Abstract
Objective
Myeloid lineage cells (MLCs) such as macrophages are known to play a key role in post-ischemic neovascularization. However, the role of MLC-derived reactive oxygen species (ROS) in this process and the chemical identity of the ROS remain unknown.
Methods and Results
Transgenic mice with MLC-specific over-expression of catalase (TgCat-MLC mice) were created on a C57BL/6 background. Macrophage catalase activity was increased 3.4-fold compared to wild-type mice. After femoral artery ligation, LASER Doppler perfusion imaging revealed impaired perfusion recovery in TgCat-MLC mice. This was associated with fewer collateral vessels, as assessed by micro CT angiography, and decreased capillary density. Impaired functional recovery of the ischemic limb was also evidenced by a 50% reduction in spontaneous running activity. The deficient neovascularization was associated with a blunted inflammatory response, characterized by decreased macrophage infiltration of ischemic tissues, and lower mRNA levels of inflammatory markers such as tumor necrosis factor-α, osteopontin, and matrix mettaloproteinase-9. In vitro macrophage migration was impaired in TgCat-MLC mice, suggesting a role for H2O2 in regulating the ability of macrophages to infiltrate ischemic tissues.
Conclusions
MLC-derived H2O2 plays a key role in promoting neovascularization in response to ischemia and is a necessary factor for the development of ischemia-induced inflammation.
Keywords: limb ischemia, reactive oxygen species, angiogenesis, hydrogen peroxide, macrophages
Neovascularization is an important repair mechanism to preserve tissue integrity after ischemia, and it is a key determinant of the final outcome of vascular occlusive diseases such as coronary or peripheral vascular atherosclerosis1, 2. The term describes a complex process that includes the sprouting of new capillary beds (angiogenesis), the transformation of small arterioles into large conductance arteries (collateral vessel formation, also named arteriogenesis), and the in-situ differentiation of endothelial progenitors into mature endothelial cells (vasculogenesis)3. In the setting of acute ischemia, it is thought that the largest contributor to blood flow recovery is provided by arteriogenesis in the form of collateral arteries3. Whereas hypoxia is the major driving force in angiogenesis, arteriogenesis seems to be more dependent on mechanical forces and inflammation4. Previous studies have shown that myeloid lineage cells (MLCs), in particular macrophages, play a central role in orchestrating the inflammatory response that promotes collateral vessel growth5-8. Several factors released by macrophages, such as reactive oxygen species (ROS), tumor necrosis factor alpha (TNF-α), osteopontin (OPN), and matrix metalloproteinases (MMPs), among others, modulate post-ischemic neovascularization9-11.
ROS such as superoxide anion (O2·-) and hydrogen peroxide (H2O2) are key regulators of physiological and pathophysiological responses in the vasculature. In excess amounts, ROS are mediators of cellular injury. In contrast, at physiological levels, ROS participate in strictly regulated redox signaling pathways, modulating cell migration, proliferation, and matrix remodeling, all of which are necessary events for neovascularization12-15. In addition, the proangiogenic effects of vascular growth factors such as vascular endothelial growth factor (VEGF) and angiopoietin-1 appear to be mediated at least in part by the production of ROS16, 17. Potential enzymatic sources of ROS in the vascular wall are diverse and include mitochondria18, xanthine oxidase19, uncoupled nitric oxide synthase20, and the NADPH oxidase isoforms present in a variety of cell types21, 22. In vivo studies using knockout mice for the gp91phox subunit of NADPH oxidase show altered neovascularization after hind limb ischemia23, thus highlighting the importance of this particular source of ROS.
Despite the strong evidence for the role of ROS as major mediators of the angiogenic response to ischemia, the specific ROS involved remains unclear. Compared to O2·-, H2O2 is uncharged, can diffuse freely across lipid membranes, and is longer-lived, which makes it a good candidate for redox signaling in the vasculature. In this study we demonstrate that H2O2 derived from inflammatory cells is an important mediator of post-ischemic neovascularization. Using a transgenic mouse model whereby levels of H2O2 in MLCs are decreased by the tissue-specific over-expression of catalase, we show impaired collateral vessel formation and functional recovery of the hind limb after ischemia. This was associated with an impaired migratory activity of catalase-overexpressing macrophages and an overall reduced inflammatory response in the ischemic tissues.
Methods
Details are provided in an expanded Methods section in the online Data Supplement, available at http://atvb.ahajournals.org.
Generation and Characterization of Transgenic Mice with Specific Over-expression of Human Catalase in MLCs
To develop mice with MLC–specific over-expression of catalase (TgCat-MLC mice), we used a Cre/LoxP system similar to that described previously24, modified to have the cre-recombinase cDNA under the control of the MLC-specific promoter Lysz. Due to the genetic design used, cells other than MLCs are expected to show green fluorescent protein (GFP) fluorescence, while selective expression of cre-recombinase in MLCs will lead to excision of the GFP gene and loss of fluorescence in this cell population (for details, please see the online methods supplement and supplemental Figure I). All mice used in this study were on a C57BL/6 background. Control mice used for all experiments, referred from now on as wild type (WT) mice, were littermates of the transgenic mice. Only male mice between 8 and 10 weeks were used. All procedures were approved by the Emory University Institutional Animal Care and Use Committee and were in compliance with the standards for the care and use of laboratory animals of the Institute of Laboratory Animal Resources, National Academy of Sciences, Bethesda, MD.
The expression of human catalase and other antioxidant enzymes was assessed by western blot of thioglycollate-induced peritoneal macrophages. Catalase activity in peritoneal macrophages was measured as previously described24. The specificity of the cre-mediated recombination in MLCs was assessed by immunocytochemistry of peritoneal cells and immunohistochemistry of lung tissues as detailed in the online supplement.
Mouse Model of Unilateral Hind limb Ischemia and Assessment of Neovascularization
Ligation and excision of the left superficial femoral artery, LASER Doppler perfusion imaging (LDPI) and micro-CT imaging were performed as previously described9. Capillary density, expressed as number of capillaries per muscle fiber, was assessed by immunostaining of gastrocnemius muscle against CD31and β-dystroglycan. Functional recovery of the ischemic limb was assessed by monitoring of spontaneous activity in cages equipped with a running wheel.
Quantification of Mobilized Endothelial Progenitor Cells (EPCs) and EPC Culture Assay
EPCs were quantified from bone marrow or peripheral blood mononuclear cells (MNCs) as Sca-1+/Flk-1+ cells by flow cytometry. EPC culture assay was performed as previously described25, 26.
Assessment of Inflammation in the Ischemic Hind Limb
At post-operative day 5, paraffin sections of gastrocnemius muscle were obtained and processed for conventional hematoxylin and eosin staining or immunostaining against the macrophages-specific marker MAC-3 or against an anti-neutrophil antibody. Levels of CD68, OPN, TNF-α, MMP-9, VEGF and SDF-1 mRNA in gastrocnemius muscle were measured by quantitative real-time PCR (qRT-PCR) and normalized to ribosomal 18S RNA content.
Macrophage Migration Assay
Macrophage migration in response to MCP-1 was assessed using a modified Boyden chamber assay as previously described9.
Measurement of H2O2 Production
H2O2 production from gastrocnemius muscle or peritoneal macrophages was measured by Amplex Red assay (Invitrogen, Carlsbad, California) as per the manufacturer’s instructions.
Statistical Analysis
Data are presented as mean ± SEM. When applicable, values were compared by Student t test or ANOVA with Bonferroni post-hoc analysis. p < 0.05 was considered to be statistically significant.
Results
Phenotypic Characterization of Transgenic Mice
The expression of human catalase in MLCs of TgCat-MLC mice was evaluated by western blot analysis of isolated peritoneal macrophages using an antibody that preferentially identifies the human enzyme24. Figure 1A demonstrates the over-expression of human catalase in TgCat-MLC macrophages. Other antioxidant enzyme systems were not altered compared to littermate WT controls (Figure 1A). To confirm that the over-expression of catalase resulted in an increase in enzymatic activity, we assayed for catalase activity in peritoneal macrophages. As shown in Figure 1B, TgCat-MLC macrophages exhibited a 3.4-fold increase in catalase activity compared to WT macrophages (1169.4 ± 241.2 vs 343.1 ± 63.4 mU/mg of protein, respectively, p < 0.05).
Figure 1. MLC-specific catalase over-expression in TgCat-MLC mice.
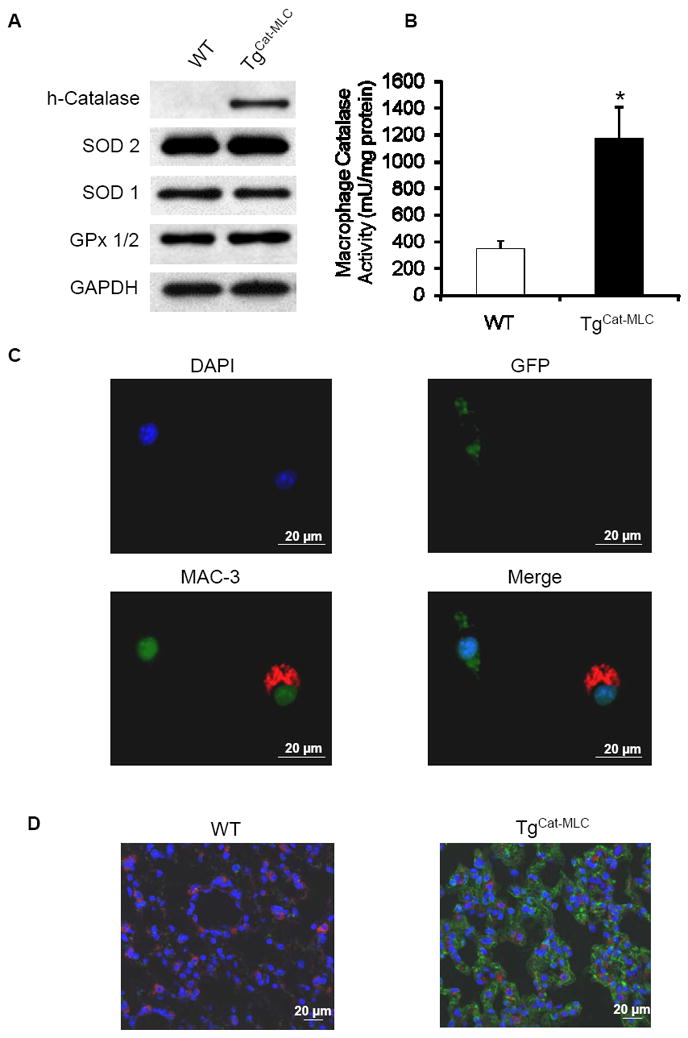
(A) Western blot analyses of peritoneal macrophages show over-expression of human catalase in TgCat-MLC mice. Levels of superoxide dismutase (SOD)-1, SOD-2, and glutathione peroxidase (GPx) 1/2 protein were not different from WT mice. Shown are representative blots of three independent experiments. (B) Macrophage catalase activity was up-regulated 3.4 fold in TgCat-MLC mice compared to WT mice. *p < 0.05. (C) Immunocytochemistry of peritoneal cells from TgCat-MLC mice shows absence of GFP fluoresce (green) in macrophages (red) but not in other cells types. Nuclei are stained with DAPI (blue). (D) Merged confocal images of lung sections stained for GFP (green), alveolar macrophages (red) and DAPI (blue). GFP fluorescence can be seen in the alveolar epithelium of TgCat-MLC but not in WT mice. The absence of co-localization between red and green (yellow) indicates selective recombination of GFP in macrophages.
To verify that the excision of the GFP cDNA by cre-recombinase (which allows for the expression of the human catalase gene) was confined to MLCs, we performed immunocytochemistry on thioglycollate-induced peritoneal cells of TgCat-MLC mice (Figure 1C), as well as immunostaining of lung sections (Figure 1D). After 72 hours of thioglycollate injection, most peritoneal leukocytes are macrophages27. Figure 1C shows that peritoneal cells stained for the macrophage specific antigen MAC-3 did not show GFP fluorescence, while cells with intrinsic GFP fluorescence were not stained for MAC-3. This absence of co-localization between GFP fluorescence and macrophage markers was also confirmed in confocal images of lung sections where, unlike the alveolar epithelium, resident alveolar macrophages lacked GFP (Figure 1D). These data demonstrate that human catalase expression is indeed confined to MLCs and not present in other cell types.
Baseline characteristics before ischemia, including muscle capillary density (supplemental Figure II) and expression of angiogenic factors such as VEGF and SDF-1 (supplemental Figure III), were not different between genotypes. There was no obvious effect of MLC–specific over-expression of catalase on number of total circulating leukocytes or percentages of granulocytes, monocytes and lymphocytes (supplemental Figure IV). Similarly, no differences in litter size, body weight, blood pressure, susceptibility to infections, or other physical descriptors were found (data not shown).
LASER Doppler Perfusion Imaging
LDPI measurements were done from day 2 through day 28 after femoral artery ligation and excision. The acquired images illustrated a significant lag in recovery in TgCat-MLC mice (Figure 2A). Quantitative perfusion measurements confirmed this discrepancy (Figure 2B). WT mice showed a steady recovery in perfusion, reaching a maximum perfusion ratio of 52 ± 5% at day 28 relative to the non-ischemic leg. In contrast, TgCat-MLC mice showed a blunted curve, which diverged from the WT curve after day 7 and reached a maximum perfusion ratio of only 37 ± 2% at day 28 (Figure 2B, p < 0.05). The difference in perfusion between WT and TgCat-MLC mice achieved statistical significance at day 14 and was maintained at the subsequent time points.
Figure 2. LDPI analysis.

(A) Representative LDPI images demonstrate the time course of ischemic limb reperfusion in TgCat-MLC and WT mice. (B) Quantitative analyses are presented as perfusion ratios (ischemic/non-ischemic leg), up to post-operative day 28. A significant lag in perfusion recovery in TgCat-MLC mice was evident at day 14 and maintained through day 28 (n = 14 to 16 per genotype). *p < 0.05 vs WT.
Micro-CT Imaging of Collateral Vessels
Micro-CT angiography was employed to quantitatively assess vascular anatomy. Qualitative observation of the 3-D image reconstructions of the vasculature performed 28 days after hind limb ischemia showed reduced collateral vessel formation in TgCat-MLC mice relative to WT (Figure 3A). Quantitative analysis of the obtained images using 3-D histomorphometric software revealed an approximate 30% reduction in the vascular volume to tissue volume ratio and a 15% reduction in vascular density in TgCat-MLC mice compared to WT (Figure 3B).
Figure 3. Analysis of collateral vessel formation, capillary density, and spontaneous motor activity.
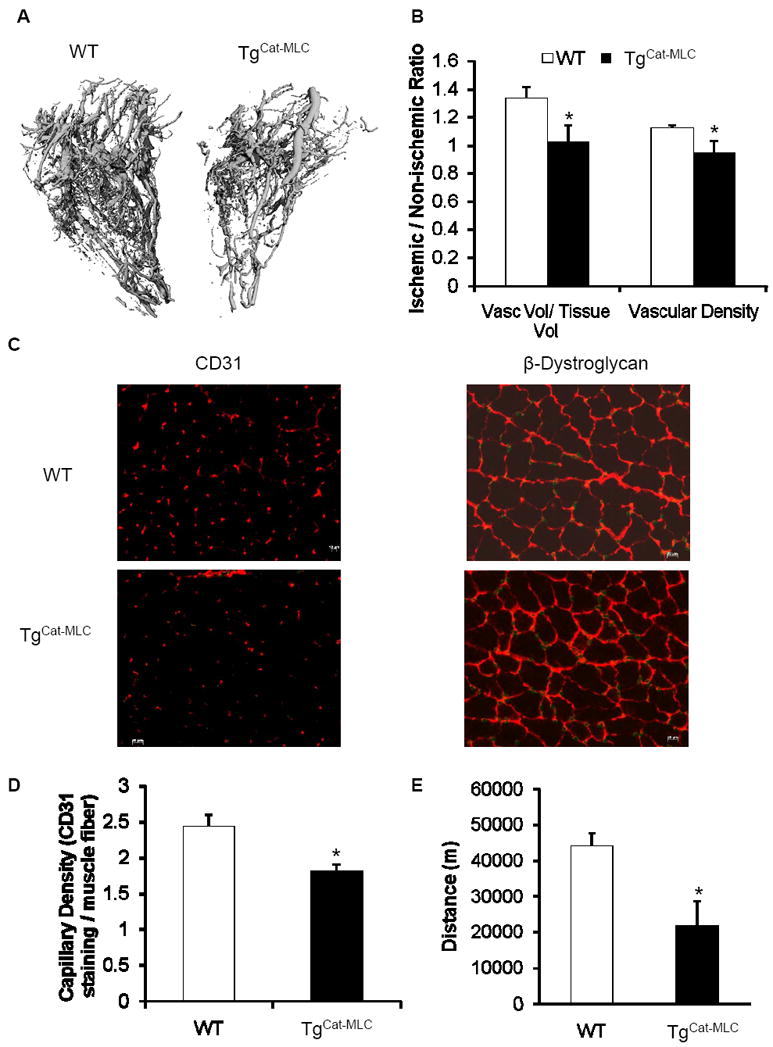
(A) Representative Micro-CT angiographies of ischemic limbs from WT and TgCat-MLC mice obtained at post-operative day 28. (B) Quantitative analysis showed decreased vascular volume / tissue volume ratios as well vascular density in TgCat-MLC mice (n = 8 per genotype). Data are expressed as fold change compared to the non-ischemic leg. *p < 0.05 vs WT. (C) Representative photomicrographs of ischemic gastrocnemius muscles stained with antibodies against CD31 or β-dystroglycan, allowing the detection of capillaries or muscle fibers, respectively, 28 days after surgery. Scale bar = 20 μm. (D) Quantification of capillary density in ischemic gastrocnemius muscle of treated mice using anti-CD 31 antibody and reported to the number of muscle fibers (n = 3 per genotype). *p < 0.05 (E) Spontaneous motor activity recorded over a 7 day period and expressed as total distance run, in meters. Monitoring began at post-operative day 28 (n = 6 per genotype). *p < 0.02.
Capillary Density Analysis
Capillary density was analyzed by immunohistochemistry using antibodies against CD31 and β-dystroglycan to detect endothelial cells and skeletal muscle fibers, respectively (Figure 3C). At post-operative day 28, gastrocnemius muscle from TgCat-MLC mice showed decreased capillary to muscle fiber ratios compared to WT (1.81 ± 0.04 vs 2.44 ± 0.16 capillaries/muscle fiber, p < 0.05, Figure 3D).
Motor Activity Monitoring
As a physiological measure of functional ischemic limb recovery, spontaneous running distance on an activity wheel was measured on postoperative days 28 through 35. Despite the hind limb ischemia, WT mice exhibited a very active profile, running more than 40 kilometers over the course of a week (Figure 3E). In contrast, TgCat-MLC mice displayed significantly decreased motor activity, as shown by a 50% reduction in total distance run relative to WT animals (21.9 ± 6.9 vs 44.1 ± 3.9 Km, p < 0.02, Figure 3E).
EPC Mobilization and Differentiation in Response to Ischemia
To investigate whether a deficiency in EPC mobilization could contribute to the impaired neovascularization in TgCat-MLC mice, we quantified EPCs by flow cytometry. Three days after hind limb ischemia, we found no differences in the number of circulating EPCs in peripheral blood or in bone marrow (supplemental Figure V). We next analyzed the differentiation potential of peripheral blood and bone marrow MNCs into EPCs in culture, as assessed by the uptake of acLDL and the binding of BS-1 lectin26. Despite the impaired neovascularization in TgCat-MLC mice, there was a trend towards an increase in the number of cultured EPCs compared to WT in peripheral blood (4.8 ± 1.0 vs 2.5 ± 0.5 cells per mm2, respectively, p = 0.21, n = 3 per genotype) and bone marrow (167.2 ± 8.3 vs 113.6 ± 16.5 cells per mm2, respectively, p = 0.16, n = 3 per genotype).
Inflammatory Response to Ischemia
The degree of collateral vessel formation after ischemia is directly related to the inflammatory response in the ischemic tissues3, 5, 7, 28. To assess whether the impaired neovascularization seen in TgCat-MLC mice was associated with different levels in inflammatory cell infiltration, we performed histological analysis of the ischemic limbs. Hematoxylin and eosin staining of ischemic distal limbs from WT mice revealed an intense infiltration of inflammatory cells surrounding areas of necrotic muscle fibers (Figure 4A). Immunostaining identified macrophages as the dominant cell type (Figure 4A insert). In contrast, TgCat-MCL mice exhibited significantly lower levels of inflammatory infiltration as compared to WT (Figure 4A). Computer-assisted quantification of the low magnification hematoxylin and eosin images revealed a ≈ 50% decrease in the infiltration of inflammatory cells in TgCat-MLC mice (Figure 4B). At the same time point (post-operative day 5), the presence of neutrophils in ischemic tissues was minimal (supplemental Figure VI).
Figure 4. Inflammatory cell infiltration and expression of inflammatory markers in ischemic tissues.

(A) Representative photomicrographs of ischemic distal limbs at post-operative day 5, stained with hematoxylin and eosin. Inserts show corresponding immunostaining for macrophages (red). Green color represents autofluorescence from muscle fibers. Insert scale bar = 100 μm. (B) Quantitative assessment of the basophilic areas of inflammatory cell infiltration from hematoxylin and eosin images, expressed as percentage of total area (n = 5 per genotype). *p < 0.04. (C) mRNA levels of the corresponding inflammatory markers were measured in ischemic and non-ischemic gastrocnemius muscle at post-operative days 5 and normalized to 18S rRNA (n = 6 per genotype). Data are expressed as fold increase compared to non-ischemic legs of WT mice. *p < 0.05 vs WT non-ischemic, **p < 0.05 vs TgCat-MLC non-ischemic, §p < 0.05 vs WT ischemic. (D) H2O2 production from ischemic and non-ischemic gastrocnemius muscle was assessed by Amplex Red assay at post-operative day 5 (n = 3 per genotype). *p < 0.02 vs WT non-ischemic, **p < 0.02 vs TgCat-MLC non-ischemic. (E) H2O2 production by peritoneal macrophages was assessed by Amplex Red assay at baseline (Ctl) or after stimulation with 10 μg/mL of LPS (LPS). *p < 0.05 vs WT Ctl, **p < 0.05 vs WT + LPS.
To further assess if the difference in inflammatory cell infiltration was associated with differential expression of inflammatory markers within the two genotypes, we performed qRT-PCR analyses of muscle tissues. As shown in Figure 4C, mRNA levels of the macrophage-specific marker CD68 was significantly lower in ischemic limbs of TgCat-MLC mice compared to WT. This is consistent with the reduced number of macrophages seen on immunohistochemistry. We also investigated the expression of other inflammatory genes known to be involved in post-ischemic neovascularization, including TNF-α, OPN, and MMP-99, 10, 11, 29. The up-regulation of these factors in response to ischemia was markedly blunted in TgCat-MLC mice compared to WT (Figure 4C), such that expression was not significantly different from the non-ischemic, control limb. A similar trend was seen for ICAM-1 expression, although it did not reach statistical significance (data not shown). There was no difference in VEGF mRNA or protein expression, or in SDF-1 mRNA levels, between ischemic limbs of WT and TgCat-MLC mice (supplemental Figure VII).
Levels of H2O2 in gastrocnemius muscle were increased in the ischemic leg compared to the non-ischemic leg in both genotypes (Figure 4D). However, there were no significant differences in H2O2 production between WT and TgCat-MLC ischemic muscle (Figure 4D). In contrast, and as expected by their increased catalase activity, production of H2O2 was lower in isolated TgCat-MLC macrophages both at baseline and after stimulation with LPS (Figure 4E).
Macrophage Migration
Many studies have demonstrated that H2O2 plays an important role in regulating cell migration in different cell types including vascular smooth muscle (VSMCs) and endothelial cells (ECs)13, 30. We therefore sought to determine whether catalase over-expression by macrophages led to changes in their migratory function. For this purpose we performed an in vitro macrophage migration assay in a modified Boyden chamber using MCP-1 as the chemotactic factor. Although stimulation with MCP-1 induced macrophage migration in both genotypes compared to media alone, TgCat-MLC cells displayed a significantly impaired migratory response compared to WT (Figure 5).
Figure 5. Macrophage migratory activity.
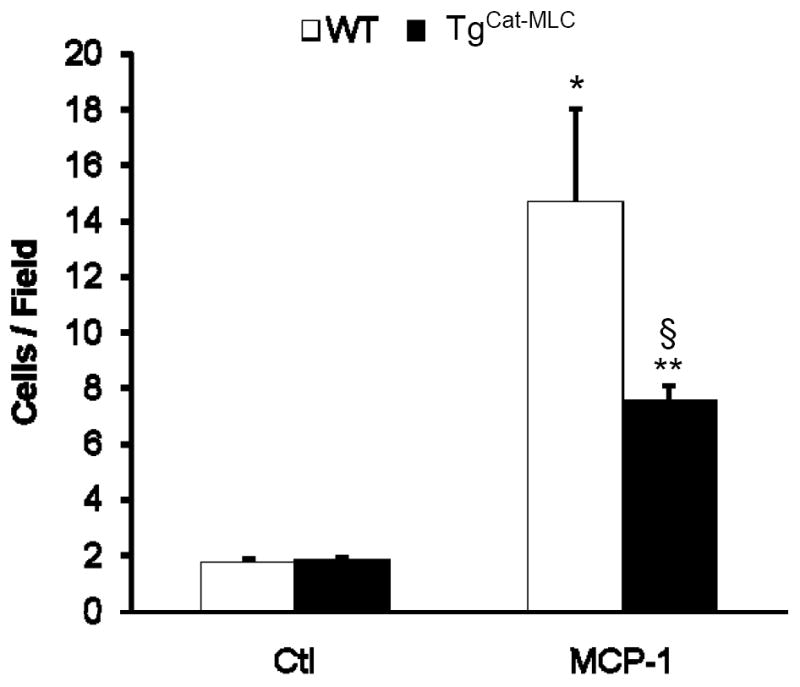
Migration of isolated peritoneal macrophages in response to DMEM media alone (Ctl) or media containing 0.5 μg/mL MCP-1. *p < 0.01 vs WT Ctl, **p < 0.01 vs TgCat-MLC Ctl, §p < 0.02 vs WT + MCP-1.
Discussion
The major finding in our study is that H2O2 derived from cells of myeloid origin plays a key role in post-ischemic neovascularization. Transgenic mice with tissue-specific over-expression of catalase in MLCs had impaired blood flow recovery, collateral vessel development, capillary density, and functional capacity of the hind limb after acute ischemia. This was associated with a blunted inflammatory response in the ischemic tissues, characterized by lower levels of inflammatory markers and reduced macrophage infiltration.
Despite the mounting evidence for the involvement of ROS as major regulators of neovascularization, the role of each specific ROS species, as well as the major cellular sources responsible for their effects, remains unclear. Several animal studies on post-ischemic neovascularization have focused on modulating O2·- levels by genetic manipulation of Nox enzymes or superoxide dismutase isoforms23, 31, 32. Such changes in O2·- are expected to be accompanied by changes in its dismutation product, H2O2. The role of H2O2 in angiogenesis has been studied in vitro, where it has been shown to induce the formation of tube-like structures by ECs33 and promote the proliferation and migration of ECs and VSMCs13-16. However, confirmation of the angiogenic potential of H2O2 in vivo is lacking.
Our study provides novel evidence for the role of MLC-derived H2O2 in promoting neovascularization in vivo, as shown by the impaired perfusion recovery in TgCat-MLC mice. The deficit in perfusion seen on LDPI analyses was explained in part by a reduction in the number of collateral vessels formed, as assessed by micro-CT. These results are in agreement with a previous report where, in an inverse model, catalase deficient mice form larger arterioles in response to angiopoietin-117. Of note, while the LDPI perfusion ratios between the ischemic and non-ischemic legs of WT mice were below 1 at 28 days, the ratios for vascular volume obtained by micro-CT were higher than 1. This suggests the existence of some degree of “ineffective” collateral vessel formation, consisting of non-connecting vessels that provide abnormal perfusion to tissues.
In addition to the effects on collateral vessels, overproduction of catalase by MLCs resulted in decreased capillary density after ischemia. The absence of differences in capillary density in the non-ischemic muscle, along with the LDPI data showing differences in perfusion only at later time points (after day 7), is consistent with an impairment in angiogenesis, as opposed to differences in preexisting collaterals34.
Although O2·- has been implicated in the mobilization of EPCs after hind limb ischemia35, we found no differences between genotypes in the number of EPCs in peripheral blood and bone marrow after surgery, suggesting that this process is not H2O2-sensitive. Surprisingly, there was a trend towards an increase in the number of EPCs cultured from TgCat-MLC MNCs compared to WT. In the setting of overall impaired neovascularization, this may represent a compensatory mechanism to overcome the deficiencies in angiogenesis and collateral vessel formation.
Finally, and most importantly, the impaired neovascularization in TgCat-MLC mice correlated with dramatic differences in functional recovery of the ischemic limb, as evidenced by a 50% reduction in spontaneous motor activity compared to their littermate controls.
The role of inflammation in promoting post-ischemic neovascularization, in particular collateral vessel formation, has been well documented by numerous groups3. Macrophages appear to play a central role in driving new vessel formation. They secrete vascular growth factors, cytokines, MMPs, and ROS, all of which promote proliferation and migration of vascular cells. Indeed, macrophage infiltration of ischemic tissues appears to be a necessary event for collateral vessel development7, and it may be related to their ability to burrow channels for vessel growth through the action of proteolytic enzymes10. Our results clearly show a significant reduction in the inflammatory response mounted by macrophages in TgCat-MLC mice, as evidenced by the immunohistochemistry analyses, as well as quantification of CD68 mRNA in ischemic tissues. This correlated with decreased expression of inflammatory markers such as OPN, TNF-α, and MMP-9; factors that have been implicated in the formation and growth of collateral arteries9, 11. In contrast, expression of VEGF and SDF-1 after ischemia were similar in both genotypes. Thus, these factors did not seem to account for the observed differences in the angiogenic response.
Since H2O2 regulates cell migration in various cell types13, we hypothesized that macrophages from TgCat-MLC mice may have an inherent deficiency in their migratory function. Results from our in vitro migration assays are consistent with this hypothesis, suggesting that the reduced number of infiltrating macrophages in TgCat-MLC mice is a consequence of their impaired ability to migrate in response to an ischemic insult.
Although MLCs from TgCat-MLC mice clearly produced less H2O2 (Figure 4E), the total amount of H2O2 released by the ischemic muscle was not significantly different than WT (Figure 4D). Other cellular sources, such as ECs, VSMCs, or skeletal muscle fibers, may produce enough H2O2 in response to ischemia to hinder any differences arising specifically from MLCs. The absence of differences in global H2O2 levels in ischemic tissues raises the possibility that H2O2 does not have a direct role in neovascularization, but acts indirectly, perhaps by impeding the access of macrophages to the area of ischemia. However, it is also possible that H2O2 directly regulates new vessel formation, but its actions depend on its specific localization at the site of macrophage infiltration. Supporting this latter notion, studies have shown that the actions of H2O2 can be localized not only as a paracrine vascular signal36, but also within different intracellular compartments37.
A limitation of our study is posed by the relative lack of cellular specificity of the lysozyme promoter. Although we found differences in macrophage-mediated inflammation in our transgenic mice, it is important to point out that some activity of the promoter is expected in other inflammatory cells of myeloid origin such as neutrophils38. These cells are known to infiltrate tissues very early and transiently after ischemia39, but their role on post-ischemic neovascularization is less clear, with studies showing diverse effects40, 41. In our model of hind limb ischemia most neutrophils were absent by post-operative day 5, which is consistent with previous reports39. At this same time point macrophage infiltration was intense, and differences in the expression of inflammatory markers between genotypes were significant. These findings demonstrate that H2O2 regulates macrophage-mediated inflammatory events that are relevant to the formation of functional collateral vessels. Although less likely, the data do not rule out a role for neutrophil-derived H2O2 in post-ischemic neovascularization.
Finally, studies show that different concentrations of ROS have disparate effects on angiogenesis. For instance, in young, healthy mice, Nox2 deficiency impairs post-ischemic neovascularization23, whereas, in older mice exposed to tobacco smoke, the same enzyme deficiency augments post-ischemic angiogenesis32. This suggests that while some level of ROS is necessary for neovascularization, an excess is detrimental. Indeed, the existence of an optimal “oxidative window” was also demonstrated by Reed et al. in the coronary circulation42. Thus, generalizing our results to conditions of increased oxidative burden require further studies.
In summary, in this study we identify H2O2 as one of the key mediators secreted by MLCs which regulates post-ischemic neovascularization. The mechanisms by which MLC-derived H2O2 stimulates collateral vessel formation involve the facilitation of macrophage infiltration in ischemic tissues and mounting of an inflammatory response. Targeting MLC-derived H2O2 could lead to improved therapies for regulating neovascularization.
Supplementary Material
Acknowledgments
None
Sources of Funding
This work was supported by American Heart Association grant 10POST4360002 to RH and National Institutes of Health grants RO1 HL090584 and P01 HL095070 to WRT
Footnotes
Disclosures
None.
References
- 1.Sabia PJ, Powers ER, Jayaweera AR, Ragosta M, Kaul S. Functional significance of collateral blood flow in patients with recent acute myocardial infarction. A study using myocardial contrast echocardiography. Circulation. 1992;85:2080–2089. doi: 10.1161/01.cir.85.6.2080. [DOI] [PubMed] [Google Scholar]
- 2.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: A 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 3.Silvestre JS, Mallat Z, Tedgui A, Levy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78:242–249. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 4.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 5.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking cc-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 7.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 8.Hoefer IE, van Royen N, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–617. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 9.Duvall CL, Weiss D, Robinson ST, Alameddine FM, Guldberg RE, Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arterioscler Thromb Vasc Biol. 2008;28:290–295. doi: 10.1161/ATVBAHA.107.158485. [DOI] [PubMed] [Google Scholar]
- 10.Johnson C, Sung HJ, Lessner SM, Fini ME, Galis ZS. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: Potential role in capillary branching. Circ Res. 2004;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goukassian DA, Qin G, Dolan C, Murayama T, Silver M, Curry C, Eaton E, Luedemann C, Ma H, Asahara T, Zak V, Mehta S, Burg A, Thorne T, Kishore R, Losordo DW. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 12.Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med. 2002;33:1047–1060. doi: 10.1016/s0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- 13.Martin AS, Griendling KK. Redox control of vascular smooth muscle migration. Antioxid Redox Signal. doi: 10.1089/ars.2009.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ushio-Fukai M. Redox signaling in angiogenesis: Role of nadph oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Griendling KK, Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. J Lab Clin Med. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 16.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing nad(p)h oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 17.Kim YM, Kim KE, Koh GY, Ho YS, Lee KJ. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res. 2006;66:6167–6174. doi: 10.1158/0008-5472.CAN-05-3640. [DOI] [PubMed] [Google Scholar]
- 18.Quijano C, Castro L, Peluffo G, Valez V, Radi R. Enhanced mitochondrial superoxide in hyperglycemic endothelial cells: Direct measurements and formation of hydrogen peroxide and peroxynitrite. Am J Physiol Heart Circ Physiol. 2007;293:H3404–3414. doi: 10.1152/ajpheart.00761.2007. [DOI] [PubMed] [Google Scholar]
- 19.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from enos: Enzymology and physiological implications. Free Radic Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 21.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of nox1 and nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 22.Babior BM. The nadph oxidase of endothelial cells. IUBMB Life. 2000;50:267–269. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- 23.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (nox2)-containing nad(p)h oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin ii-induced hypertension is mediated by vascular smooth muscle cell-derived h2o2. Hypertension. 2005;46:732–737. doi: 10.1161/01.HYP.0000182660.74266.6d. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. Cd31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: Novel role of nonendothelial cd31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. Hmg-coa reductase inhibitors (statins) increase endothelial progenitor cells via the pi 3-kinase/akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–2009. [PubMed] [Google Scholar]
- 28.Tressel SL, Kim H, Ni CW, Chang K, Velasquez-Castano JC, Taylor WR, Yoon YS, Jo H. Angiopoietin-2 stimulates blood flow recovery after femoral artery occlusion by inducing inflammation and arteriogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1989–1995. doi: 10.1161/ATVBAHA.108.175463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoefer IE, van Royen N, Rectenwald JE, Bray EJ, Abouhamze Z, Moldawer LL, Voskuil M, Piek JJ, Buschmann IR, Ozaki CK. Direct evidence for tumor necrosis factor-alpha signaling in arteriogenesis. Circulation. 2002;105:1639–1641. doi: 10.1161/01.cir.0000014987.32865.8e. [DOI] [PubMed] [Google Scholar]
- 30.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 31.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular sod in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 32.Haddad P, Dussault S, Groleau J, Turgeon J, Michaud SE, Menard C, Perez G, Maingrette F, Rivard A. Nox2-containing nadph oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1522–1528. doi: 10.1161/ATVBAHA.109.191437. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, Kuroiwa Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ets-1 in endothelial cells. Life Sci. 1999;64:249–258. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 34.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 35.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M. Role of nox2-based nadph oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103:212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 37.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin i by phosphorylation allows localized h(2)o(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using lysmcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 39.Behm CZ, Kaufmann BA, Carr C, Lankford M, Sanders JM, Rose CE, Kaul S, Lindner JR. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation. 2008;117:2902–2911. doi: 10.1161/CIRCULATIONAHA.107.744037. [DOI] [PubMed] [Google Scholar]
- 40.Wagner EM, Sanchez J, McClintock JY, Jenkins J, Moldobaeva A. Inflammation and ischemia-induced lung angiogenesis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L351–357. doi: 10.1152/ajplung.00369.2007. [DOI] [PubMed] [Google Scholar]
- 41.Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, Kojima H, Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 42.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin ii on coronary collateral growth. Arterioscler Thromb Vasc Biol. 2008;28:61–67. doi: 10.1161/ATVBAHA.107.154294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


