Abstract
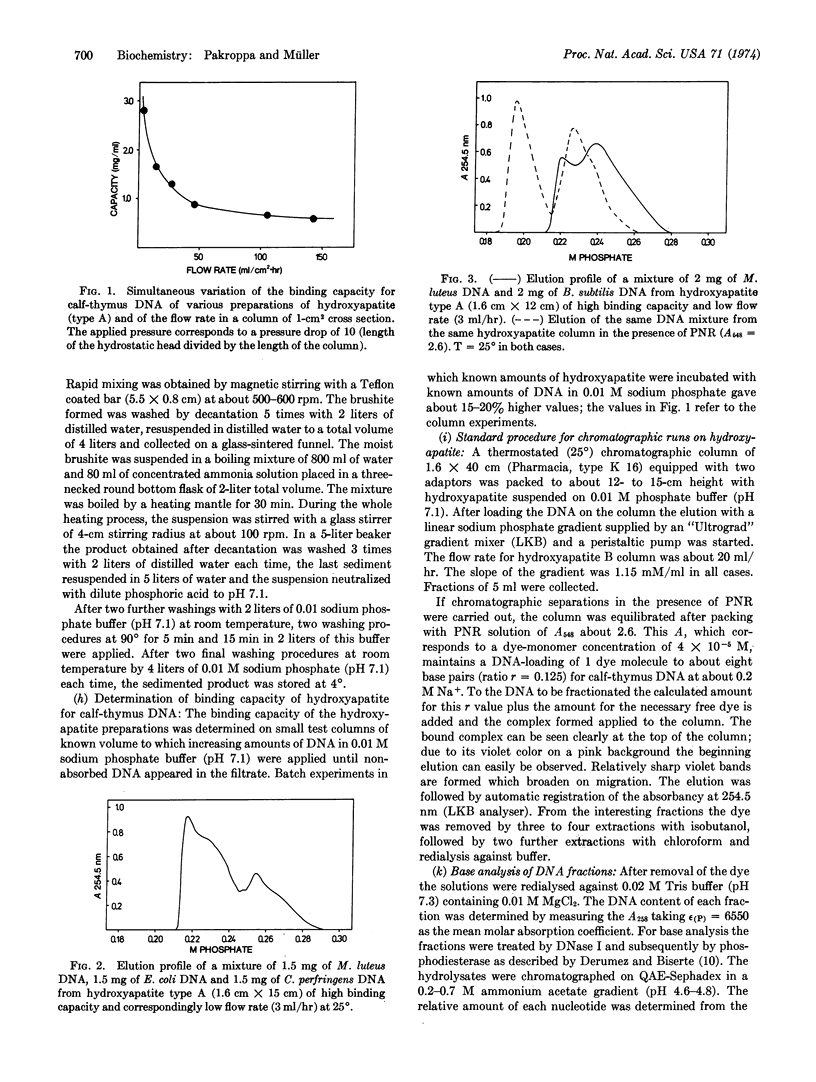
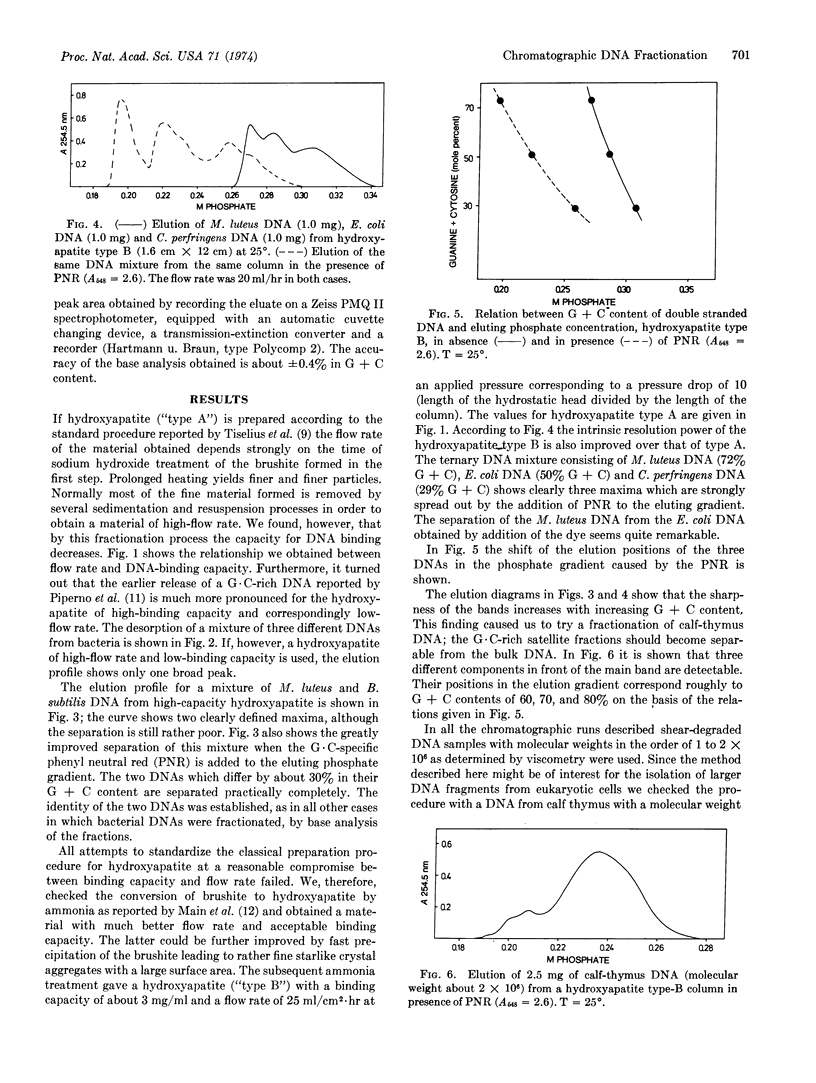
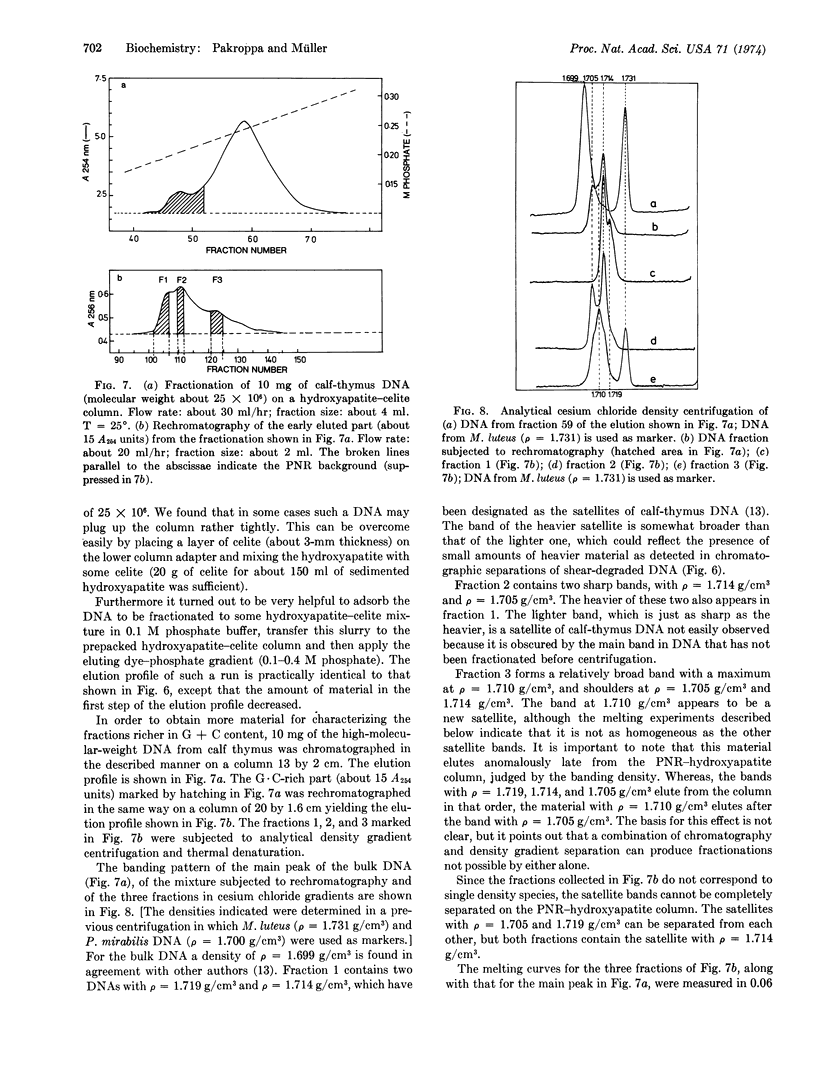
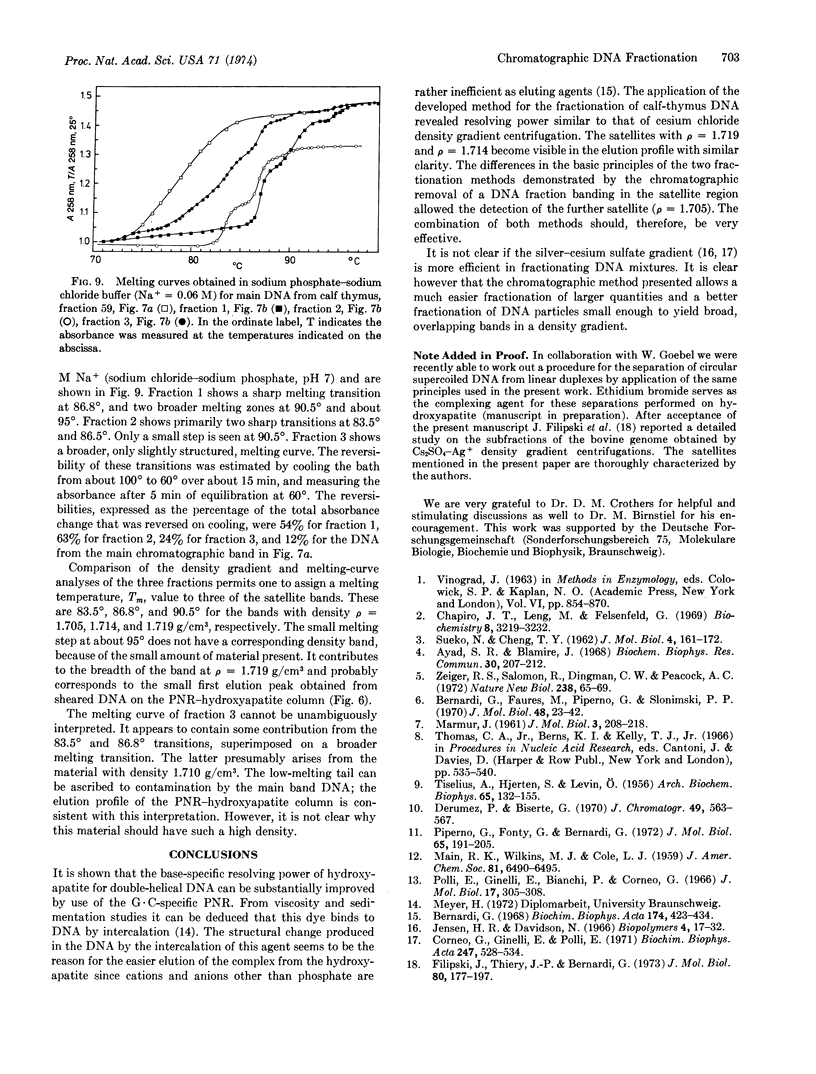
We describe a chromatographic technique for separating mixtures of DNA of varying G + C content. The method employs a specially prepared high-capacity hydroxyapatite and a G·C-specific DNA ligand (2-methyl-3-amino-7-dimethylamino-5-phenyl-phenazinium cation, abbreviated PNR). DNA molecules rich in G·C pairs bind larger amounts of this dye and are eluted earlier from the hydroxyapatite column than are molecules rich in A·T pairs. The dye can easily be removed from DNA by dialysis or solvent extraction after the chromatographic separation. The resolution of the method approaches that of CsCl density gradient separation, and the capacity of the column is much larger than that of a typical density gradient experiment. Elution of the DNA is not dependent on molecular weight, so samples of different molecular weight can be separated on the basis of G + C content. The technique should be especially useful for separating G·C-rich minor components from DNA obtained form eukaryotic cells, as demonstrated by a fraction of DNA from calf thymus.
Keywords: chromatography, bacterial DNA, satellites, DNA-dye binding
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S. R., Blamire J. Fractionation of Bacillus subtilis DNA by use of poly-L-lysine kieselguhr columns. Biochem Biophys Res Commun. 1968 Feb 15;30(3):207–212. doi: 10.1016/0006-291x(68)90436-1. [DOI] [PubMed] [Google Scholar]
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. I. Chromatography of native DNA. Biochim Biophys Acta. 1969 Feb 18;174(2):423–434. doi: 10.1016/0005-2787(69)90273-1. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Faures M., Piperno G., Slonimski P. P. Mitochondrial DNA's from respiratory-sufficient and cytoplasmic respiratory-deficient mutant yeast. J Mol Biol. 1970 Feb 28;48(1):23–42. doi: 10.1016/0022-2836(70)90216-0. [DOI] [PubMed] [Google Scholar]
- Corneo G., Ginelli E., Polli E. Renaturation properties and localization in heterochromatin of human satellite DNA's. Biochim Biophys Acta. 1971 Nov 19;247(4):528–534. doi: 10.1016/0005-2787(71)90689-7. [DOI] [PubMed] [Google Scholar]
- Derumez P., Biserte G. Microméthode pour la détermination de la composition nucléotidique des ADN. J Chromatogr. 1970 Jun 24;49(3):563–567. doi: 10.1016/s0021-9673(00)93680-x. [DOI] [PubMed] [Google Scholar]
- Filipski J., Thiery J. P., Bernardi G. An analysis of the bovine genome by Cs2SO4-Ag density gradient centrifugation. J Mol Biol. 1973 Oct 15;80(1):177–197. doi: 10.1016/0022-2836(73)90240-4. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- Piperno G., Fonty G., Bernardi G. The mitochondrial genome of wild-type yeast cells. II. Investigations on the compositional heterogeneity of mitochondrial DNA. J Mol Biol. 1972 Mar 28;65(2):191–205. doi: 10.1016/0022-2836(72)90276-8. [DOI] [PubMed] [Google Scholar]
- Polli E., Ginelli E., Bianchi P., Corneo G. Renaturation of calf thymus satellite DNA. J Mol Biol. 1966 May;17(1):305–308. doi: 10.1016/s0022-2836(66)80114-6. [DOI] [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- Shapiro J. T., Leng M., Felsenfeld G. Deoxyribonucleic acid-polylysine complexes. Structure and nucleotide specificity. Biochemistry. 1969 Aug;8(8):3219–3232. doi: 10.1021/bi00836a014. [DOI] [PubMed] [Google Scholar]
- Zeiger R. S., Salomon R., Dingman C. W., Peacock A. C. Role of base composition in the electrophoresis of microbial and crab DNA in polyacrylamide gels. Nat New Biol. 1972 Jul 19;238(81):65–69. doi: 10.1038/newbio238065a0. [DOI] [PubMed] [Google Scholar]



