Abstract
The Dominantly Inherited Alzheimer’s Network Trials Unit (DIAN-TU) was formed to direct the design and management of interventional therapeutic trials of international DIAN and autosomal dominant Alzheimer’s disease (ADAD) participants. The goal of the DIAN-TU is to implement safe trials that have the highest likelihood of success while advancing scientific understanding of these diseases and clinical effects of proposed therapies. The DIAN-TU has launched a trial design that leverages the existing infrastructure of the ongoing DIAN observational study, takes advantage of a variety of drug targets, incorporates the latest results of biomarker and cognitive data collected during the observational study, and implements biomarkers measuring Alzheimer’s disease (AD) biological processes to improve the efficiency of trial design. The DIAN-TU trial design is unique due to the sophisticated design of multiple drugs, multiple pharmaceutical partners, academics servings as sponsor, geographic distribution of a rare population and intensive safety and biomarker assessments. The implementation of the operational aspects such as home health research delivery, safety magnetic resonance imagings (MRIs) at remote locations, monitoring clinical and cognitive measures, and regulatory management involving multiple pharmaceutical sponsors of the complex DIAN-TU trial are described.
Keywords: Alzheimer’s disease, Amyloid-beta (Aβ), Autosomal dominant, Amyloid deposition, Clinical trial
1. Introduction
Alzheimer’s disease (AD) is a growing public and financial healthcare crisis. AD afflicts approximately 18 million people worldwide according to the World Health Organization (WHO) (Sato et al., 2007). By 2025, this estimate is projected to grow to 34 million people, with the highest increase expected among developing countries. AD causes loss of memory cognitive function, and ultimately independence, putting a heavy personal and financial burden on the patient and the family. Because of the severity and increasing prevalence of the disease, better treatments are urgently needed.
Autosomal dominant Alzheimer’s disease (ADAD) has informed the field of AD research about the molecular and biochemical mechanisms that are believed to underlie the pathological basis of AD. Furthermore, mutations from autosomal dominant AD have provided animal and cellular models that are utilized to develop anti-amyloid-beta drugs considered as AD therapeutic agents. Due to the rarity of ADAD, the Dominantly Inherited Alzheimer Network (DIAN; U19 AG032438) was launched in 2008 to establish an international, multicenter registry of individuals at risk or with a known causative mutation of AD in the amyloid precursor protein (APP), presenilin 1 (PSEN1), or presenilin 2 (PSEN2) genes (Morris et al., 2012). The DIAN is now well established, having enrolled more than 330 participants. Interim cross-sectional analyses indicate a cascade of AD biomarker changes that begin at least 20 years before symptomatic onset of disease (Bateman et al., 2012).
The pathogenesis and pathophysiology of AD are best understood in the ADAD population. The DIAN study evaluates participants at entry and longitudinally thereafter with clinical and cognitive batteries, structural, functional, metabolic, and amyloid imaging protocols, and biological fluid (blood and cerebrospinal fluid) collection with the goal of determining the sequence of changes in presymptomatic gene carriers who are destined to develop AD. Because the clinical and pathological phenotypes of dominantly inherited AD appear similar to those for the far more common late-onset “sporadic” AD, the nature and sequence of brain changes in dominantly inherited AD are also likely relevant for sporadic AD. Many disease modifying therapies currently in development target amyloid-beta (Aβ), which is believed to be the initiator and earliest change in the AD process. We now have the opportunity to test whether drugs that were developed using animal models incorporating ADAD mutations will have similar biological and clinical effects in ADAD participants, testing fundamental questions of the potential efficacy of anti-Aβ agents. The AD pathological processes can be tracked with biomarkers (Blennow et al., 2012; May et al., 2011; Ostrowitzki et al., 2012; Salloway et al., 2009) to assess drugs developed to target these pathological processes (e.g., increased Aβ production, Aβ deposition, etc.). It is unlikely that a drug which does not engage its target in the brain will be effective in slowing cognitive decline (i.e., if the drug does not do what it was designed to do, it would have only a random chance of working) (Bateman and Klunk, 2008). Early analysis of DIAN longitudinal study has provided a suggestive algorithm of biomarker change in the ADAD population over time before the estimated age of onset of cognitive impairment (Bateman et al., 2012). This reinforces the rationale that biomarker changes are ongoing in presymptomatic ADAD participants and allows the estimation of effect size for clinical trials. Building on this information, the DIAN-TU trial will measure the effects of drugs on a comprehensive set of AD biomarkers (e.g., amyloid deposition, cerebrospinal fluid (CSF) Aβ and tau, magnetic resonance imaging (MRI) brain atrophy, and positron emission tomography (PET) imaging with 2-[18F] fluoro-2-deoxy-D-glucose (FDG PET)) to determine if a drug is likely to have a cognitive benefit in a subsequent cognitive endpoint trial.
2. Trial design
2.1. Design
The DIAN-TU trial leverages several operational efficiencies in testing multiple drugs in a single trial while addressing challenges of managing a global trial of a geographically dispersed rare population. Very few eligible participants live within driving distance of sites possessing the necessary biomarker technology, requiring air travel and hotel stays for some visits, complex remote management and use of multiple local vendors for drug delivery and safety monitoring. The DIAN-TU has developed principles which serve as a guide in the design and execution of this trial: trial integrity, safety, scientific goals, reduced burden on participants, balanced assessments across drug arms, streamlined operations, efficiency, and protecting the confidentiality of participants and pharmaceutical industry partners.
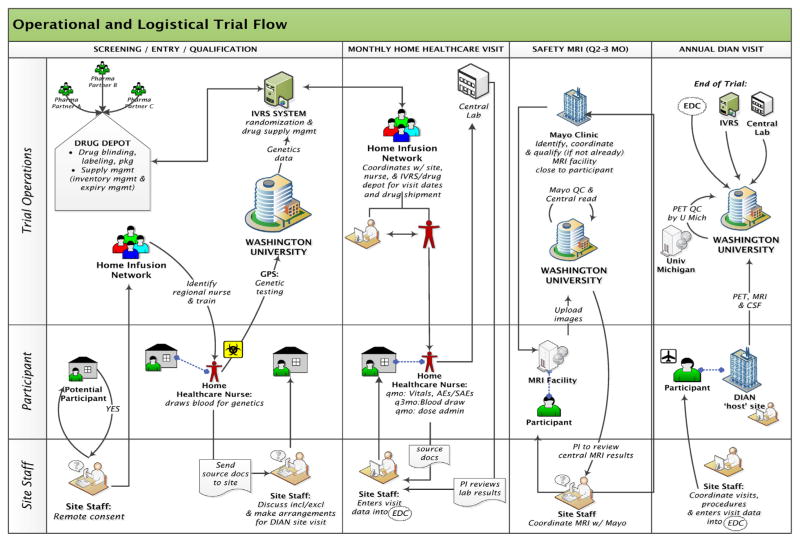
Regulatory agencies support trial designs that incorporate multiple biomarkers and cognitive measures to determine the potential benefits in a trial and have indicated that biomarker improvements with a ‘subtle cognitive benefit’ could support application for approval in the ADAD population (Huang et al., 2001). Several drug mechanisms will be tested across a group of mutations increasing the generalizability of the trial results. The advantage of rapid results regarding multiple targets’ responsiveness to various drugs increases the complexity of the trial operations due to logistical challenges of drug delivery and administration, randomization, regulatory operations and contracting (Fig. 1).
Fig. 1.
Operational and logistical trial flow.
The DIAN-TU phase 2 trial was launched in December 2012 with two anti-Aβ monoclonal antibodies that target different Aβ species. The trial design includes the potential to add additional drug arms should promising compounds become available. Inclusion of a pooled placebo group increases the efficiency of the trial design enabling 50% fewer subjects needed than if separate placebo groups were used. The pooled placebo design also increases participants’ odds of receiving active drug compared to traditional designs, thus potentially improving recruitment and retention. The use of multiple drugs in one trial increases the probability of identifying effective drug(s) and mechanism(s) of action to test the amyloid hypothesis while efficiently utilizing the available pool of subjects.
The trial design is a randomized, double blind placebo controlled trial of an anti-aggregated Aβ antibody, a soluble anti-Aβ antibody, and a pooled placebo group in a total of 120 asymptomatic to mildly symptomatic ADAD mutation carriers. Participants will receive a monthly injection or infusion for two years to determine target engagement in the central nervous system (CNS) and downstream AD biomarkers. Each drug arm will be compared to the pooled placebo with the drug specific primary outcome of PiB-PET for the anti-fibrillar Aβ antibody, and unbound free CSF Aβ42 and Aβ40 for the anti-soluble Aβ antibody. Secondary outcomes of neurodegenerative AD biomarkers will also be assessed. The trial design was optimized to account for the restricted numbers of participants and the relatively large number of promising disease modifying therapeutics.
2.1.1. Recruitment and retention
DIAN-TU sites will recruit participants from the existing DIAN cohort in the observational study, from the DIAN Expanded Registry (www.dianxr.org) and referrals from partnering clinicians and investigators (sites with eligible participants that do not have the required facilities, staff or sufficient population but want to provide their patients the opportunity to participate). DIAN participants who enroll in the trial will be monitored closely by the Sponsor (DIAN-TU Trial Clinical Operations) and Quintiles and the Contract Research Organization (CRO) in order to manage potential safety issues (radiation, amyloid-related imaging abnormality characterized by vasogenic edema (ARIA-E) and amyloid-related imaging abnormality characterized by hemorrhage (ARIA-H)), participant burden (multiple lumbar punctures [LPs]), assessment fatigue (multiple cognitive batteries and overall evaluation burden at each clinic visit) and scientific integrity (overlapping psychometrics) issues.
Most current DIAN participants (~80%) do not know their mutation status nor wish to know their own mutation status. Therefore, non-carriers and carriers who do not know their mutation status will be allowed in the trial to avoid required genetic disclosure for participation. Non-carriers will all receive placebo, but will not be included in the placebo comparison of carriers with or without active drug. Approximately 35% of currently enrolled DIAN observational participants do not have a mutation and are not at risk for ADAD, thus we predict about 35% of the DIAN-TU trial may be non-carriers.
2.1.2. Participant involvement
Participants in the DIAN-TU will undergo the following procedures at intervals detailed in the Schedule of events (Table 1): informed consent, documentation of disease/disorder, medical/treatment history, clinical assessment (clinical dementia rating sum of boxes, mini-mental state exam, functional assessment questionnaire, neuropsychiatric inventory questionnaire), physical and neurological exams, questionnaires, blood draw, pregnancy testing, cognitive testing, amyloid positron emission tomography (PET) with PiB and florbetapir, fluorodeoxyglucose (18F) PET, 3-Tesla magnetic resonance imaging, lumbar puncture, and study drug administration. Participants are required to travel to a qualified site and stay for 4 days to undergo these procedures.
Table 1.
Schedule of assessments.
| Procedure: | Visit Freq.
|
|||
|---|---|---|---|---|
| Baseline, Yr 1 & 2 | Every 6 mo. | Every 3 mo. | Every 1 mo. | |
| Informed consent | X(b-line) | |||
| Vitals & Med/Tx History | X | X | ||
| Clinical Assessment | X | |||
| PE & Neuro Exam | X | |||
| Genetics/APOE | X(b-line) | |||
| Pregnancy testing | X | X | ||
| 12-lead ECG | X | X | X | |
| Hem/Chem/LFTs/UA | X | X | X | |
| Safety MRI | X | X | ||
| C-SSRS | X | X | ||
| Cognitive Testing | X | |||
| vMRI, PET: PIB & FDG | X | |||
| Blood (pK, biomarkers) | X | |||
| LP-CSF | X | |||
| AE/SAE Assessment | X | X | ||
| Randomization | X(b-line) | |||
| Study drug admin | X | X | ||
AE: adverse event; CSF: cerebrospinal fluid; C-SSRS: columbia suicide severity rating scale; LP: lumbar puncture; SAE: serious adverse event; LFT: liver function test; UA: urinalysis; HEM: hematology; CHEM: chemistry; PE: physical exam; vMRI: volumetric magnetic resonance imaging;
2.1.3. Regulatory sponsor: Washington University in St. Louis
Simultaneous testing of multiple investigational agents from different pharmaceutical companies necessitated that Washington University serve as the regulatory sponsor of the trial and open a unique ‘trial’ investigational new drug (IND). This reduced the regulatory oversight burden on sites and regulatory agencies with one, rather than multiple sets of regulatory documents and institutional review board submissions. One sponsor further ensures that all drug arms are working in parallel and on the same timeline. By eliminating redundancy with multiple sponsors, processes were streamlined and more efficient, which will improve the integrity of the trial and scientific and medical findings.
2.1.4. Drug delivery
A central drug packaging and distribution organization was utilized for all drugs to allow for efficient packaging and consistent labeling with distribution to Europe, North America and Australia. Each drug in the DIAN-TU trial has a unique method of storage, preparation and administration. Due to the geographic dispersement of participants the trial is utilizing a central pharmacy in the U.S. for the reconstitution process of some drugs for home administration. The prepared drug must be shipped from the central pharmacy to the home nurse in a temperature controlled and monitored package within a short timeframe following detailed supply chain and accountability procedures.
Difficulties of appropriate drug shipment and blood samples to site labs are a challenge due to coordination of dry ice procurement and courier arrangements. Due to the younger, generally employed population, many of the home visits are conducted in the evening or on weekends and labs are drawn and must be shipped overnight to the central lab, some on dry ice. Multiple carriers are being utilized to accommodate the specific requirement of each participant’s visit. Determining which courier to use involves an assessment of the local courier availability, day of the week, time of the visit completion, and services available in the participant’s locality, e.g., courier personnel trained in hazardous material physically present to accept the package.
Drug-compatible supplies (specific material types and infusion pumps) and equipment (multiple-setting portable centrifuges) had to be identified, procured and provided to the home health research nurses to account for the flexibility needed for different study procedures while also performing consistently across all home visits. Study-specific training of all home health research nurses is critical, including conducting training on how to schedule and time visits, complete source documents, communication escalation and interface with the site to maintain appropriate oversight and study drug handling and coordination of same-day delivery.
Across several teams there are targeted systems, processes and communication pathways in place to facilitate visit scheduling and drug delivery, visit conduct, and data transmission with patient safety rooted in each step. The nurses are trained on the patient population, study drug preparation, administration and potential adverse effects, ICH/GCP (international conference on harmonization/good clinical practice), IATA handling, SAE (serious adverse event) reporting and clear trigger points for rapid to immediate escalation depending on the issue (Appendix A).
2.1.5. Central monitoring of clinical and cognitive measures
Central monitoring of clinical and cognitive measures was implemented for accuracy and consistency across the study and ultimate data integrity. An audio review process has been created to provide feedback to raters and retraining if needed. Reviews will be conducted in a timely manner after the assessment has taken place so the feedback is meaningful to the rater.
2.1.6. Home-based trial activities
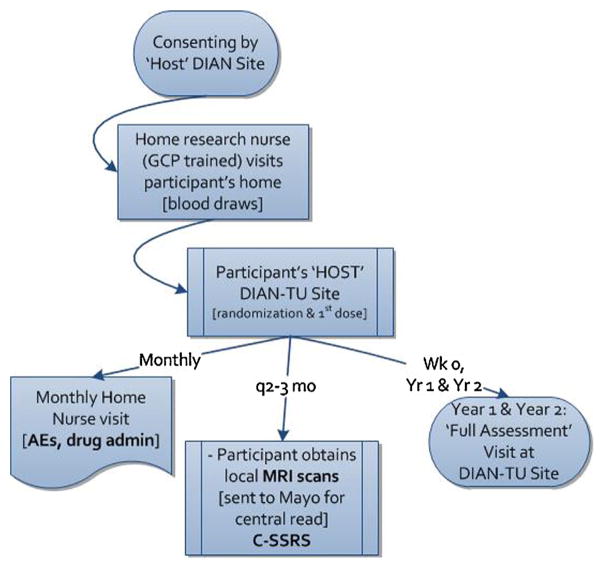
Because most participants live at a distance from their ‘host’ DIAN-TU trial site, the trial is designed so that many trial activities are performed near or even in the participants’ homes. Participants will be consented by their ‘host’ DIAN-TU trial site 1–2 months prior to the baseline visit. This will enable coordination, training and qualification of a regional home research nurse and safety MRI center close to the participant’s home. Approximately four weeks prior to the baseline visit, the home research nurse will confirm consent and draw the participant’s blood for clinical safety labs and genetic testing. Once the results are confirmed as meeting inclusion criteria, arrangements for the baseline visit at the host DIAN-TU trial site will be made (Fig. 2).
Fig. 2.
Participant visit and consent flow.
Participants will travel to their host DIAN-TU trial site for the baseline visit; this visit will typically take three to four days for all of the assessments and procedures to be completed. After the consent is again reviewed with the participant in person, all baseline assessments are obtained (Table 1) prior to dosing. Once all procedures are completed and inclusion and exclusion criteria are verified, the participant will be randomized and will receive the first dose of active drug or placebo. Clinical factors in scheduling baseline and annual visits include necessity of formal reading of MRI before the first dose is administered, arranging neuropsychometric testing for mornings rather than later in the day when participants are fatigued, and managing logistics to insure that participants are eligible for participation in the trial before more invasive procedures are done, insuring adequate timing between PET scans and insuring that the LP is performed after the MRI.
For intravenous or subcutaneous administration, participants will continue to receive monthly administration of study drug or placebo at their host DIAN-TU trial site if in close proximity, or in their home via the home research nurse. At each monthly visit, either the site study coordinator or home research nurse will assess the participant for any adverse events and serious adverse events, take vital signs and administer study drug. Additional post-dose observation periods may occur and all necessary source documentation and drug accountability forms will be completed. Approximately every three months, the participant will also have a safety MRI performed at either their DIAN-TU site or the previously identified local center. MRI images will be read centrally to monitor for any abnormalities including amyloid-related imaging abnormalities (ARIA) (Sperling et al., 2011). Approximately every six months, participants will have their blood drawn for safety and biomarker labs, complete abbreviated cognitive battery assessments (by a smaller, dedicated set of traveling nurses for consistency in administration) and have an electrocardiogram (ECG) which will be centrally read by Quintiles Cardiovascular Safety Division. At weeks 52 and 104, participants will visit their host site for the full battery of assessments performed at baseline; including vMRI, PiB-PET, FDG PET, lumbar puncture, and cognitive testing. The annual assessments will take three to four days to conduct. A follow-up phone call or in-home nursing visit will be completed three months after the final visit (last dose).
2.1.7. Home research nurses
Delivering trial activities to the home will enable ADAD families that live remotely from a DIAN-TU trial site the opportunity to participate. Visits can be scheduled at times that are convenient for the participant and least disruptive to their life and schedule (as many potential subjects will be working and have young children). Previous studies show (Gregg et al., 1986; Karlawish et al., 2008) that this decreases the participant dropout rate due to inconvenience.
2.1.8. Safety MRIs
Safety MRIs at approximately three-month intervals have been required by regulators for anti-Aβ drug trials. Participants will be able to obtain their safety MRI at a location close to their home to minimize inconvenience. Mayo Clinic Aging and Dementia Research Institute (Mayo-ADIR/MRI qualification), the site study staff, and the DIAN-TU Imaging Core identify a nearby qualified DIAN site, ADNI site, ADCS site, or an alternative, suitable 3-Tesla MR scanner which Mayo-ADIR can qualify for this purpose. The MRI center incorporates the required protocol for the scan and becomes the designated location for that participant and other nearby participants. For consistent readings across the study, all safety scans will be centrally read by investigators at Mayo-ADIR. The initial baseline scan will be performed with a 24–hour turnaround of the central read to ensure no exclusion criteria are met prior to first dosing.
3. Discussion
Persons carrying fully-penetrant ADAD mutations therefore provide a unique opportunity to test the ability of novel agents to prevent the onset of clinical AD. However, such trials present unique logistical and ethical issues which need to be considered in their design (Bateman et al., 2011; Ringman et al., 2009). The importance of potentially delaying or possibly preventing dementia in asymptomatic individuals is widely recognized. Diverse and committed collaborations between academia, patient advocacy groups, participant populations, pharmaceutical companies and regulatory agencies have contributed to the design and implementation of the DIAN-TU trial. Excellent communication and dedication have been the key to launching the DIAN-TU trial in a twelve-month time frame. A successful trial could advance the entire field and pave the way for prevention and or possibly treatment in both ADAD and likely the more common late-onset AD.
Supplementary Material
Acknowledgments
We gratefully acknowledge the Dominantly Inherited Alzheimer Network (DIAN; U19 AG032438; JC Morris, PI), the DIAN Pharma Consortium (Biogen Idec, Elan, Eli Lilly, Genentech, Hoffman La-Roche, Janssen, Mithridion, Novartis, Pfizer, Sanofi-Aventis), the Alzheimer’s Association, all of the Cores of the DIAN-TU, and the participants and their families to whom we are especially indebted. The DIAN-TU is supported by funding from the Alzheimer’s Association and the DIAN Pharma Consortium.
Appendix A. Supplementary data
Supplementary data (Abbreviations/glossary of terms) associated with this article can be found, in the online version, at doi:10.1016/j.neurol.2013.07.017.
Footnotes
This manuscript is dedicated to the memory of Denise Heinrichs, devoted mother who led by gracious example. She is an inspiration for dominantly inherited Alzheimer’s disease families, researchers and doctors.
Disclosure of interests
R.J.B. has consulted for Pfizer, DZNE, Probiodrug AG, Medscape, En Vivo (SAB) and has research grants with AstraZeneca, Merck and Eli Lilly in the past year. Washington University and R.J.B. have a financial interest in C2 N Diagnostics, which uses the SILK methodology in human studies. R.J.B. is a co-inventor on U.S. patent 7,892,845 “Methods for measuring the metabolism of neurally derived biomolecules in vivo.” Washington University, with R.J.B. are co-inventors, has also submitted the U.S. non-provisional patent application “Methods for measuring the metabolism of CNS derived biomolecules in vivo,” serial #12/267,974. R.J.B. is co-inventor on U.S. Provisional Application 61/728,692 “Methods of diagnosing amyloid pathologies using analysis of amyloid-beta enrichment kinetics”.
D.M.C. has consulted for Pfizer, Genzyme, Millennium, PML Consortium, Amgen, Quintiles, Arnold Todara & Welch, W. Holt Smith Attorney, Biogen Idec, Cytheris, FDA hearing, Genentech, GSK and has research grants with Bavarian Nordic, Lilly, Roche and Biogen Idec.
References
- Bateman RJ, Klunk WE. Measuring target effect of proposed disease-modifying therapies in Alzheimer’s disease. Neurotherapeutics. 2008;5(3):381–90. doi: 10.1016/j.nurt.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, et al. Autosomal dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3(1):1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Rinne JO, Salloway S, Wei J, Black R, Grundman M, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate alzheimer disease. Arch Neurol. 2012;69(8):1002–10. doi: 10.1001/archneurol.2012.90. [DOI] [PubMed] [Google Scholar]
- Gregg RE, Zech LA, Schaefer EJ, Stark D, Wilson D, Brewer HB., Jr Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. 1986;78(3):815–21. doi: 10.1172/JCI112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZH, Lin CY, Oram JF, Mazzone T. Sterol efflux mediated by endogenous macrophage ApoE expression is independent of ABCA1. Arterioscler Thromb Vasc Biol. 2001;21(12):2019–25. doi: 10.1161/hq1201.100242. [DOI] [PubMed] [Google Scholar]
- Karlawish J, Cary MS, Rubright J, Tenhave T. How redesigning AD clinical trials might increase study partners’ willingness to participate. Neurology. 2008;71(23):1883–8. doi: 10.1212/01.wnl.0000336652.05779.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, et al. Robust central reduction of amyloid-β in humans with an orally available non-peptidic β-secretase inhibitor. J Neurosci. 2011;31(46):16507–16. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Aisen PS, Bateman RJ, Benzinger TL, Cairns NJ, Fagan AM, et al. Developing an international network for Alzheimer research: the dominantly inherited alzheimer network. J Clin Invest. 2012;2(10):975–84. doi: 10.4155/cli.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, et al. Mechanism of amyloid removal in patients with alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69(2):198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Grill J, Rodriguez-Agudelo Y, Chavez M, Xiong C. Commentary on “a roadmap for the prevention of dementia II: Leon Thal Symposium 2008” Prevention trials in persons at risk for dominantly inherited Alzheimer’s disease: opportunities and challenges. Alzheimers Dement. 2009;5(2):166–71. doi: 10.1016/j.jalz.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–70. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Ishihama Y, Oda Y. Quantitative proteomics of mouse brain and specific protein-interaction studies using stable isotope labeling. Methods Mol Biol. 2007;359:53–70. doi: 10.1007/978-1-59745-255-7_4. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7(4):367–85. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.