Abstract
During embryonic neural development, axon tips (“growth cones”) are guided through a dynamic three-dimensional (3-D) landscape by soluble chemotropic factors and by immobilized, growth-permissive or growth-inhibiting contact cues present in the extracellular matrix and on the surface of surrounding cells. It has been difficult to probe the search algorithms of growth cones in response to multiple contact cues during 3-D navigation using traditional two-dimensional (2-D) substrates. Here, we present an in vitro study in which the axons of murine embryonic cortical neurons are challenged with competing growth options, using 3-D substrates that feature variations in permissiveness and microtopography. As 3-D substrates, we used poly-d-lysine (PDL) coatings on microfabricated steps of polydimethylsiloxane (PDMS) and complementary features of Matrigel. We found that axons display a preference for PDL over Matrigel and for the straightest path within a distance consistent with the exploratory range of the growth cone. When these two preferences are in conflict, axons choose to grow straight into Matrigel; when the straight path is not permissive, the axon turns in the direction that minimizes the turning angle. These results suggest that growth cones make 3-D navigation decisions by integrating permissiveness and topographical cues.
Keywords: Axon guidance, Cell culture, Embryonic, PDMS, Matrigel, Surface micropatterning, Surface microstructure
Introduction
To establish the intricate neuronal network of an adult brain, neurons must correctly project their axons to their synaptic targets during embryonic development. Studies in the last two decades have revealed that extracellular signals including short-range contact cues and long-range diffusible factors are critical to guide the axon navigation process [1]. In particular, the axonal tip, also referred to as the “growth cone”, senses permissive or inhibitory cues along its growth path constrained by a particular topographical arrangement of extracellular matrix (ECM) and other cells [2–7].
Microfabrication technologies, which enable a precise design of the chemical composition of the surface, have been used for a long time to direct axon growth in vitro ([8,9], reviewed in [10–12]). Surface micropatterns of adhesive molecules have been used to probe the permissiveness of various substrates [13–18]. Surface microtopography has also been shown to affect axon growth on various substrates and neuronal cell types [19–26]. In these studies, the axon tip was presented with only one surface cue (either a biochemical or a topographical variation), thus the responses of growth cones to multiple cues on 3-D substrata are largely unknown.
Here, we report a study of the growth paths chosen by axon tips after encountering abrupt changes in substrate composition and/or topography. Our results suggest that axons actively search for the straightest permissive path and take into account both biochemical and topographical information simultaneously during navigation in a 3-D environment.
Materials and methods
Fabrication of surfaces containing microtopographical features
The surfaces were made in polydimethylsiloxane (PDMS) by replica-molding from a photolithographically-patterned master [27] using the large-aspect-ratio SU-8 photoresist as described elsewhere [28]. SU-8 patterns were created from the same photomask in different heights; the heights were measured with a surface profilometer (KLA-Tencor, model P15, San Jose, California). A mixture of PDMS prepolymer and curing agent (10:1 (wt/wt), Sylgard 184, Dow-Corning) was cast against the silicon “master” and cured for 24 h at 65°C to ensure complete cross-linking.
Surface treatment
For neuron cultures on PDMS substrates, PDMS surfaces were oxidized by exposure to an oxygen plasma (Branson/IPC 2000 barrel etcher, 150 W, 1 Torr) for 30 s, immediately bathed in 10 µg/mL poly-d-lysine (PDL, M.W. = 70,000–150,000, Sigma, St. Louis, MO) for 1 h at room temperature, and then rinsed and dried overnight. Sometimes substrates were further coated with 25–50 µg/mL laminin (Sigma) for 1 h. For controls, flat substrates such as glass coverslips (Fischer Scientific, Pittsburgh, PA) were coated with 10 µg/mL PDL (and, sometimes, further coated with 25–50 µg/mL laminin) as above.
To study axon guidance by surface biochemical cues, micropatterns of fluorescently-labeled PDL or laminin (Alexa Fluor® 546 protein labeling kit, A10237 from Molecular Probes, Eugene, OR) were created on a bare glass background using an elastomeric stencil to mask the glass substrate during PDL or laminin adsorption. The stencil was made by exclusion molding from the SU-8 masters as described elsewhere [29]. We also made micropatterns of alternating lanes of labeled PDL and growth factor-reduced Matrigel (BD Biosciences, San Jose, CA). Matrigel is a solubilized basal membrane extract with laminin and collagen IV as major ECM components; Matrigel gels when warmed at room temperature or higher but stays liquid when diluted 1:10 in PBS. To produce the micropatterns we followed the subsequent steps: (a) uniform coating of the coverslips with fluorescently-labeled PDL, rinsing and drying; (b) assembling a PDMS device featuring a set of parallel microchannels on top of the PDL coating; (c) allowing an oxygen plasma (1 min) to penetrate the microchannels in order to etch away the PDL layer on the areas not covered by PDMS; (d) introducing 1:10 Matrigel:PBS into the microchannels, causing adsorption of the Matrigel proteins on the areas etched by the oxygen plasma; and (e) flushing the microchannels with PBS and manually removing the PDMS microchannels from the substrate, which revealed alternating lines of Matrigel and labeled PDL. For these studies, coverslips coated uniformly with 1:10 Matrigel:PBS (1 h adsorption and triple rinse) and coverslips coated with PDL were used as controls. Based on experiments with fluorescently-labeled laminin or PDL using the same etching and filling protocol (data not shown), we estimate that the coverage of microfluidically-patterned 1:10 Matrigel is similar to the coverage on the unpatterned control Matrigel surfaces.
Neuronal culture
E11–E14 mouse embryos were harvested from timed-pregnant white Swiss Webster female mice (ATL-Harlan, Kent, WA) in accordance with a protocol approved by the University of Washington Animal Care and Use Committee, and decapitated in ice-cold, oxygenated artificial cerebrospinal fluid (ACSF; in mM: NaCl 119, KCl 2.5, MgCl2 1.3, CaCl2 2.5, NaH2PO4 1, NaHCO3 26.2, glucose 11) [30]. Cortical cell cultures were prepared using protocols as described in [31]. After mechanical dissociation, cells were plated on PDL (or laminin)-coated glass coverslips or PDMS substrates at density of 100/mm2 in culture medium containing Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with B-27 (1X) (Invitrogen), 100 U/mL penicillin–streptomycin (Invitrogen) and 0.5 mM Gluta-Max (Invitrogen). The cell density of 100/mm2 was chosen based on published protocol [32] and our own experiences for better cell survival and health. Due to the specific time-point of tissue harvest and the use of serum-free Neurobasal medium, the majority of cells in the cultures became neurons as confirmed by Microtubule-associated Protein-2 immunostaining.
As a neuronal 3-D growth-supporting gel substrate we used the growth factor-reduced Matrigel. When warmed to room temperature or higher, Matrigel forms a biologically active gel matrix resembling the cellular basal membrane [33]. A neuronal suspension in supplemented Neurobasal medium was mixed with freshly thawed Matrigel at equal volumes, resulting in a final cell density of 1 × 104 cells/mL. The cell-containing Matrigel was then plated onto PDL-coated microgrooved PDMS substrates or, as a control, in a polystyrene tissue culture dish. After allowing the cells to settle for 5–10 min at room temperature (at which Matrigel gels slowly), the cell cultures were transferred to a humidified 37°C, 5% CO2 incubator for 30 min to allow for Matrigel to completely gel. Medium was then added on top of the gel. All cultures were kept in the supplemented Neurobasal medium in the incubator, with one third of the medium changed to fresh pre-warmed Neurobasal medium every 3–4 days.
Immunocytochemistry and immunofluorescence microscopy
α-tubulin immunostaining procedures for cells without gel were performed as described in [31]. Mouse anti-bovine-α-tubulin monoclonal antibody and Alexa Fluor® 488 goat anti-mouse IgG were purchased from Molecular Probes. For cells in Matrigel, incubation times were prolonged for all steps to allow for diffusion into and out of the gel: fixation for 1 h, permeabilization for 30 min, primary antibody binding for 12 h at 4°C, secondary antibody binding for 4 h, and every rinsing step for 30 min. Following staining, the coverslips or PDMS microdevices were mounted with the Slow Fade Light mounting kit (Molecular Probes) onto glass microscope slides and imaged using a 12-bit cooled-CCD camera (ORCA ER; Hamamatsu, Hamamatsu City, Japan) and standard fluorescence microscopy equipment. To trace the growth of some axons in the Z-direction, series of images were obtained by changing focus plane with an automated stage controlled by MetaMorph software (Molecular Devices, Sunnyvale, CA).
Data analysis
For quantitative analysis, in any given imaging field only neurites that arrived at the border of PDMS “walls” (i.e., the vertical surfaces at the edges of a groove) were counted and scored as either “cross” or “turn”. For each condition, experiments were repeated at least 3 times (3 different batches of cell cultures from different isolations), resulting in a typical number of axons counted n on the order of hundreds. Such redundancy is desirable in order to minimize the potential error from human counting and from the effect of fasciculation (the tendency of axons to follow the paths found by previous axons) on the data. Data are expressed as mean ± SEM. Statistical differences were determined using Student’s t test.
Results and discussion
Axon guidance by surface biochemical cues
Several neuronal cell types are known to grow axons in vitro following surface-immobilized, micropatterned biochemical cues such as laminin or polylysine tracks [16,18,34–39]. Mice cortical neurogenesis occurs between about E11.5 and E17, when post-mitotic neurons migrate from cortical ventricular zone through intermediate zone to final destinations in the cortical plate [40]. Due to the specific time-point of tissue harvest (E11–E14) and the use of serum-free Neurobasal medium (which inhibits glial differentiation and division), at the time of imaging the majority of cells in the cultures were neurons. Prior to studying neuronal responses on substrates featuring multiple simultaneous cues, we confirmed that the mouse embryonic cortical neurons used for this study displayed a similar guidance behavior in response to a single permissive biochemical cue: as exemplified in Fig. 1C, the adhesion and neurite outgrowth of neurons is confined within the regions that are coated with PDL (or laminin, data not shown) since untreated glass could not support growth of these neurons (data not shown).
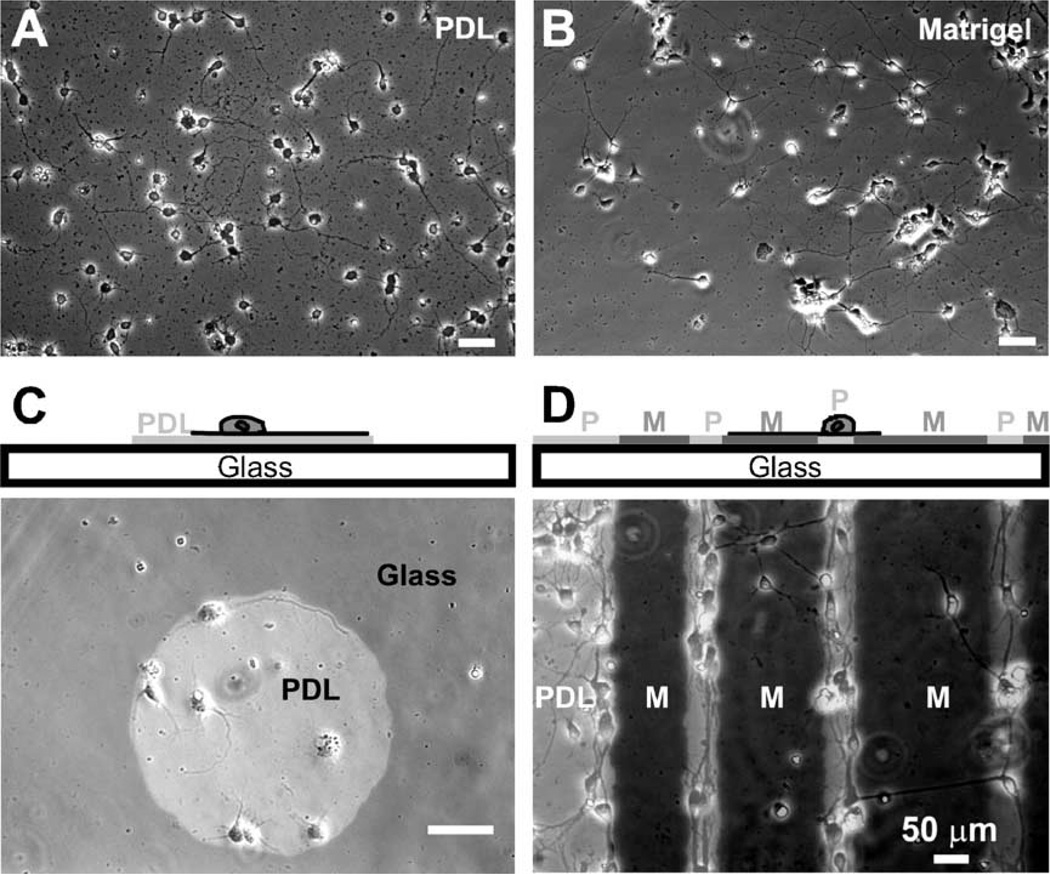
Fig. 1.
Axon guidance by surface biochemical cues. (A) Control 1: cells grown on poly-d-lysine (PDL)-coated glass coverslips. The cells were dissociated at embryonic day 14 (E11) and images were taken after 4 days in vitro (DIV). (B) Control 2: cells grown on diluted Matrigel (1:10 in PBS)-coated glass coverslips. (C) Cross-sectional schematic representation (top) and overlay image (bottom) of phase-contrast (cells) and fluorescence (substrate composition) images illustrating the guidance of neurites by surface poly-d-lysine (PDL) patterns on bare glass background. The fluorescently-labeled PDL islands (light gray circle) were created by exposing the glass substrate to PDL through a PDMS stencil mask (see Materials and methods). The neurites (E13, 3 DIV) are observed to prefer PDL over bare glass substrate. (D) Cross-sectional schematic representation (top) and overlay image (bottom) of neurons (E11, 4 DIV) on glass coverslips patterned with alternating lanes of labeled PDL (light gray regions) and a diluted (1:10 in PBS) solution of Matrigel (see Materials and methods for detailed procedures). As shown, the majority of neurons preferred to adhere and grow on PDL regions, although some somas adhered on Matrigel regions and some neurons extended axons from PDL to Matrigel regions.
We also studied neuronal growth on planar micropatterns of two different growth-permissive biochemical substrates (PDL and diluted Matrigel, both adsorbed onto glass; Matrigel is a basal lamina extract composed of several ECM proteins [33,41]; see Materials and methods for details). We found that on micropatterned surfaces more neurons preferred to adhere their soma and grow axons on PDL regions as compared to the adjacent Matrigel regions (Fig. 1D). When arriving at the border of PDL/Matrigel, many axons that had originated on PDL regions turned at the border inside the PDL areas, while others continued to extend into Matrigel regions (Fig. 1D). Combined with the observation that some somas adhered on Matrigel regions and neurons grew well on both types of non-patterned surfaces (Figs. 1A and B), these results indicate that adsorbed Matrigel coatings are permissive to growth but less permissive than PDL coatings.
Axon guidance by microtopographical cues
The growth response of several neuronal cell types (but not cortical neurons) has been shown to depend on the substrate topography [20,21,23–26]. To present surface topographical cues to the axons of our embryonic cortical neurons, we cultured neurons on micromolded PDMS substrates containing grooves of various depths. PDMS was chosen because it can be easily and repeatedly replicated from a master mold at low cost and because of its transparency and biocompatibility; on PDL-coated PDMS, but not on uncoated PDMS, cells attached and extended neurites at growth rates very similar to those on control PDL-coated glass coverslips (see supplemental Fig. S1). To quantitatively investigate the mechanism of topographical guidance, we systematically studied the turning behavior of axons whose growth had been challenged by the presence of a step (or groove) in their growth path. The depth of the grooves (i.e., the height of the steps, h) ranged from h = 2.5 Am to h = 69 µm (2.5, 4.6, 11, 15, 22, 44 and 69 µm). In the range of the tested groove widths (50–350 µm), the width of the grooves did not influence turning behavior (data not shown).
Axons turn at the edges of deep grooves but not at the edges of shallow grooves
In response to steps of h = 22–69 µm, the vast majority of axons (e.g. 96.0% ± 1.9% for h = 22 µm, 99.0% ± 0.2% for h = 44 µm, and 97.2% ± 1.1% for h = 69 µm, with number of axons counted n = 811, 473, and 270, respectively) appeared to be guided by surface topography by turning and remaining inside the grooves (Figs. 2A–C) or staying on the top surface (the “plateaus”) (Figs. 2D–F). Since the PDMS substrates (including the walls) were uniformly coated with PDL, for these experiments the turning behavior was exclusively due to surface topography. Obviously, neurites encountering the edge of a groove could not continue to grow straight into the liquid nor into solid PDMS, so they had to turn, either on the same plane or up/down onto the groove walls. Importantly, the observed turning angle for steps of h = 22 µm or higher was almost always the minimal angle of the possible turning choices. For example, an axon that approached the edge at a 25° angle (with the edge line) would turn 25° on the same plane – it would not turn 155° on the same plane nor turn 90° down or up the wall – resulting in growth along the edge of the vertical wall (top edge for on-plateau neurons and bottom edge for in-groove neurons).
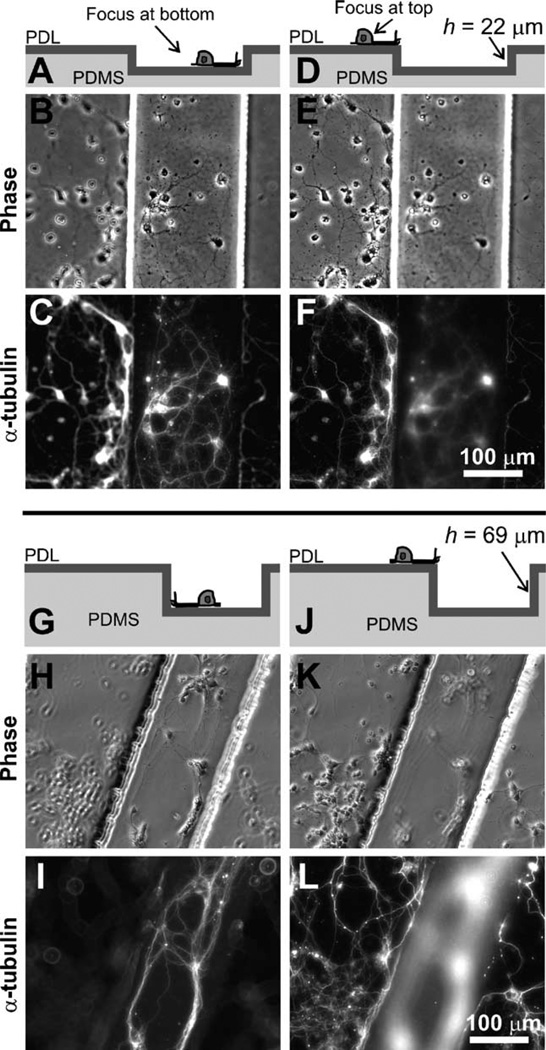
Fig. 2.
Axons turn at the edges of deep microfabricated steps. Cross-sectional schematic representation (top row), phase-contrast (middle row) and corresponding α-tubulin immunofluorescence micrographs (bottom row) illustrating the typical axon turning behavior at the edges of PDL-coated deep PDMS grooves (depth h = 22–69 µm). For h = 22 µm, most axons (whether inside the grooves (A–C) or on the top plateau surface (D– F)) were guided by the surface topography and turned at the wall; a similar guidance trend was observed for h = 69 µm (G–L).
On the other hand, almost all neurons on shallow grooves, 98.3% ± 0.9% for h = 2.5 µm (n = 474) and 93.0% ± 1.3% for h = 4.6 µm (n = 924), disregarded the topographical steps and could extend axons freely into and out of the grooves (Fig. 3). This marked disregard for topography in shallow grooves is in clear contrast with previous reports from Rajnicek et al. [21] that nanometer-scale topographical steps could align the neurites of embryonic Xenopus spinal cord neurons (parallel) and rat hippocampal neurons (parallel and perpendicularly). It also contrasts with reports from Clark et al. that chick embryo hemisphere neurons aligned to a single step of 1 to 5 µm in depth [20] or repeated 2 µm-deep, 8 µm-wide grooves separated by 20 µm-wide ridges [19], although the findings that increasing step heights reduces the percentage of axon crossing were similar. The discrepancy between the three findings may be due to (a) differences in neuronal cell type (murine cortical E11–14 vs. rat hippocampal E16 or Xenopus spinal cord vs. chick cerebral E8); (b) polylysine coverage/affinity for the substrate (resulting in adhesiveness differences); (c) edge sharpness; (d) substrate compliance (elastomeric PDMS vs. stiff quartz or Perspex plastic); and/or (e) width of the gaps (Rajnicek et al. report ridge widths of 1 to 4 µm). It is possible that cells get sensitized to shallower groove depths with decreasing gap widths [42,43]. A recent report [43] found rat embryonic hippocampal axons followed the orthogonal patterns of poly-l-lysine-coated silicon pillars with 1.5 µm gaps, but not the patterns of pillars with 4.5 µm gaps. In our tested range of groove widths (50–350 µm), however, the width did not influence turning behavior.
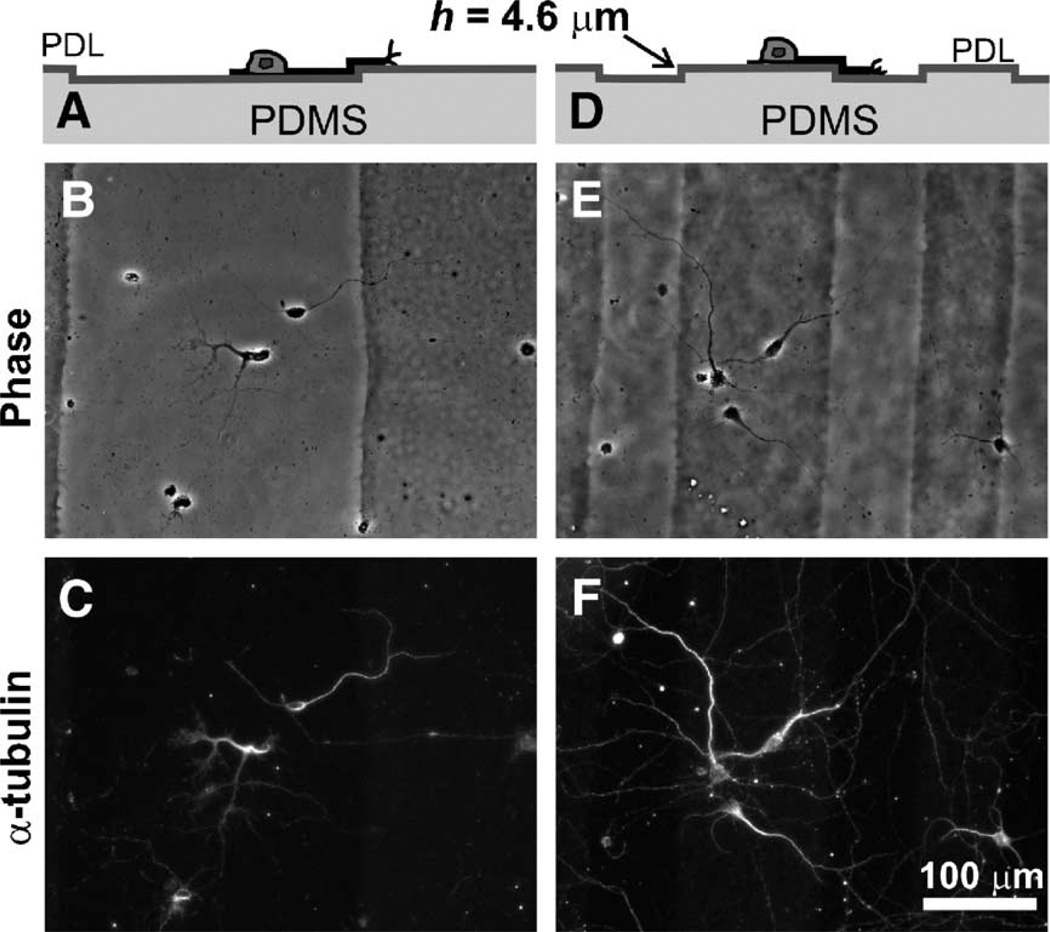
Fig. 3.
Axons disregard shallow steps. Cross-sectional schematic representation (top row), phase-contrast (middle row) and corresponding α-tubulin immunofluorescence micrographs (bottom row) illustrating typical neurons cultured on 4.6-µm-deep, PDL-coated PDMS grooves. Note that neurons (both on the bottom surface of the grooves (A–C) and on the plateau surfaces (D–F)) ignored the topographical steps, extending axons freely into and out of the grooves.
The threshold in step height for topographically-induced axon turning is ∼10 µm
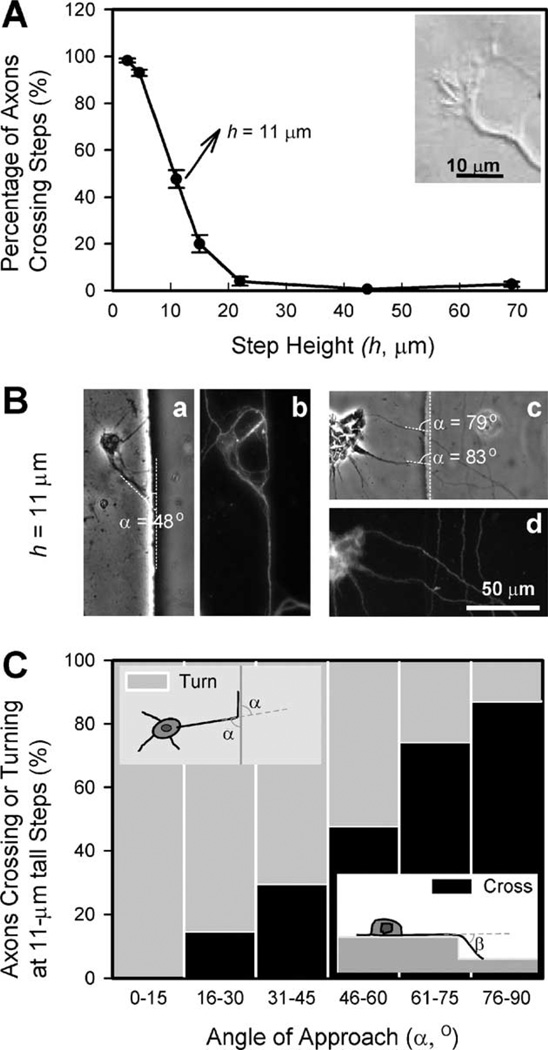
In contrast to the strong but opposite effects of deep and shallow steps, steps of intermediate heights (h = 11–15 µm) showed an intermediate effect. Some axons (whether inside the grooves or on the plateaus) were guided by the surface topography and turned along the wall, but others disregarded the topography and were able to overcome the steps, advancing to the upper or lower surfaces (Fig. 4). The shallower the grooves were, the more neurons disregarded the walls: 20.0% ± 3.8% of neurites for h = 15 µm (n = 684) and 47.7% ± 3.9% for h = 11 µm (n = 593). Fig. 5A shows a plot of the percentage of neurites that crossed borders as a function of h. Thus, the threshold in step height (i.e. the value of h for which we observe a turning percentage of 50%) is ∼10–11 µm.
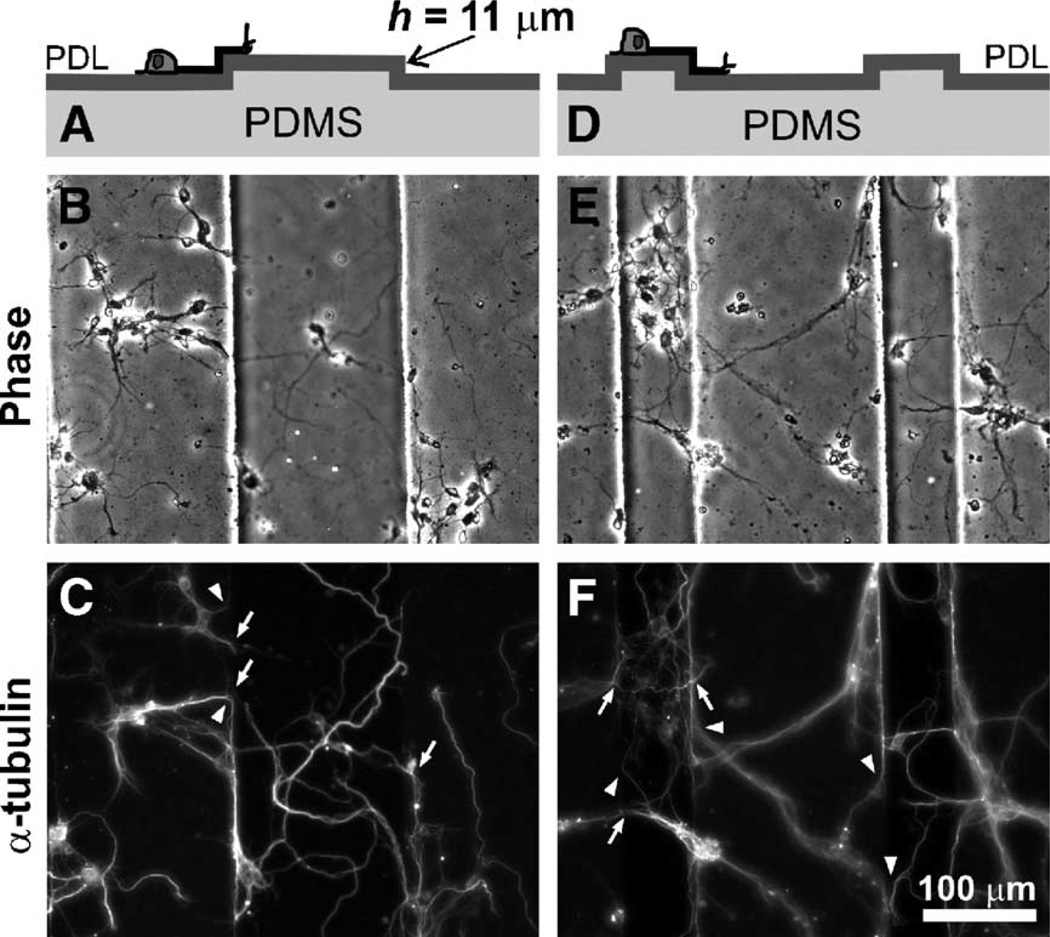
Fig. 4.
Mixed responses of axons to steps of intermediate depths. Cross-sectional schematic representation (top row), phase-contrast (middle row) and corresponding α-tubulin immunofluorescence micrographs (bottom row) illustrating the typical axon turning behavior at the edges of 11-µm-deep PDL-coated PDMS grooves. Some axons (whether inside the grooves (A–C) or on the plateaus (D–F)) crossed the edges to the upper or lower surfaces (white arrows in the graph), while others turned and grew along the edges (arrowheads).
Fig. 5.
Summary of neuronal responses to microfabricated steps. (A) Percentage of axons that cross a step as a function of step height h. The graph shows that the percentage of axons that disregard the micro-topography increases as the groove depth decreases, with a threshold at ∼11 µm. The inset shows a Hoffman DIC image of an axon from a murine cortical neuron (E13, 3 DIV). Note that the size of the growth cone is ∼10 µm, similar to the threshold in step size for topographical guidance. (B) Two sets of phase-contrast (a, c) and corresponding α-tubulin immunofluorescence images (b, d) illustrating two types of responses on 11-µm-high steps as a function of the angle at which the axon tip reaches the step edge (“angle of approach”, a, see inset schematic in C, top view). (C) Percentage of axons that overcome an 11-µm-high step as a function of α. We hypothesize that axons do not need to bend 90° to cross the step, rather they can gently slope up or down the step forming an angle β (“bridging angle”, see inset schematic, side view) with the original axon direction. In agreement with this hypothesis, the graph shows that as α increases axons are more likely to choose to bend the angle β and disregard the step, bridging onto the next plane.
To explain these results, we hypothesize that the height for which the vertical wall is no longer an obstacle for axons to overcome the step is determined by the 3-D exploratory range of the growth cone. Several pieces of evidence support this hypothesis: (1) growth cones are not permanently anchored to substrates, thus they are inherently capable of 3-D exploration (see supplemental movie V1); (2) the threshold (∼10 µm) in step size for which half of the axons ignore the topographical change is similar to the typical in-plane width of a growth cone (see Fig. 5A inset); and (3) we have also observed that axons are occasionally able to grow through fluid gaps and bridge 20 µm-wide, 50 µm-deep PDMS grooves (data not shown). A definite proof of this hypothesis could, in principle, be obtained with live-cell 3-D imaging techniques (e.g., confocal microscopy of GFP-expressing neurons).
The mixed responses of axons to steps of intermediate depths (h = 11 and 15 µm) may be due partially to the unavoidable variability in growth cones sizes, growth dynamics, neuronal types, and local surface conditions, or partially to the fact that growth cones do not seem to need to bend 90° in order to overcome a small step (contrary to the largest steps). In shallow grooves, when axons overcame a step they appeared to slope gently up or down the wall (judging from focal changes). We reasoned that the incidence of turning should be a function of the angle of approach (α in Fig. 5B, see inset schematic in Fig. 5C) because above a certain value of α the axon should display its preference to grow as straight as possible by bridging to the next plane, bending an angle β < 90° (“bridging angle”, see inset schematic in Fig 5C). Fig. 5C confirms that α strongly affects axon turning for the 11-µm steps: as α increases, it becomes increasingly more difficult for axons to turn along the edge than to bridge to the next plane. Since the range of angles of approach for which the percentage of turning axons reaches 50% is α = 45–60°, we conjecture that β may be on the order of ∼45–60° for h = 11 µm. From Fig. 5C, we conclude that the general observation that axons have a preference for the minimal-bending angle still holds, even for axons that bridge different planes.
Axon guidance by simultaneous microtopographical and biochemical cues
During development, axons are surrounded by a changing 3-D environment consisting of multiple guidance cues, including soluble and immobilized biochemical factors in various topographical arrangements; the spatiotemporal integration of all the guidance signals ultimately dictates the growth and turning response of the axon. To investigate axon decision-making in response to combined topographical and biochemical contact cues, we cultured neurons on PDL-coated PDMS microgrooves covered with the gel matrix Matrigel (growth factor-reduced).
In our experiments, the mixture of cell suspension and Matrigel liquid precursor (not yet gelled) was allowed to settle for 5–10 min before being transferred to a 37°C incubator for complete gelling. In this period of time, some cells had time to settle onto the PDL-coated PDMS surface; the rest of the cells were fully embedded in Matrigel and observed in higher focal planes, extending axons randomly in all three dimensions. Neurons fully embedded in Matrigel (not attached to the surface) typically showed a polarized bright soma under phase-contrast microscopy (see Supplemental Fig. S2). In contrast, neurons that settled onto the PDMS surface, despite being covered and surrounded by Matrigel, always displayed the phase-contrast morphology typical of an attached cell and grew axons on the plane of the surface (Fig. 6). This preference for surface-immobilized PDL over the 3-D gel is similar to the aforementioned preference for adsorbed PDL over adsorbed Matrigel regions in planar micropatterns (see Fig. 1B). Thus, a preliminary conclusion would be that axons prefer to adhere to PDL rather than to Matrigel.
Fig. 6.
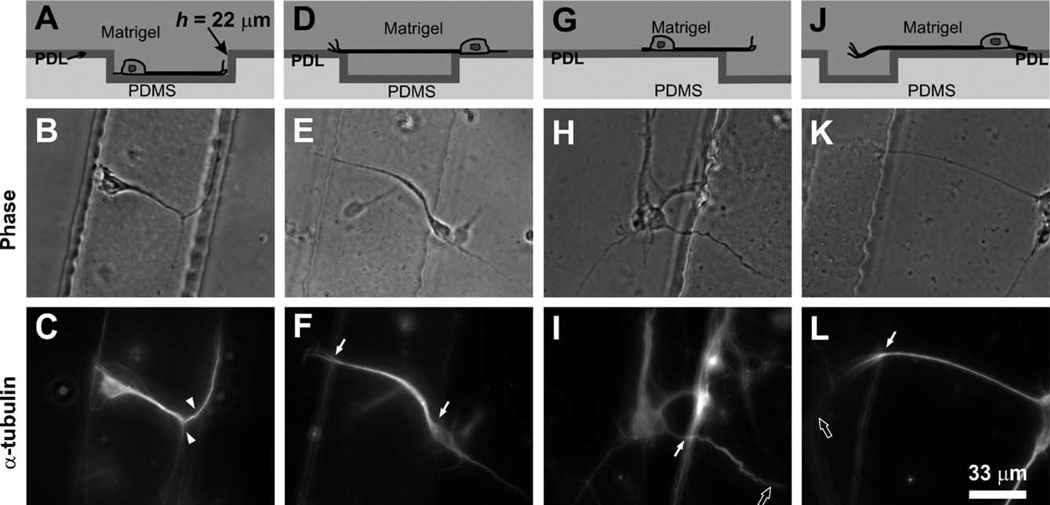
Axon growth on microstructured PDMS substrates with competing topographical and biochemical cues. Cross-sectional schematic representation (top row), phase-contrast (middle row) and corresponding α-tubulin immunofluorescence micrographs (bottom row) illustrating four typical axon growth patterns observed on PDL-coated, microstructured PDMS substrates covered with the growth-supporting gel Matrigel. When neurons were grown on substrates containing 22 µm-deep grooves, axons (both inside the grooves (A–C) and on the top plateau surface (G–L)) initially preferred PDL to Matrigel and kept growing attached to the PDL-coated PDMS surface. As axons reached the groove walls, in-groove axons were guided by topographical features and turned (arrowheads in C) along the wall (A–C), while on-plateau axons extended into Matrigel (D–F: crossing the gel and landing onto the other side of grooves; G–I and J–L: extending out of focus deeper into the gel) (solid white arrows indicating axons that cross the edges and hollow white arrows indicating axons that are out of focus).
To challenge the generality of that conclusion, we followed the growth of substrate-attached neurons on Matrigel-covered substrates containing topographical steps of h = 22 µm, 44 Am, or 69 Am. Here we distinguish between the neurons that settled inside the grooves (“in-groove neurons”) and those that settled on top of the grooves (“on-plateau neurons”). Naturally, the axons of in-groove neurons only had two choices when the growth cone encountered the wall: to grow up the wall or to stay inside the groove; we found that the axon typically continued to grow along the bottom edge of the wall (a topographical guidance virtually identical to that observed at the bottom of grooves in the absence of Matrigel, see Figs. 6A–C). In contrast, when the growth cone of an “on-plateau” axon reached the edge of the plateau, in effect it was presented with two surface biochemical cues (PDL on the PDMS surface and Matrigel ahead) and one surface topographical cue (the step of the groove). Thus, the axon faced two growth choices: 1) “take off” and grow into Matrigel, or 2) stay attached to the PDL surface and turn a certain angle to the right, to the left, or 90° downward into the groove. We found that, upon arrival at the edge, almost all of on-plateau axons preferred to extend into Matrigel (Figs. 6D–L) rather than turning along the PDL-coated groove edge (97.7 ± 1.6% for h = 22 µm (n = 107), 100.0 ± 0.0% for h = 44 µm (n = 54), and 99.0 ± 1.0% for h = 69 µm (n = 140)). This straight growth behavior contrasts sharply with the micro-topography-guided turning behavior observed on grooves in the absence of Matrigel (Fig. 2). The straightness of the 3-D growth was similar to that of growth on PDMS plateaus: some axons crossed the gel and landed onto the opposing plateau (Figs. 6D–F) while other axons (Figs. 6G–I and J– L) grew into and gradually changed height in Matrigel. For smaller step heights (3.5, 11, 15 µm) covered with Matrigel, on-plateau axons also extend straight into Matrigel judging from differences in focal planes (data not shown).
The observation that on-plateau axons “take off” straight into Matrigel from the edge of the plateau also contrasts with the observation that the same neurons do not take off into Matrigel before reaching the edge of the plateau. The two observations combined demonstrate a clear preference by the axon to grow in a direction that minimizes turning (which we term a “straightness preference”). As shown above, axons also manifest this straightness preference on grooves in the absence of Matrigel when the growth cone ignores a shallow step or when it chooses the minimal-angle path in front of a deep step. Therefore, independently of whether the groove is covered by fluid or Matrigel, at the edge of a step the axon is inevitably conflicted between its “permissiveness preference” (for PDL) and its “straightness preference”. Hence we may speak of an “overall preference” that integrates both preferences and essentially defines the growth cone’s decision for the “best” option. On planar micropatterns (Fig. 1), this integration process has a clear effect when the permissiveness terminates abruptly (as in the PDL-glass borders, Fig. 1A) but, predictably, it has a more subtle effect when the permissiveness of two adjacent regions is only slightly different (i.e., axons are often seen to ignore the PDL-Matrigel borders, see Fig. 1B).
In growth cones, actin filaments and microtubules (MTs) are the cytoskeletal components responsible for locomotion and are the ultimate targets of directional signaling [44]. The observed axonal preference for minimal bending angles may reflect an intrinsic property of cytoskeletal organization. To better understand the intracellular mechanisms of axon growth and guidance, further investigations on cytoskeleton regulation and mechanics will be needed.
Axon guidance mechanisms share similarities with those of axon branching (also an important phenomenon in shaping neuronal morphology and neural connections), as reviewed in [45]. In our experiments, we sometimes observe the presence of lateral growth cones, which could be the sites for axon branching or eventual turning points for axons. For example, in the developing corpus callosum, efferent axons pause underneath the contralateral cortex target region, then regrow primary axons with the growth cone remnant left behind. The interstitial branches grow from the growth cone remnant, and eventually complete the callosal connection after the primary axons regress [46]. In the corticospinal tract, interstitial branches also innervate target neurons [47]. Lateral growth cones and axon turning may also contribute to the turning responses of axons to surface chemical or topographical boundaries.
In conclusion, we have demonstrated a cell culture system where axon 3-D growth in response to two simultaneous contact cues can be studied. We propose that axons have an intrinsic tendency to explore in 3-D and that, at least in the absence of chemotropic factors, the decision to extend in a given direction is made by the growth cone by integrating its preferences to grow straight and its preference to grow on the most permissive substrate. Extracellular signals act through intracellular pathways, leading to a subsequent concerted activity of microtubule and actin cytoskeleton, thus enabling the growth cone to navigate towards precise targets through complex microenvironments.
Supplementary Material
Acknowledgments
We thank Dr. Marc Tessier-Lavigne, Dr. Douglas Currie, and Thomas Keenan for their technical help and insightful discussions, and Dr. Lisa Horowitz for critical comments on the manuscript. This work was supported by the National Institute of Biomedical Imaging and Bioengineering grant #EB003307 and by the National Science Foundation Career Award to A.F. Photolithography was done at Washington Technology Center.
Abbreviations
- 3-D
three-dimensional
- MT
Microtubule
- PDL
poly-d-lysine
- PDMS
polydimethylsiloxane
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.yexcr.2005.10.007.
References
- 1.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 2.Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu. Rev. Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo Y, Raper JA. Inhibitory factors controlling growth cone motility and guidance. Curr. Opin. Neurobiol. 1994;4:648–654. doi: 10.1016/0959-4388(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 4.Keynes RJ, Cook GM. Repulsive and inhibitory signals. Curr. Opin. Neurobiol. 1995;5:75–82. doi: 10.1016/0959-4388(95)80090-5. [DOI] [PubMed] [Google Scholar]
- 5.Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu. Rev. Cell Dev. Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- 6.Silver J, Sidman RL. A mechanism for the guidance and topographic patterning of retinal ganglion cell axons. J. Comp. Neurol. 1980;189:101–111. doi: 10.1002/cne.901890106. [DOI] [PubMed] [Google Scholar]
- 7.Krayanek S, Goldberg S. Oriented extracellular channels and axonal guidance in the embryonic chick retina. Dev. Biol. 1981;84:41–50. doi: 10.1016/0012-1606(81)90368-7. [DOI] [PubMed] [Google Scholar]
- 8.Letourneau PC. Cell-to-substratum adhesion and guidance of axonal elongation. Dev. Biol. 1975;44:92–101. doi: 10.1016/0012-1606(75)90379-6. [DOI] [PubMed] [Google Scholar]
- 9.Kleinfeld D, Kahler KH, Hockberger PE. Controlled outgrowth of dissociated neurons on patterned substrates. J. Neurosci. 1988;8:4098–4120. doi: 10.1523/JNEUROSCI.08-11-04098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung DR, Kapur R, Adams T, Giuliano KA, Mrksich M, Craighead HG, Taylor DL. Topographical and physicochemical modification of material surface to enable patterning of living cells. Crit. Rev. Biotechnol. 2001;21:111–154. doi: 10.1080/20013891081700. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Tourovskaia A, Folch A. Biology on a chip: microfabrication for studying the behavior of cultured cells. Crit. Rev. Biomed. Eng. 2003;31:423–488. doi: 10.1615/critrevbiomedeng.v31.i56.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey JM, Feldman EL. Substrate patterning: an emerging technology for the study of neuronal behavior. Exp. Neurol. 2003;184:S89–S96. doi: 10.1016/s0014-4886(03)00392-3. [DOI] [PubMed] [Google Scholar]
- 13.Gundersen RW. Sensory neurite growth cone guidance by substrate adsorbed nerve growth factor. J. Neurosci. Res. 1985;13:199–212. doi: 10.1002/jnr.490130114. [DOI] [PubMed] [Google Scholar]
- 14.Hammarback JA, Letourneau PC. Neurite extension across regions of low cell–substratum adhesivity: implications for the guidepost hypothesis of axonal pathfinding. Dev. Biol. 1986;117:655–662. doi: 10.1016/0012-1606(86)90334-9. [DOI] [PubMed] [Google Scholar]
- 15.Lemmon V, Burden SM, Payne HR, Elmslie GJ, Hlavin ML. Neurite growth on different substrates-permissive versus instructive influences and the role of adhesive strength. J. Neurosci. 1992;12:818–826. doi: 10.1523/JNEUROSCI.12-03-00818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark P, Britland S, Connolly P. Growth cone guidance and neuron morphology on micropatterned laminin surfaces. J. Cell Sci. 1993;105:203–212. doi: 10.1242/jcs.105.1.203. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzawa M, Tokumitsu S, Knoll W, Liesi P. Molecular gradient along the axon pathway is not required for directional axon growth. J. Neurosci. Res. 1998;53:114–124. doi: 10.1002/(SICI)1097-4547(19980701)53:1<114::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Esch T, Lemmon V, Banker G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J. Neurosci. 1999;19:6417–6426. doi: 10.1523/JNEUROSCI.19-15-06417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990;108:635–644. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- 20.Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour. I. Simple step cues. Development. 1987;99:439–448. doi: 10.1242/dev.99.3.439. [DOI] [PubMed] [Google Scholar]
- 21.Rajnicek A, Britland S, McCaig C. Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions neuronal age and cell type. J. Cell Sci. 1997;110:2905–2913. doi: 10.1242/jcs.110.23.2905. [DOI] [PubMed] [Google Scholar]
- 22.Rajnicek A, McCaig C. Guidance of CNS growth cones by substratum grooves and ridges: effects of inhibitors of the cytoskel-eton calcium channels and signal transduction pathways. J. Cell Sci. 1997;110:2915–2924. doi: 10.1242/jcs.110.23.2915. [DOI] [PubMed] [Google Scholar]
- 23.Nagata I, Kawana A, Nakatsuji N. Perpendicular contact guidance of CNS neuroblasts on artificial microstructures. Development. 1993;117:401–408. doi: 10.1242/dev.117.1.401. [DOI] [PubMed] [Google Scholar]
- 24.Merz M, Fromherz P. Polyester microstructures for topographical control of outgrowth and synapse formation of snail neurons. Adv. Mater. 2002;14:141–144. [Google Scholar]
- 25.Cyster LA, Parker KG, Parker TL, Grant DM. The effect of surface chemistry and nanotopography of titanium nitride (TiN) films on primary hippocampal neurones. Biomaterials. 2004;25:97–107. doi: 10.1016/s0142-9612(03)00480-0. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney MJ, Chen RR, Tan J, Saltzman WM. The influence of microchannels on neurite growth and architecture. Biomaterials. 2005;26:771–778. doi: 10.1016/j.biomaterials.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Xia YN, Kim E, Whitesides GM. Micromolding of polymers in capillaries: applications in microfabrication. Chem. Mater. 1996;8:1558–1567. [Google Scholar]
- 28.Folch A, Ayon A, Hurtado O, Schmidt MA, Toner M. Molding of deep polydimethylsiloxane microstructures for microfluidics and biological applications. J. Biomech. Eng. 1999;121:28–34. doi: 10.1115/1.2798038. [DOI] [PubMed] [Google Scholar]
- 29.Tourovskaia A, Barber T, Wickes BT, Hirdes D, Grin B, Castner DG, Healy KE, Folch A. Micropatterns of chemisorbed cell adhesion-repellent films using oxygen plasma etching and elastomeric masks. Langmuir. 2003;19:4754–4764. [Google Scholar]
- 30.Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Sul JY, Haydon PG. A calcium-induced calcium influx factor nitric oxide modulates the refilling of calcium stores in astrocytes. J. Neurosci. 2003;23:10302–10310. doi: 10.1523/JNEUROSCI.23-32-10302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price PJ, Brewer GJ. Serum-free media for neural cell cultures: adult and embryonic. In: Fedoroff S, Richardson A, editors. Protocols for Neural Cell Culture. Totowa, NJ: Humana Press; 2001. pp. 255–264. [Google Scholar]
- 33.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 34.Gundersen RW. Response of sensory neurites and growth cones to patterned substrata of laminin and fibronectin in vitro. Dev. Biol. 1987;121:423–431. doi: 10.1016/0012-1606(87)90179-5. [DOI] [PubMed] [Google Scholar]
- 35.Hammarback JA, McCarthy JB, Palm SL, Furcht LT, Letourneau PC. Growth cone guidance by substrate-bound laminin pathways is correlated with neuron-to-pathway adhesivity. Dev. Biol. 1988;126:29–39. doi: 10.1016/0012-1606(88)90235-7. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzawa M, Liesi P, Knoll W. Chemically modifying glass surfaces to study substratum-guided neurite outgrowth in culture. J. Neurosci. Methods. 1996;69:189–196. doi: 10.1016/S0165-0270(96)00052-0. [DOI] [PubMed] [Google Scholar]
- 37.James CD, Davis R, Meyer M, Turner A, Turner S, Withers G, Kam L, Banker G, Craighead H, Isaacson M, Turner J, Shain W. Aligned microcontact printing of micrometer-scale poly-l-lysine structures for controlled growth of cultured neurons on planar microelectrode arrays. IEEE Trans. Biomed. Eng. 2000;47:17–21. doi: 10.1109/10.817614. [DOI] [PubMed] [Google Scholar]
- 38.Yeung CK, Lauer L, Offenhausser A, Knoll W. Modulation of the growth and guidance of rat brain stem neurons using patterned extracellular matrix proteins. Neurosci. Lett. 2001;301:147–150. doi: 10.1016/s0304-3940(01)01628-7. [DOI] [PubMed] [Google Scholar]
- 39.Chang JC, Brewer GJ, Wheeler BC. A modified microstamping technique enhances polylysine transfer and neuronal cell patterning. Biomaterials. 2003;24:2863–2870. doi: 10.1016/s0142-9612(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 40.Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- 41.Taub M, Wang Y, Szczesny TM, Kleinman HK. Epidermal growth factor or transforming growth factor alpha is required for kidney tubulogenesis in matrigel cultures in serum-free medium. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4002–4006. doi: 10.1073/pnas.87.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtis A, Wilkinson C. Topographical control of cells. Biomaterials. 1997;18:1573–1583. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 43.Dowell-Mesfin NM, Abdul-Karim MA, Turner AM, Schanz S, Craighead HG, Roysam B, Turner JN, Shain W. Topographically modified surfaces affect orientation and growth of hippocampal neurons. J. Neural Eng. 2004;1:78–90. doi: 10.1088/1741-2560/1/2/003. [DOI] [PubMed] [Google Scholar]
- 44.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 45.Dent EW, Tang F, Kalil K. Axon guidance by growth cones and branches: common cytoskeletal and signaling mechanisms. Neuroscientist. 2003;9:343–353. doi: 10.1177/1073858403252683. [DOI] [PubMed] [Google Scholar]
- 46.Halloran MC, Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J. Neurosci. 1994;14:2161–2177. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Leary DD, Bicknese AR, De Carlos JA, Heffner CD, Koester SE, Kutka LJ, Terashima T. Target selection by cortical axons: alternative mechanisms to establish axonal connections in the developing brain. Cold Spring Harbor Symp. Quant. Biol. 1990;55:453–468. doi: 10.1101/sqb.1990.055.01.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.