Abstract
Objective
To determine the effects of different hormonal levels on endometrial biochemical development during ovulation induction for assisted reproduction technology (ART) cycles.
Design
Prospective controlled study.
Setting
University center.
Patient(s)
Nine women during a natural cycle (control) and 9 oocyte donors (treated) during an ART cycle.
Intervention(s)
At the time consistent with day 3 embryo transfer (LH+5 in control, hCG+5 in treated), transvaginal ultrasound, endometrial biopsy, and blood sampling were performed. Real-time reverse-transcription polymerase chain reaction was used to measure mRNA levels for insulin receptor (InsR), type I IGF receptor (IGFRI), prolactin receptor (PRL-R), androgen receptor (AR), TSH receptor (TSHR), nuclear receptors for T3 and T4 (TRα1, TRα2, and TRβ1), iodothyronine deiodinase (DIO2), and 1,25-dihydroxyvitamin D3 receptor (VDR) in the endometrial tissue.
Main Outcome Measure(s)
Biochemical endometrial development.
Result(s)
IGFRI mRNA levels were 69% lower in treated patients than in control subjects, 0.12 ± 0.005 pg/μg RNA versus 0.39 ± 0.01 pg/μg RNA. TSHR mRNA was 57% lower, 2.6 ± 0.1 fg/μg RNA versus 6.0 ± 0.2 fg/μg RNA. TRα1 and TRα2 mRNA did not change, but TRβ1 mRNA levels were 63% higher. DIO2 mRNA was 63% lower, 1.2 ± 0.07 pg/μg RNA versus 3.2 ± 0.2 pg/μg RNA. InsR mRNA levels, despite being 68% lower in treated patients, did not reach significance, and PRL-R, AR, and VDR did not significantly change.
Conclusion(s)
Exposure of the endometrium to ovarian stimulation appears to influence insulin and thyroid hormone signaling pathways in the decidua at day 3 embryo transfer, whereas prolactin, androgen, and vitamin D pathways are uninfluenced. These findings echo the known delayed endometrial maturation during ovarian stimulation.
Keywords: Endometrium, development, decidua, ART, hormone receptors, GnRH-antagonist, real time RT-PCR
Endometrial decidualization represents a process of morphologic and biochemical changes that occur after ovulation and is mainly under the control of the sex hormones estrogen and progesterone (P). Endometrial response to sex steroids is modulated by a variety of substances which remain incompletely characterized. During ovulation induction for assisted reproduction technology (ART), decidualization of the endometrium is known to be impaired compared with natural cycles. In particular, endometrial development is delayed by 2–3 days, as demonstrated by biochemical and imaging studies (1, 2). We have previously reported the morphologic follicular pattern of the endometrium (trilaminar vs. homogeneous hyperechoic) during ovulation induction for ART, noting that it persisted longer than in natural cycles imaged during the same time period, which reflects delayed endometrial development (2). These results were consistent with our previous imaging studies on endometrial development during ART (3). The morphologic changes were concomitant with a significant increase in the expression of progesterone receptor B (PR-B), unchanged progesterone receptor A (PR-A), and slightly (nonsignificantly) increased estrogen receptors α and β (ER-α, ER-β) (2). These biochemical and morphologic endometrial changes occurred in the presence of elevated serum levels of both E2 and P and decreased levels of FSH and LH.
The androgen receptor (AR) is widely expressed in female reproductive tissues, including the endometrium (4). Although P is indispensable for the endometrial differentiation into decidua, a growing body of evidence indicates that AR and androgen signaling are also decisive in this process (5). Androgen signaling is an essential component of normal endometrial physiology, and its modification, such as in polycystic ovary syndrome (PCOS), primary ovarian insufficiency, or advanced maternal age, is associated with reproductive failure (5). Androgens might play a critical role in the decidualization process at the time of embryo implantation and trophoblast invasion by protecting from oxidative stress (6).
Insulin and insulin-like growth factors 1 and 2 (IGF1 and IGF2) act as endocrine, paracrine, and autocrine factors that regulate cellular activities such as proliferation, survival, and differentiation. Their actions are mediated by insulin receptor (InsR) and type I IGF receptor (IGFRI) and their hybrid forms (7). InsR-1 seems to be more prominent in muscle and fat, InsR-2 in liver and pancreatic cells, and InsR-3 and InsR-4 seem to have only marginal importance in insulin actions (8). In the endometrium, insulin and IGF1 promote their actions mostly in the proliferative period, whereas IGF2 promotes proliferation in the secretory phase (9) and its actions become more prominent during placentation and pregnancy, where it enhances the proteolytic activity of pregnancy-associated plasma protein A (10). InsR and IGFRI are up-regulated in the secretory phase of the natural cycle in what seems to be a result of P action (11). Activation of IGFRI causes cell proliferation, and its overexpression has been reported in hyper-plastic endometrium and endometrial carcinoma (12). Type II IGF receptor (IGFRII) is considered to be a scavenger receptor for IGF2 only, and it is unclear whether it is able to initiate a signaling cascade. Even if not always by a direct effect, estrogen and P are known to modulate insulin and IGF1 and IGF2, their binding proteins, and their receptors.
Human secretory-phase, but not proliferative-phase, endometrium has been shown to produce prolactin (PRL), which is virtually identical to pituitary-produced PRL (13). The production process is in part regulated by P (14). In fact, in the absence of P, the stromal cells in the decidua do not secrete PRL. However, PRL is not indispensable to promote a decidual reaction in the endometrium in vivo, because the principal hormone regulating this process is P. Prolactin exerts its paracrine and autocrine actions through the PRL receptor, PRL-R, which is structurally similar to growth hormone, leukemia-inhibiting factor, and leptin receptors (15). In the decidua it alternatively acts as a growth factor and an immunomodulator (16).
The endometrium has also been shown to be a site of extrathyroidal production of T3 and T4. In fact, TSH receptors (TSHR) and iodothyronine deiodinases (DIO2 and DIO3) have been shown in the endometrium of different mammals, in addition to the nuclear receptors for T3 and T4 (TRα1, TRα2, and TRβ1) (17, 18). DIO2 is the most abundant enzyme that converts T4, which is produced in the thyroid gland, to the more potent T3, whereas DIO3 inactivates T3 to T2 (19). In the presence of T3, thyroid receptors bind to response elements in the promoters of target genes and form coactivator complexes to activate transcription. The effects of this receptor-ligand binding are complex, because they are organ, tissue, and cell specific. Regulation of synthesis and activation-deactivation processes of the endometrial thyroid signaling pathway is regulated by pituitary TSH and seems also to be regulated locally by P (18, 20). Thyroid production of T4 and T3 is up-regulated by E2, which also increases production of thyroid-binding globulin (TBG), and by hCG, through LH receptor–mediated increased TRH production in the hypothalamus (21). This endocrine pathway has been described in early pregnancy, and the effects of the increased availability of thyroid hormones have not been clarified. Thyroid production of T3 and T4 contributes to the availability of thyroid hormones in the endometrium and adds to the complexity of endometrial development.
The active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3), is an essential regulator of mineral homeostasis and has a modulatory role in female reproduction and the immune system (22, 23). It was demonstrated that 1,25-(OH)2D3 can be synthesized in the decidua and placenta by action of the CYP27B1 enzyme (24). 1,25-(OH)2D3 has multiple functions which are regulated through its receptor VDR, which has been isolated in endometria and decidua (25). It has been hypothesized that 1,25-(OH)2D3 supports successful implantation of the embryo by attenuating decidual T-cell function (26).
In the present study, we sought to investigate whether the high serum hormonal levels and the delayed endometrial maturation in ART cycles would modulate insulin, PRL, thyroid, and vitamin D endometrial signaling pathways in the secretory period. Specifically, we evaluated InsR, IGFRI, PRL-R, AR, TSHR, TRα1, TRα2, TRβ1, DIO2, and VDR mRNA levels in the endometrium of women during natural and ART cycles.
Materials and Methods
This study represents further analysis of endometrial biopsy samples obtained during a previous study from our group (2). The conduct of the previous study was approved by the Wayne State University Human Investigation Committee. Each of the subjects gave written informed consent to participate in the study. The study protocol has been published previously and is briefly reviewed here. Eleven normal volunteers (all had conceived once before enrollment) and 11 oocyte donors underwent transvaginal ultrasonography, endometrial sampling using a flexible pipelle, and blood sampling on day 3 after ovulation (LH+5 for natural cycles, hCG+5 for ART cycles). Endometrial tissue could not be obtained from two volunteers and two oocyte donors, who were excluded from the analyses. Ultrasound confirmed ovulation and the appearance of a corpus luteum. The oocyte donors underwent ovarian stimulation with gonadotropins and ganirelix acetate for pituitary down-regulation (Antagon; Organon). Gonadotropins were administered from stimulation day 1 until the day of the hCG trigger, following a step-up protocol. Ganirelix acetate (0.25 mg daily) was added beginning the day when at least one follicle reached 14 mm in diameter and continued until, and including, the day of hCG administration. hCG was administered when at least two follicles were >18 mm in diameter. Oocyte retrieval was performed 35–36 hours after hCG administration.
Endometrial tissue samples were snap frozen in liquid nitrogen within 15 minutes from the biopsy procedure and stored at −72°C. Total RNA was extracted from the tissues with the RNeasy mini-kit (Qiagen) per the manufacturer's protocol. RNA quality was determined using the Nanodrop 2000i (Thermo Scientific). RNA used for this study had an A260/A280 of ∼2:1, indicating its integrity. A 20-μL cDNA reaction was then prepared with the use of the Quantitect Reverse Transcription Kit (Qiagen). We used real-time reverse transcription polymerase chain reaction (real-time RT-PCR) to measure mRNA levels for InsR (all subtypes), IGFRI, PRL-R, AR, TSHR, TRα1, TRα2, TRβ1, DIO2, and VDR in the endometrial tissue samples.
Optimal oligonucleotide primer pairs for real-time RT-PCR amplification of reverse-transcribed cDNA were selected with the aid of the software program Beacon Designer (Premier Biosoft). Human oligonucleotide primers, which amplify variable portions of the protein coding regions, were used. Sequences of the oligonucleotides used for amplification of the receptors are presented in Table 1.
Table 1.
Sequences of the human hormone receptors primers studied.
| Locus | Sense (5′-3′) | Antisense (3′-5′) |
|---|---|---|
| β-Actin | GCATTGTTACAGGAAGTC | TTACATAATTTACACGAAAGC |
| AR | AGATGGGCTTGACTTTCCCAGAAAG | ATGGCTGTCATTCAGTACTCCTGGA |
| InsR | ATGCGTTATTCCTGAGTG | GAGGTTGCCATTATGACT |
| IGFR1 | CAACAGCAGTAAGAAGAA | GATGAATATCTGAACCGTAA |
| DIO2 | AGTAACCATATCACCTCTC | TGTATCAGTTCCTTCTCAA |
| TRα1 | GATGGAATTGAAGTGAAT | AGGTAACTAGGGATATAC |
| TRα2 | CCAGGCAGAAATAGTTGT | CTTGGGAAACAGACTCAT |
| TRβ1 | ACATAGTATTACCTCACA | AAGATACAACCTGGATAA |
| TSHR | AGATGTCTATGAACTGATTGA | ACCATTGTGAGTAGTGTAG |
| PRL-R | CATTCCAGAAGTACCCTCAAAGAC | TGTGAATCCCTGCGTAGGCA |
| VDR | TGGCAGATTTAGTGAAAG | TACATTGGTTGACTTGAC |
Note: AR = androgen receptor; DIO2 = iodothyronine deiodinase; IGFRI = type I IGF receptor; InsR = insulin receptor; PRL-R = prolactin receptor; TRα1, TRα2, TRβ1 = nuclear receptors for T3 and T4; TSHR = TSH receptor; VDR = 1,25-dihydroxyvitamin D3 receptor.
Real-time RT-PCR was performed with the Quantitect Sybr Green RT-PCR kit (Qiagen) and a Cepheid 1.2f Detection System. Each reaction was 25 μL, consisting of 12.5 μL 2× Quantitect Sybr Green RT-PCR master mix, 1 μL cDNA template, and 0.2 μmol/L each target-specific primer that was designed to amplify a part of the gene of interest. To quantify each target transcript, a standard curve was constructed with the use of a tenfold dilution series of β-actin standard plasmid (Invitrogen). We used β-actin as housekeeping gene in previous studies on peritoneal fibroblasts (2, 27). Other studies have reported on the stability of β-actin in endometrial samples (28). Real-time RT-PCR measures PCR amplification as it occurs, thus allowing precise quantification of gene expression. The PCR reaction conditions for InsR, IGFRI, PRL-R, AR, TSHR, TRα1, TRα2, TRβ1, DIO2, and VDR, were programmed for each primer as follows: an initial cycle was performed at 95°C for 14, 15, 18.3, and 18.3 minutes, followed by 45 cycles of denaturation at 95°C for 15 seconds, annealing for 30 seconds at 50°C, 53°C, 58°C, and 60°C, and then product synthesis at 72°C for 30 seconds. A control containing all of the reaction components except for the template was included in all experiments. The amount of mRNA was then normalized to β-actin. This process was repeated three times for each receptor in each endometrial sample. The three measurements were then averaged into a single value for the analysis. There was no effect of any of the hormones studied on the expression of the housekeeping gene, β-actin (data not shown).
Serum levels of E2, P, PRL, TSH, FSH, and LH evaluated for all study patients were obtained from our database as previously reported (2).
Mann-Whitney U test and Spearman correlations (SPSS v21.0 statistical package for Windows) were used, with 95% confidence intervals (CIs), to define internal estimates associated with each probability.
Results
Eighteen RNA samples were available for the endometrial receptors and the serum hormone analyses. Table 2 summarizes the ovarian stimulation characteristics of the patients who underwent ART cycles for oocyte donation. The duration of ovarian stimulation was 10.0 ± 1.0 days (95% CI 9–10).
Table 2.
Ovarian simulation characteristics of the oocyte donors.
| Parameter | Mean±SD | 95% CI |
|---|---|---|
| Total FSH dose (IU) | 1,732 ± 790 | 1,178–2,285 |
| Total LH-like dose (IU) | 595 ± 427 | 297–894 |
| Duration of ovarian stimulation (d) | 10 ± 1.0 | 9.1–10.3 |
| Duration of GnRH-antagonist use (d) | 4.3 ± 1.2 | 3.5–5.2 |
| Start day of GnRH-antagonist | 6 ± 1 | 6–7 |
| No. oocytes retrieved | 18 ± 4 | 14–21 |
Note: CI = confidence interval.
Table 3 compares the characteristics of the two groups of women at the time of endometrial sampling (LH+5 and hCG+5). In both groups, serum levels of FSH, LH, and E2 were significantly different on the day of endometrial sampling compared with cycle day 3. FSH and LH levels showed changes opposite of E2 levels.
Table 3.
Characteristics (mean ± SD) of the two groups of women, normal volunteers (control group) and oocyte donors (treated group), at the time of endometrial sampling (LH+5 and hCG+5).
| Parameter | Normal volunteers | Oocyte donors | 95% CI of the difference | P valuea |
|---|---|---|---|---|
| Age (y) | 31.8.2 ± 5.8 | 26.2 ± 4.5 | 2.9–8.2 | <.05 |
| Cycle day at endometrial sampling | 18.0 ± 1.0 | 15.0 ± 1.0 | 2.1–3.7 | <.001 |
| Serum FSH (mIU/mL) | 4.2 ± 1.8 | 1.6 ± 0.6 | −3.4 to −1.7 | <.005 |
| Serum LH (mIU/mL) | 3.1 ± 2.5 | 0.4 ± 0.5 | −3.7 to −1.6 | <.02 |
| Serum estradiol (pg/mL) | 99.1 ± 33.2 | 1,801 ± 856 | 1,235–2,169 | <.001 |
| Serum progesterone (ng/mL) | 8.5 ± 4.7 | 107.5 ± 42.2 | 72.9–125.2 | <.001 |
| Serum prolactin (ng/mL) | 12.2 7.6 | 19.9 5.5 | 4.0–11.1 | ns |
| Serum testosterone (ng/mL) | 47.6 ± 25.3 | 90.7 ± 38.8 | 27.1–59.1 | <.03 |
| Serum TSH (nIU/L) | 1.3 ± 0.5 | 2.4 ± 1.7 | 0.6–1.6 | ns |
Note: CI = confidence interval; ns = not significant.
Mann-Whitney U test.
Serum testosterone levels were significantly increased in treated patients, but AR mRNA levels showed a nonsignificant increase from 27.7 ± 12.1 fg/μg RNA in control subjects to 33.2 ± 22.8 fg/μg RNA in treated patients.
Serum PRL remained stable through ovarian stimulation, and PRL-R mRNA levels were nonsignificantly lower in control subjects, 16.1 ± 5.4 fgμg RNA, than in treated patients, 12.0 ± 2.4 fg/μg RNA.
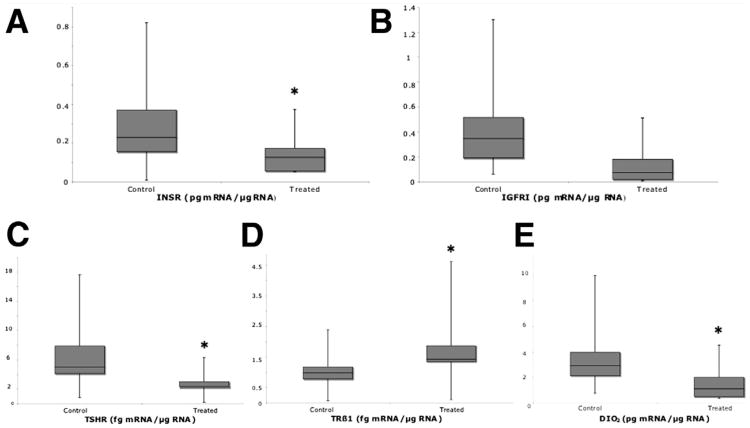
IGFRI mRNA levels changed 69% from 0.39 ± 0.01 pg/μg RNA in control subjects to 0.12 ± 0.005 pg/μg RNA in treated patients (P=.04) (Figs. 1A and B). Despite InsR being 59% lower in treated patients, 0.11 ± 0.005 pg/μg RNA, than in control subjects, 0.27 ± 0.02 pg/μg RNA, the difference was not significant.
Figure 1.
(A) IGFRI, (B) InsR, (C) TSHR, (D) TRβ1, and (E) DIO2 mRNA levels in human endometrium at the time of hypothetical embryo transfer in ovarian stimulation cycles (1, treated) and in natural unstimulated menstrual cycles (2, control). *P<.05 (Mann-Whitney U test).
TSHR mRNA levels changed 57% from 6.0 ± 0.2 fg/μg RNA in control subjects to 2.6 ± 0.1 fg/μg RNA in treated patients (P=.005; Figs. 1C and D). TRα1 and TRα2 mRNA levels did not change, but TRβ1 levels were 63% higher, from 0.97 ± 0.08 fg/μg RNA in control subjects to 1.7 ± 0.09 fg/μg RNA in treated patients (P=.006; Fig. 1E). DIO2 levels changed 63% from 3.2 ± 0.2 pg/μg RNA in control subjects to 1.2 ± 0.07 pg/μg RNA in treated patients (P=.02) (Fig. 2C). These variations occurred despite unchanged serum TSH levels.
VDR mRNA levels remained stable through ovarian stimulation: 65 ± 2.8 fg/μg RNA in control subjects and 62 ± 2.9 fg/μg RNA in treated patients (ns).
We used Spearman correlations to find a statistical relationship between the significantly different endometrial receptors and the serum levels of the hormones studied. The variable InsR was included in the correlation tests because of its remarkable lower levels in treated patients. Across all patients, serum E2 was the hormone correlated with all hormone receptors (InsR: r = −0.80; P=.01; IGFRI: r = −0.83; P=.003; TSHR: r = −0.71; P=.02; TRβ1: r = 0.88; P=.004) and deiodinase changes (DIO2: r = −0.86; P=.001). Serum P was inversely correlated with TSHR (r = −0.67;P=.03) and DIO2 (r= 0.77;P= .006), and testosterone was correlated directly with TRβ1 (r = 0.83; P=.04) and inversely with IGFRI (r = −0.76; P=.03). Serum FSH was correlated with TSHR (r = 0.77; P=.009) and LH inversely correlated with TRβ1 (r = −0.77; P= .03). In the treated group considered alone, we found no significant relationship of serum E2, P, and FSH with any of the receptors. However, among the mRNA levels, expression of DIO2 showed a positive relationship with InsR (r = 0.79; P= .001) and IGFRI (r = 0.88; P< .001), and TSHR showed an indirect relationship with TRβ1 (r = −0.9; P=.007).
Discussion
Exposure of the endometrium to ovarian stimulation with gonadotropins and the GnRH antagonist ganirelix acetate caused decidual down-regulation of the insulin and thyroid hormone signaling pathways, except for TRβ1, and PRL, androgen, and vitamin D pathways remained unchanged. In addition, these changes were correlated with the increase in serum E2 and P. Our results should be interpreted in line with our previous findings of a delayed endometrial development during ART cycles as reflected by persistence of a trilaminar appearance of the endometrium on ultrasound and the up-regulation of PR-B and its stimulatory effects.
The lower mRNA levels of IGFRI and InsR in the present study was similar to what occurred in opposite conditions of proliferative phase endometria of women with PCOS (29). It has been postulated that, in patients with PCOS, hyperinsulinemia would cause insulin binding to IGFRI, in addition to InsR, and this would be responsible for cell proliferation and its outcomes, endometrial hyperplasia, and carcinoma. We speculate that the lower mRNA levels of IGFRI (activated by IGF1, IGF2, and, with less affinity, insulin), and InsR (which can be activated by either insulin or IGF2), being opposite to what occurs in the secretory phase in normal endometrium (11), would impair differentiation and decidualization during ART cycles.
The role of PRL in the decidua and placenta has not been completely elucidated; however, it has been shown to be of foremost importance to regulate initial placentation (16). Similarly to PRL-R, AR mRNA levels also were unchanged. AR mRNA levels are expected to decline during the early secretory phase of the cycle but remain expressed in the decidua (30): P has been shown to play a pivotal role in AR expression regulation (31). Our results regarding endometrial PRL-R and AR during ovulation induction would, again, show a marginal influence by E2 or P fluctuations, because there was no change in expression despite the high E2 and P serum levels (Table 3) (2).
During the early decidual differentiation of the endometrium in women undergoing ovulation induction, we found that TSHR and DIO2 mRNA levels were lower, TRβ1 levels were higher, and TRα1 and TRα2 mRNA levels remained unchanged compared with control subjects. Although the individual role of TRα and TRβ in the endometrium remains to be elucidated, TRα seems to be more abundant in the secretory phase and TRβ in the proliferative phase (17). From our results, we can speculate that ovulation induction induces a down-regulation of thyroid hormone endometrial production by decreasing TSHR and by increasing the nuclear receptor TRβ1, which is normally expressed during the proliferative period (17). DIO2, the most abundant enzyme that converts T4 to T3, was lower during stimulated than during natural cycles, further indicating that ovarian stimulation down-regulates endometrial thyroid hormone production. The clinical implications of our findings remain to be clarified; however, they underscore the importance of having normal serum levels of T3 and T4 for women undergoing ovulation induction for ART. With the reduced ability to produce thyroid hormones in the endometrium and the increased request (up-regulation of TRβ1), it seems important to at least maintain systemic euthyroidism. In addition, we found the changes among TSHR, DIO2, IGFRI, and InsR to be directly correlated, and all of them inversely correlated with serum estrogen.
VDR expression did not significantly change in the secretory endometrium. This could reflect the delayed endometrial maturation in ART cycles or the physiologic role of vitamin D as it progressively becomes more prominent in the decidua and placenta.
The present study carries some limitations: The number of samples was calculated based on P receptor expression variation; a specific impact on endometrial development of individual medications could not be assessed; and assessment of endometrial development beyond LH+5 was not performed, thus precluding insight on the day of implantation. In addition, normal volunteers were significantly older than the oocyte donors: This could have influenced our results because of a physiologic earlier LH surge and ovulation (however, oocyte donors still showed a significantly earlier hCG+5 date). Nonetheless, important clinical implications of our results arise from the positive and negative relationship of the endometrial receptors expression with serum hormonal levels and within the various receptors expressions. We showed that endometrial receptor changes during ART are concomitant and might, at least in part, be caused by the increase in serum E2 (IGFRI, InsR, DIO2, TRβ1, and TSHR) and P (DIO2 and TSHR).
In conclusion, these findings further characterized the suboptimal endometrial development achieved in ART cycles, where regulation of specific endometrial receptors differs from the natural cycle. These findings substantiate a delayed maturation of the endometrium during ovulation induction which could lead to the observed decreased implantation rates in ART with fresh nondonor cycles. This delay was also suggested by genomic studies that linked ART to suboptimal endometrial receptivity (1, 32, 33). The clinical implications of the present study would include titration of ovarian stimulation not only to yield the optimal number of harvested oocytes, but also to achieve serum hormonal levels that would promote an optimal endometrial development and better pregnancy outcomes with fresh cycles. In addition, we propose cancellation of fresh embryo transfer and freezing or vitrification of either eggs or embryos and postponing the transfer to more suitable endometrial development such as reached during natural cycles or controlled endometrial maturation. This model was initially introduced in the 1980s and was recently tested and confirmed to be succesful (34).
Acknowledgments
Supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant no. K 12 HD-01254-11), National Institutes of Health, Bethesda, Maryland, and in part by an institutional grant from the University of Tennessee Health Science Center, Memphis, Tennessee.
Footnotes
L.D. has nothing to disclose. R.A.U. has nothing to disclose. N.M.F. has nothing to disclose. M.P.D. has nothing to disclose. G.M.S. has nothing to disclose.
References
- 1.Horcajadas JA, Mínguez P, Dopazo J, Esteban FJ, Domínguez F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93:4500–10. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 2.Detti L, Saed GM, Fletcher NM, Kruger ML, Brossoit M, Diamond MP. Endometrial morphology and modulation of hormone receptors during ovarian stimulation for assisted reproductive technology cycles. Fertil Steril. 2011;95:1037–41. doi: 10.1016/j.fertnstert.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detti L, Yelian FD, Kruger ML, Diamond MP, Puscheck EE. Endometrial thickness dynamics and morphologic characteristics during pituitary downregulation with antagonists in assisted reproductive technology cycles. J Ultrasound Med. 2008;27:1591–6. doi: 10.7863/jum.2008.27.11.1591. [DOI] [PubMed] [Google Scholar]
- 4.Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum Reprod. 1992;7:1461–6. doi: 10.1093/oxfordjournals.humrep.a137595. [DOI] [PubMed] [Google Scholar]
- 5.Cloke B, Christian M. The role of androgens and the androgen receptor in cycling endometrium. Mol Cell Endocrinol. 2012;358:166–75. doi: 10.1016/j.mce.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Kajihara T, Tochigi H, Prechapanich J, Uchino S, Itakura A, Brosens JJ, et al. Androgen signaling in decidualizing human endometrial stromal cells enhances resistance to oxidative stress. Fertil Steril. 2012;97:185–91. doi: 10.1016/j.fertnstert.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Siddle K, Urso B, Niesler CA, Cope DL, Molina L, Surinya KH, et al. Specificity in ligand binding and intracellular signaling by insulin and insulin-like growth factor receptors. Biochem Soc Trans. 2001;29:513–25. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- 8.Patel N, Huang C, Klip A. Cellular location of insulin-triggered signals and implications for glucose uptake. Pflugers Arch. 2006;451:499–510. doi: 10.1007/s00424-005-1475-6. [DOI] [PubMed] [Google Scholar]
- 9.Ganeff C, Chatel G, Munaut C, Frankenne F, Foidart JM, Winkler R. The IGF system in in-vitro human decidualization. Mol Hum Reprod. 2009;15:27–38. doi: 10.1093/molehr/gan073. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, et al. The insulin-like growth factor IGF system and human decidualization (IGF)–dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A. 1999;96:3149–53. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 12.McCampbell AS, Broaddus RR, Loose DS, Davies PJ. Overexpression of the insulin-like growth factor I receptor and activation of the AKT pathway in hyperplastic endometrium. Clin Cancer Res. 2006;12:6373–8. doi: 10.1158/1078-0432.CCR-06-0912. [DOI] [PubMed] [Google Scholar]
- 13.Clements J, Whitfeld P, Cooke N, Healy D, Matheson B, Shine J, et al. Expression of the prolactin gene in human decidua-chorion. Endocrinology. 1983;112:1133–4. doi: 10.1210/endo-112-3-1133. [DOI] [PubMed] [Google Scholar]
- 14.Wang JD, Zhu JB, Shi WL, Zhu PD. Immunocytochemical colocalization of progesterone receptor and prolactin in individual stromal cells of human decidua. J Clin Endocrinol Metab. 1994;79:293–7. doi: 10.1210/jcem.79.1.8027244. [DOI] [PubMed] [Google Scholar]
- 15.Taga T, Kishimoto T. Cytokine receptors and signal transduction. FASEB J. 1992;6:3387–96. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- 16.Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr Rev. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- 17.Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril. 2011;95:230–7. doi: 10.1016/j.fertnstert.2010.06.079. [DOI] [PubMed] [Google Scholar]
- 18.Galton VA, Martinez E, Hernandez A, St Germain EA, Bates JM, St Germain DL. The type 2 iodothyronine deiodinase is expressed in the rat uterus and induced during pregnancy. Endocrinology. 2001;142:2123–8. doi: 10.1210/endo.142.5.8169. [DOI] [PubMed] [Google Scholar]
- 19.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 20.Catalano RD, Critchley HO, Heikinheimo O, Baird DT, Hapangama D, Sherwin JR, et al. Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in human endometrium. Mol Hum Reprod. 2007;13:641–54. doi: 10.1093/molehr/gam021. [DOI] [PubMed] [Google Scholar]
- 21.Fritz MA, Speroff L. Reproduction and the thyroid. In: Fritz MA, Speroff L, editors. Clinical gynecologic endocrinology and infertility. 8th. Philadelphia: Lippincott Williams & Wilkins; pp. 885–99. [Google Scholar]
- 22.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004;26:21–8. doi: 10.1002/bies.10368. [DOI] [PubMed] [Google Scholar]
- 23.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 24.Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1α,25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. 1979;281:317–9. doi: 10.1038/281317a0. [DOI] [PubMed] [Google Scholar]
- 25.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, et al. Targeted ablation of the 25-hydroxyvitamin D 1ahydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebut-Bonneton C, Demignon J. Effects of 1,25-dihydroxyvitamin D3 on in vitro lymphocyte reactions: arguments for a role at the maternofetal interface. Gynecol Obstet Invest. 1991;32:134–8. doi: 10.1159/000293014. [DOI] [PubMed] [Google Scholar]
- 27.Detti L, Saed G, Jiang Z, Diamond MP. Differential expression of estrogen, progesterone, androgen, and prolactin receptors in in vitro human fibroblasts isolated from normal peritoneum and adhesions. J Assist Reprod Genet. 2008;25:245–50. doi: 10.1007/s10815-008-9230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bersinger NA, Wunder DM, Birkhäuser MH, Mueller MD. Gene expression in cultured endometrium from women with different outcomes following IVF. Mol Hum Reprod. 2008;14:475–84. doi: 10.1093/molehr/gan036. [DOI] [PubMed] [Google Scholar]
- 29.Fornes R, Ormazabal P, Rosas C, Gabler F, Vantman D, Romero C, et al. Changes in the expression of insulin signaling pathway molecules in endometria from polycystic ovary syndrome women with or without hyperinsulinemia. Mol Med. 2010;16:129–36. doi: 10.2119/molmed.2009.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mertens HJ, Heineman MJ, Theunissen PH, de Jong FH, Evers JL. Androgen, estrogen and progesterone receptor expression in the human uterus during the menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 2001;98:58–65. doi: 10.1016/s0301-2115(00)00554-6. [DOI] [PubMed] [Google Scholar]
- 31.Slayden OD, Nayak NR, Burton KA, Chwalisz K, Cameron ST, Critchley HO, et al. Progesterone antagonists increase androgen receptor expression in the rhesus macaque and human endometrium. J Clin Endocrinol Metab. 2001;86:2668–79. doi: 10.1210/jcem.86.6.7606. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Lee KF, Ng EH, Yeung WS, Ho PC. Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Ferti Steril. 2008;90:2152–64. doi: 10.1016/j.fertnstert.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Haouzi D, Assou S, Mahmoud K, Tondeur S, Rème T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24:1436–45. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozenthawed embryo transfer cycles. Fertil Steril. 2013;99:389–92. doi: 10.1016/j.fertnstert.2012.09.044. [DOI] [PubMed] [Google Scholar]