-03-3516. [DOI] [PubMed] [Google Scholar]
- 21.Ganten TM, et al. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:2640–2646. doi: 10.1158/1078-0432.CCR-05-2635. [DOI] [PubMed] [Google Scholar]
- 22.Berg D, et al. Enforced covalent trimerization increases the activity of the TNF ligand family members TRAIL and CD95L. Cell death and differentiation. 2007;14:2021–2034. doi: 10.1038/sj.cdd.4402213. [DOI] [PubMed] [Google Scholar]
- 23.Wajant H, et al. Engineering death receptor ligands for cancer therapy. Cancer letters. 2011 doi: 10.1016/j.canlet.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Muller N, et al. Superior serum half life of albumin tagged TNF ligands. Biochemical and biophysical research communications. 2010;396:793–799. doi: 10.1016/j.bbrc.2010.04.134. [DOI] [PubMed] [Google Scholar]
- 25.Kim TH, et al. PEG-transferrin conjugated TRAIL (TNF-related apoptosis-inducing ligand) for therapeutic tumor targeting. Journal of controlled release: official journal of the Controlled Release Society. 2012;162:422–428. doi: 10.1016/j.jconrel.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim TH, et al. PEGylated TNF-related apoptosis-inducing ligand (TRAIL) analogues: pharmacokinetics and antitumor effects. Bioconjugate chemistry. 2011;22:1631–1637. doi: 10.1021/bc200187k. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, et al. A sulfate polysaccharide/TNF-related apoptosis-inducing ligand (TRAIL) complex for the long-term delivery of TRAIL in poly(lactic-co-glycolic acid) (PLGA) microspheres. The Journal of pharmacy and pharmacology. 2013;65:11–21. doi: 10.1111/j.2042-7158.2012.01564.x. [DOI] [PubMed] [Google Scholar]
- 28.Hellwig CT, Rehm M. TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies. Molecular cancer therapeutics. 2012;11:3–13. doi: 10.1158/1535-7163.MCT-11-0434. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, et al. Combination antibody-based cancer immunotherapy. Cancer science. 2007;98:1297–1302. doi: 10.1111/j.1349-7006.2007.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amm HM, et al. Combined modality therapy with TRAIL or agonistic death receptor antibodies. Cancer biology & therapy. 2011;11:431–449. doi: 10.4161/cbt.11.5.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichikawa K, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nature medicine. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 32.Zheng L, et al. Adeno-associated virus-mediated doxycycline-regulatable TRAIL expression suppresses growth of human breast carcinoma in nude mice. BMC cancer. 2012;12:153. doi: 10.1186/1471-2407-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang M, et al. Synergistic antitumor effect of AAV-mediated TRAIL expression combined with cisplatin on head and neck squamous cell carcinoma. BMC cancer. 2011;11:54. doi: 10.1186/1471-2407-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, et al. Adenovirus-mediated TRAIL expression and downregulation of Bcl-2 expression suppresses non-small cell lung cancer growth in vitro and in vivo. International journal of molecular medicine. 2012;30:358–364. doi: 10.3892/ijmm.2012.998. [DOI] [PubMed] [Google Scholar]
- 35.Norian LA, et al. Eradication of metastatic renal cell carcinoma after adenovirus-encoded TNF-related apoptosis-inducing ligand (TRAIL)/CpG immunotherapy. PloS one. 2012;7:e31085. doi: 10.1371/journal.pone.0031085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim CY, et al. Preclinical studies for pharmacokinetics and biodistribution of Ad-stTRAIL, an adenovirus delivering secretable trimeric TRAIL for gene therapy. Experimental & molecular medicine. 2011;43:580–586. doi: 10.3858/emm.2011.43.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, et al. The effect of lentivirus-mediated expression of tumor necrosis factor related apoptosis-inducing ligand and shRNA against Bcl-2 on the growth of lymphoma cells. Leukemia & lymphoma. 2012;53:710–717. doi: 10.3109/10428194.2011.631158. [DOI] [PubMed] [Google Scholar]
- 38.Hingtgen SD, et al. A novel molecule integrating therapeutic and diagnostic activities reveals multiple aspects of stem cell-based therapy. Stem Cells. 2010;28:832–841. doi: 10.1002/stem.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu TC, et al. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nature clinical practice. Oncology. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 40.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer gene therapy. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 41.Tamura K, et al. Multimechanistic tumor targeted oncolytic virus overcomes resistance in brain tumors. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:68–77. doi: 10.1038/mt.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corsten MF, Shah K. Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. The lancet oncology. 2008;9:376–384. doi: 10.1016/S1470-2045(08)70099-8. [DOI] [PubMed] [Google Scholar]
- 43.Schichor C, et al. Mesenchymal stem cells and glioma cells form a structural as well as a functional syncytium in vitro. Experimental neurology. 2012;234:208–219. doi: 10.1016/j.expneurol.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 44.Zielske SP, et al. Radiation increases invasion of gene-modified mesenchymal stem cells into tumors. International journal of radiation oncology, biology, physics. 2009;75:843–853. doi: 10.1016/j.ijrobp.2008.06.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SM, et al. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells. 2010;28:2217–2228. doi: 10.1002/stem.543. [DOI] [PubMed] [Google Scholar]
- 46.Shah K. Mesenchymal stem cells engineered for cancer therapy. Advanced drug delivery reviews. 2012;64:739–748. doi: 10.1016/j.addr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasportas LS, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi SA, et al. Therapeutic efficacy and safety of TRAIL-producing human adipose tissue-derived mesenchymal stem cells against experimental brainstem glioma. Neuro-oncology. 2011;13:61–69. doi: 10.1093/neuonc/noq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S, et al. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials. 2012;33:4907–4916. doi: 10.1016/j.biomaterials.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 50.Kim SW, et al. Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:415–427. doi: 10.1158/1078-0432.CCR-12-1568. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Quintanilla J, et al. In vivo Imaging of the Therapeutic Efficacy and Fate of Bimodal Engineered Stem Cells in Malignant Brain Tumors. Stem Cells. 2013 doi: 10.1002/stem.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu YL, et al. Mesenchymal stem cells as a novel carrier for targeted delivery of gene in cancer therapy based on nonviral transfection. Molecular pharmaceutics. 2012;9:2698–2709. doi: 10.1021/mp300254s. [DOI] [PubMed] [Google Scholar]
- 53.Bagci-Onder T, et al. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer research. 2011;71:154–163. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 54.Corsten MF, et al. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer research. 2007;67:8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- 55.Loebinger MR, et al. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer research. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee HJ, et al. A Therapeutic Strategy for Metastatic Malignant Fibrous Histiocytoma Through Mesenchymal Stromal Cell-Mediated TRAIL Production. Annals of surgery. 2012 doi: 10.1097/SLA.0b013e3182710401. [DOI] [PubMed] [Google Scholar]
- 57.Maksimovic-Ivanic D, et al. Resistance to TRAIL and how to surmount it. Immunologic research. 2012;52:157–168. doi: 10.1007/s12026-012-8284-8. [DOI] [PubMed] [Google Scholar]
- 58.Dimberg LY, et al. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene. 2012 doi: 10.1038/onc.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li F, et al. In vitro and in vivo growth inhibition of drug-resistant ovarian carcinoma cells using a combination of cisplatin and a TRAIL-encoding retrovirus. Oncology letters. 2012;4:1254–1258. doi: 10.3892/ol.2012.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sayers TJ, Murphy WJ. Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy. Cancer immunology, immunotherapy: CII. 2006;55:76–84. doi: 10.1007/s00262-005-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagci-Onder T, et al. Real-time imaging of the dynamics of death receptors and therapeutics that overcome TRAIL resistance in tumors. Oncogene. 2012 doi: 10.1038/onc.2012.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bangert A, et al. Histone deacetylase inhibitors sensitize glioblastoma cells to TRAIL-induced apoptosis by c-myc-mediated downregulation of cFLIP. Oncogene. 2012;31:4677–4688. doi: 10.1038/onc.2011.614. [DOI] [PubMed] [Google Scholar]
- 63.Nebbioso A, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nature medicine. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 64.Kauer TM, et al. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nature neuroscience. 2012;15:197–204. doi: 10.1038/nn.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reagan MR, et al. Stem Cell Implants for Cancer Therapy: TRAIL-Expressing Mesenchymal Stem Cells Target Cancer Cells In Situ. Journal of breast cancer. 2012;15:273–282. doi: 10.4048/jbc.2012.15.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahbarizadeh F, et al. Nanobody; an old concept and new vehicle for immunotargeting. Immunological investigations. 2011;40:299–338. doi: 10.3109/08820139.2010.542228. [DOI] [PubMed] [Google Scholar]
- 67.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nature biotechnology. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 68.Muyldermans S. Single domain camel antibodies: current status. Journal of biotechnology. 2001;74:277–302. doi: 10.1016/s1389-0352(01)00021-6. [DOI] [PubMed] [Google Scholar]
- 69.Stieglmaier J, et al. Selective induction of apoptosis in leukemic B-lymphoid cells by a CD19-specific TRAIL fusion protein. Cancer immunology, immunotherapy: CII. 2008;57:233–246. doi: 10.1007/s00262-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van de Water JA, et al. Therapeutic stem cells expressing variants of EGFR-specific nanobodies have antitumor effects. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16642–16647. doi: 10.1073/pnas.1202832109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wheatley MA, et al. Cellular signal transduction can be induced by TRAIL conjugated to microcapsules. Journal of biomedical materials research. Part A. 2012;100:2602–2611. doi: 10.1002/jbm.a.34189. [DOI] [PubMed] [Google Scholar]
- 72.Perlstein B, et al. TRAIL conjugated to nanoparticles exhibits increased anti-tumor activities in glioma cells and glioma stem cells in vitro and in vivo. Neuro-oncology. 2013;15:29–40. doi: 10.1093/neuonc/nos248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herbst RS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 74.Hotte SJ, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 75.Forero-Torres A, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer biotherapy & radiopharmaceuticals. 2010;25:13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wakelee HA, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camidge DR. Apomab: an agonist monoclonal antibody directed against Death Receptor 5/TRAIL-Receptor 2 for use in the treatment of solid tumors. Expert opinion on biological therapy. 2008;8:1167–1176. doi: 10.1517/14712598.8.8.1167. [DOI] [PubMed] [Google Scholar]
- 78.Younes A, et al. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin’s lymphoma. British journal of cancer. 2010;103:1783–1787. doi: 10.1038/sj.bjc.6605987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garraway LA, Lander ES. Lessons from the cancer genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 80.Piras V, et al. Enhancing apoptosis in TRAIL-resistant cancer cells using fundamental response rules. Scientific reports. 2011;1:144. doi: 10.1038/srep00144. [DOI] [PMC free article] [PubMed] [Google Scholar]