Abstract
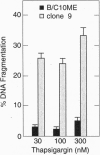
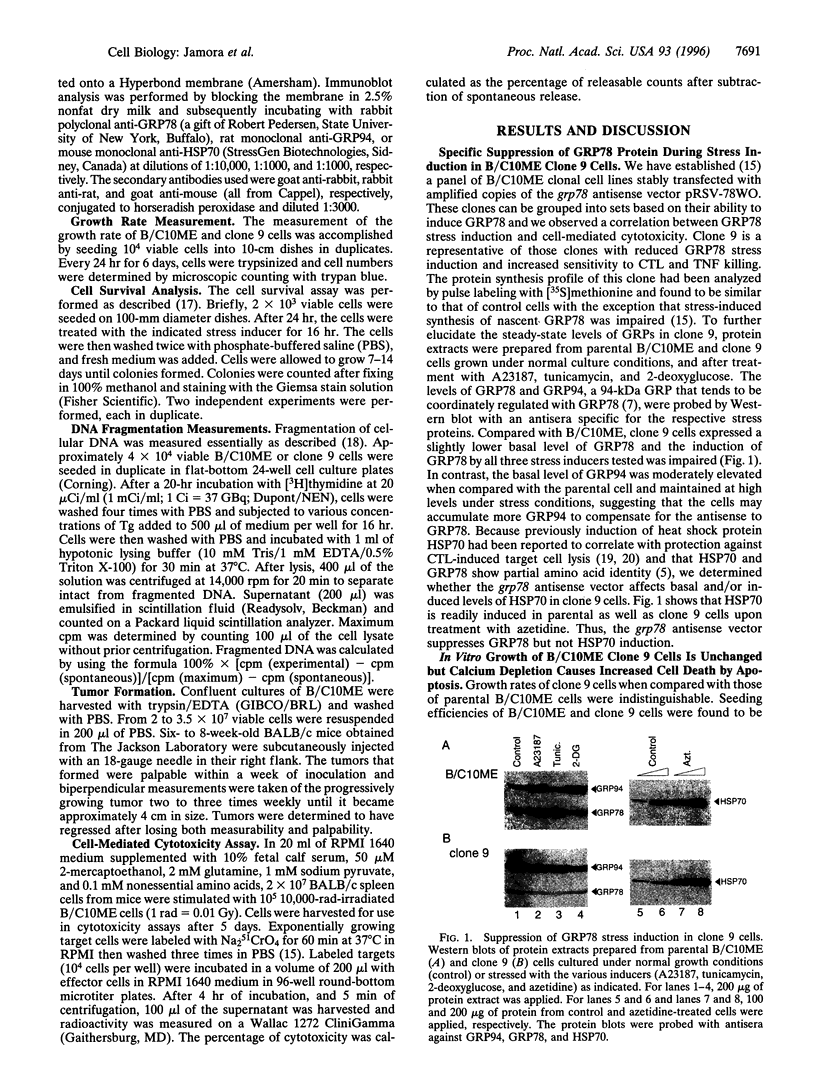
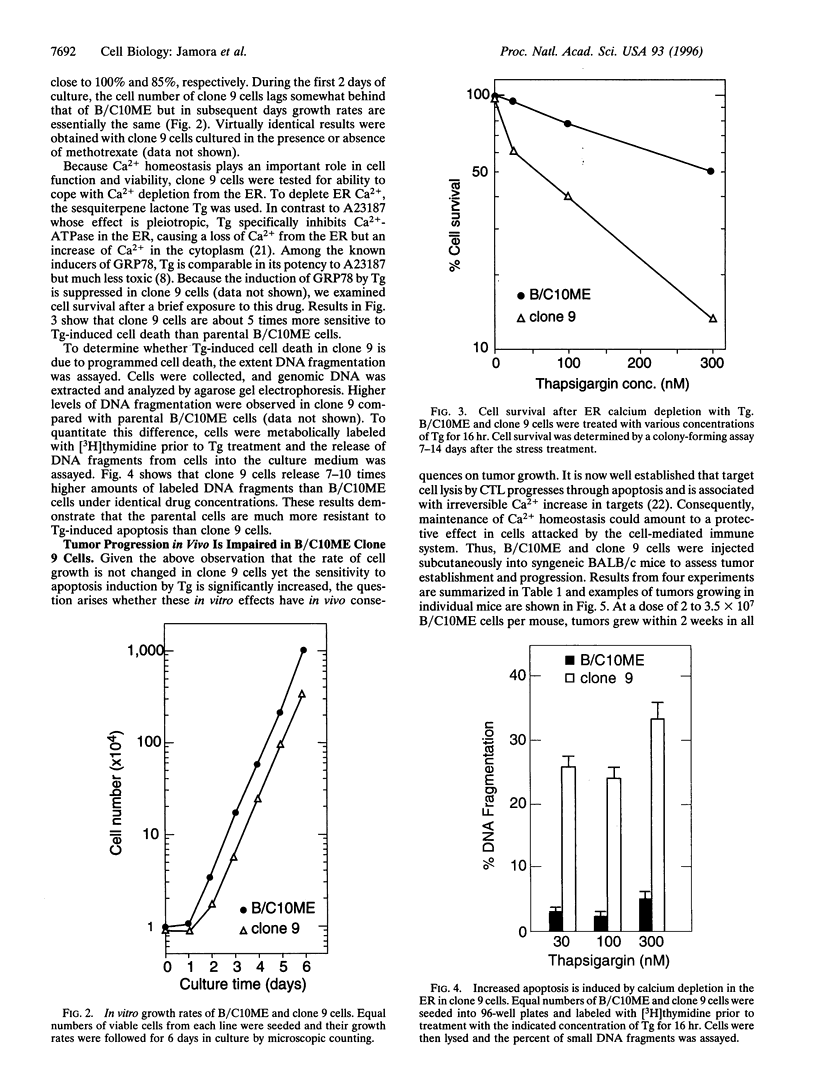
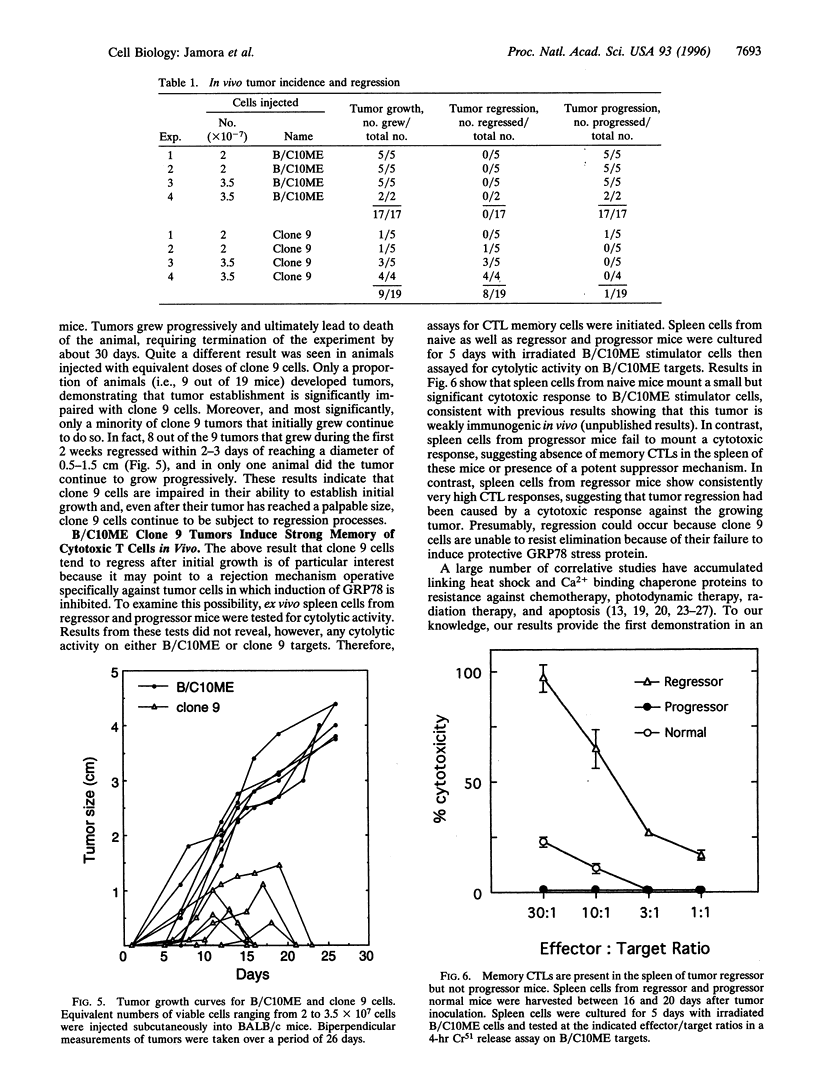
Stress protein GRP78/BiP is highly induced in progressively growing tumors and has recently been shown to exert a protective role against lysis by cytotoxic T cells and tumor necrosis factor in vitro. This raises the question whether the in vitro observed protective function of GRP78/BiP translates into the in vivo situation in which tumors grow progressively, killing the host. Herein we report that molecular inhibition of GRP78/BiP induction in the fibrosarcoma B/C10ME, while not affecting in vitro cell proliferation, causes a dramatic increase in apoptotic cell death upon Ca2+ depletion of the endoplasmic reticulum. When B/C10ME cells incapable of inducing GRP78/BiP are injected into mice, tumors are initially formed that, however, regress presumably due to a cytotoxic T-cell response demonstrable by a strong in vitro response to the tumor with spleen cells of regressor mice. Since sensitivity to apoptosis is key to tumor rejection, these results may point to new approaches to the therapy of cancer via regulation of stress protein GRP78/BiP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cai J. W., Henderson B. W., Shen J. W., Subjeck J. R. Induction of glucose regulated proteins during growth of a murine tumor. J Cell Physiol. 1993 Feb;154(2):229–237. doi: 10.1002/jcp.1041540204. [DOI] [PubMed] [Google Scholar]
- Dowd D. R., MacDonald P. N., Komm B. S., Haussler M. R., Miesfeld R. L. Stable expression of the calbindin-D28K complementary DNA interferes with the apoptotic pathway in lymphocytes. Mol Endocrinol. 1992 Nov;6(11):1843–1848. doi: 10.1210/mend.6.11.1336124. [DOI] [PubMed] [Google Scholar]
- Flieger D., Riethmüller G., Ziegler-Heitbrock H. W. Zn++ inhibits both tumor necrosis factor-mediated DNA fragmentation and cytolysis. Int J Cancer. 1989 Aug 15;44(2):315–319. doi: 10.1002/ijc.2910440221. [DOI] [PubMed] [Google Scholar]
- Gazit G., Kane S. E., Nichols P., Lee A. S. Use of the stress-inducible grp78/BiP promoter in targeting high level gene expression in fibrosarcoma in vivo. Cancer Res. 1995 Apr 15;55(8):1660–1663. [PubMed] [Google Scholar]
- Gomer C. J., Ferrario A., Rucker N., Wong S., Lee A. S. Glucose regulated protein induction and cellular resistance to oxidative stress mediated by porphyrin photosensitization. Cancer Res. 1991 Dec 15;51(24):6574–6579. [PubMed] [Google Scholar]
- Hendershot L. M., Ting J., Lee A. S. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol Cell Biol. 1988 Oct;8(10):4250–4256. doi: 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Jättelä M., Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med. 1993 Jan 1;177(1):231–236. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koong A. C., Chen E. Y., Lee A. S., Brown J. M., Giaccia A. J. Increased cytotoxicity of chronic hypoxic cells by molecular inhibition of GRP78 induction. Int J Radiat Oncol Biol Phys. 1994 Feb 1;28(3):661–666. doi: 10.1016/0360-3016(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Lee A. S. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992 Apr;4(2):267–273. doi: 10.1016/0955-0674(92)90042-b. [DOI] [PubMed] [Google Scholar]
- Li L. J., Li X., Ferrario A., Rucker N., Liu E. S., Wong S., Gomer C. J., Lee A. S. Establishment of a Chinese hamster ovary cell line that expresses grp78 antisense transcripts and suppresses A23187 induction of both GRP78 and GRP94. J Cell Physiol. 1992 Dec;153(3):575–582. doi: 10.1002/jcp.1041530319. [DOI] [PubMed] [Google Scholar]
- Li W. W., Alexandre S., Cao X., Lee A. S. Transactivation of the grp78 promoter by Ca2+ depletion. A comparative analysis with A23187 and the endoplasmic reticulum Ca(2+)-ATPase inhibitor thapsigargin. J Biol Chem. 1993 Jun 5;268(16):12003–12009. [PubMed] [Google Scholar]
- Li X. A., Lee A. S. Competitive inhibition of a set of endoplasmic reticulum protein genes (GRP78, GRP94, and ERp72) retards cell growth and lowers viability after ionophore treatment. Mol Cell Biol. 1991 Jul;11(7):3446–3453. doi: 10.1128/mcb.11.7.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little E., Ramakrishnan M., Roy B., Gazit G., Lee A. S. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1994;4(1):1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993 Mar 5;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987 Aug;6(8):2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsamooj P., Notario V., Dritschilo A. Enhanced expression of calreticulin in the nucleus of radioresistant squamous carcinoma cells in response to ionizing radiation. Cancer Res. 1995 Jul 15;55(14):3016–3021. [PubMed] [Google Scholar]
- Rozhin J., Robinson D., Stevens M. A., Lah T. T., Honn K. V., Ryan R. E., Sloane B. F. Properties of a plasma membrane-associated cathepsin B-like cysteine proteinase in metastatic B16 melanoma variants. Cancer Res. 1987 Dec 15;47(24 Pt 1):6620–6628. [PubMed] [Google Scholar]
- Shen J., Hughes C., Chao C., Cai J., Bartels C., Gessner T., Subjeck J. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3278–3282. doi: 10.1073/pnas.84.10.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara S., Nowicki M., Xie S., Song H. J., Dennert G. Effects of stress on lysability of tumor targets by cytotoxic T cells and tumor necrosis factor. J Immunol. 1990 Sep 15;145(6):1991–1998. [PubMed] [Google Scholar]
- Sugawara S., Takeda K., Lee A., Dennert G. Suppression of stress protein GRP78 induction in tumor B/C10ME eliminates resistance to cell mediated cytotoxicity. Cancer Res. 1993 Dec 15;53(24):6001–6005. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulatowski L. M., Lam M., Vanderburg G., Stallcup M. R., Distelhorst C. W. Relationship between defective mouse mammary tumor virus envelope glycoprotein synthesis and GRP78 synthesis in glucocorticoid-treated mouse lymphoma cells. Evidence for translational control of GRP78 synthesis. J Biol Chem. 1993 Apr 5;268(10):7482–7488. [PubMed] [Google Scholar]
- Wei Y. Q., Zhao X., Kariya Y., Teshigawara K., Uchida A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol Immunother. 1995 Feb;40(2):73–78. doi: 10.1007/BF01520287. [DOI] [PMC free article] [PubMed] [Google Scholar]