Abstract
Heart failure with preserved ejection fraction (HFpEF) is recognized as a major cause of cardiovascular morbidity and mortality. An ability to identify patients with HFpEF who are at increased risk for adverse outcome can facilitate their more careful management. We studied the patients having heart failure (HF) using data from the Heart Failure Adherence and Retention Trial (HART). HART enrolled 902 NYHA Class II or III patients who had been recently hospitalized for HF to study the impact of self-management counseling on the primary outcome of death or HF hospitalization. In HART 208 patients had HFpEF and 692 had HFrEF (heart failure with reduced ejection fraction), and were followed for median of 1080 days. Two final multivariate models were developed. In patients having HFpEF, predictors of primary outcome were: male sex (OR 3.45, p=0.004), NYHA class III (OR 3.05, p=0.008), distance covered on 6-minute walk test (6-MWT) of< 620 feet (OR 2.81, p=0.013), and <80% adherence to prescribed medications (OR 2.61, p=0.018). In patients having HFrEF, the predictors were: being on diuretics (OR 3.06, p=0.001), having ≥ 3 comorbidities (OR 2.11, p=0.0001), distance covered on 6-MWT of < 620 feet (OR 1.94, p=0.001), NYHA class III (OR 1.90, p=0.001) and age > 65 years (OR 1.63, p=0.01). In conclusion, indicators of functional status(6-MWT and NYHA class) were common to both HFpEF and HFrEF patients while gender and adherence to prescribed therapy were unique to patients having HFpEF in predicting death or HF hospitalization.
Keywords: heart failure with preserved ejection fraction, predictors, death, heart failure related hospitalization
Introduction
Given the increasing recognition of heart failure with preserved ejection fraction (HFpEF) as a growing and difficult-to-treat clinical problem, the identification of predictors of adverse outcome can help to identify those patients who are at the highest risk and who would benefit from more personalized and aggressive management. To be useful, such predictors should be easy to identify in routine clinical practice, thereby making them potentially valuable in personalizing the approach to patient care, monitoring disease progression, and evaluating therapeutic effectiveness. Comparing these predictors amongst patients having HFpEF and heart failure with reduced ejection fraction (HFrEF) can further our understanding of the differences in the two subtypes of heart failure (HF).
Methods
We analyzed data from Heart failure Adherence and Retention Trial (HART). HART was a single-center, multiple-hospital, partially blinded, randomized controlled behavioral trial that was based in the Chicago metropolitan area. HART was designed to assess the impact of self-management counseling versus education alone on the composite primary outcome of death or HF hospitalizations in patients with HFrEF or HFpEF1,2. HART enrolled a total of 902 patients. Of the patients who could be classified, 692 had HFrEF and 208 had HFpEF. Details on patient enrollment, data collection and follow up within HART have been reported elsewhere1. Briefly, patients having HF were recruited through inpatient and outpatient screening and through referrals from cardiologists and internists. The recruitment continued from October 2001 through October 2004. The follow-up was completed in May 2007. All patients were receiving some form of active HF treatment, including diuretics, for the previous 3 months. HFrEF was defined as ejection fraction of ≤ 40% by echocardiography, radiographic ventriculography, or radionuclide ventriculography. HFpEF was defined as ejection fraction > 40% by 1 of the 3 above listed methods and 1 or more previous hospitalizations for HF.
Baseline data were collected on demographics, medications, co-morbidities and adherence to medications. The median follow up period was 1080 days. Primary endpoints were ascertained through blind adjudication by a designated team of cardiologists 2. All patients, or in the case of death, their family members, were contacted every 3 months by telephone to ascertain occurrence of a death or hospitalization. Reports of death were confirmed by medical record, death certificate, emergency medical services record, or queries from the Social Security Death Index. HF admissions were adjudicated by the presence of shortness of breath, peripheral edema, or chest radiographic evidence of pulmonary edema without evidence of another disease process accounting for symptoms or signs. HF admissions were confirmed if the patient responded to HF therapy or had a documented decrease in left ventricular function.
Medication adherence was tested using electronic pill cap monitoring. The patient was asked to place a month’s supply of an angiotensin-converting enzyme (ACE) inhibitor [Angiotensin Receptor Blocker (ARB), beta-blocker or diuretics, if the patient was not taking an ACE inhibitor] into a Medication Event Monitoring System electronic pill cap container (MEMS V Trackcap; AARDEX, Zug, Switzerland). They were taught to use it for the ensuing month. Adherence to drug therapy was defined via the percentage of pills taken relative to pills prescribed, with a cut-point of <80% indicating non-adherence. NYHA (New York Heart Association) class was assessed by the treating physicians at the time of enrolment and during follow up. Six-Minute walk test (6-MWT) was performed by measuring the distance that patients could walk over a period of 6 minutes. For analysis, distance covered on 6-MWT was dichotomized at the lowest tertile 2. Glomerular Filtration Rate (GFR) was calculated using the Cockroft - Gault equation. Diabetes was self-reported at the time of enrolment and during follow up. Other comorbid conditions which were assessed included previous myocardial infarction, hypertension, cancer, stroke, arthritis, lung disease, liver disease, asthma, sleep apnea and Parkinson’s disease. Depression was assessed using Geriatric Depression Scale with a score of > 10 having high sensitivity and specificity for diagnosing depression. Other psychosocial factors, which were assessed using standardized questionnaires, included quality of life, purpose in life and social support1.
Statistical analyses began with a description of the baseline characteristics in overall population of 900 patients, and then a comparison of patients with HFpEF and HFrEF. To identify predictors of the primary outcome (Death or HF hospitalization), univariate unadjusted odds ratios reflecting risk for the primary endpoint were computed for each of the baseline factors separately in those with HFpEF and HFrEF. Next, and again separately, for subgroups having HFpEF and HFrEF, a backward stepwise multivariate elimination strategy was employed with all factors with an unadjusted odds ratio marginally different from 1 (i.e., p<0.40) included in a saturated model and then iteratively assessed for elimination. The criterion for remaining in the model was p ≤ 0.15. Both Likelihood-ratio and Hosmer-Lemeshow tests were used to assess model fit. Unadjusted Kaplan-Meier curves were used to evaluate the time to event over the follow up period for patients having HFpEF.
Results
Table 1 presents baseline patient characteristics of the total cohort and the differences in baseline characteristics between patients with HFpEF and HFrEF. The average age of all the patients in the trial was 63.6 years, 47% were women and 60% were Caucasian. A total of 74 (36%) of the 208 patients having HFpEF had a primary event. Of these, 48 (29%) were hospitalized for HF and 39 (20%) died. Of the 48 patients who were hospitalized for HF, 27 (56%) were hospitalized once and 21 (44%) were hospitalized more than once during the follow up. A total of 259 (37%) of the 692 patients having HFrEF had a primary event. Of these, 162 (23%) were hospitalized for HF and 148 (21%) died. Of the 162 who were hospitalized, 86 (53%) were hospitalized once and 76 (47%) were hospitalized more than once during follow up. These numbers do not add up to the total with primary event because those who were hospitalized and later died were counted only once. Table 2 presents the univariate odds of primary event by baseline characteristic in patients having HFpEF and HFrEF.
Table 1.
Baseline characteristics of HART patients
| Characteristic | All patients (N=900) | HFpEF (N=208) | HFrEF (N=692) |
|---|---|---|---|
| Age (years)***, mean (SD) | 63.6 (13.5) | 67.3 (13.0) | 62.4 (13.4) |
| Women*** | 426 (47%) | 136 (65%) | 290 (42%) |
| Minority race/ethnicity | 361 (40%) | 79 (38%) | 282 (41%) |
| ≤ High school education | 393 (44%) | 82 (39%) | 311 (45%) |
| Annual family income < $30,000 | 426 (52%) | 106 (56%) | 320 (50%) |
| Married or living with someone else as if unmarried*** | 502 (56%) | 93 (45%) | 409 (60%) |
| In treatment arm | 450 (50%) | 107 (51%) | 343 (50%) |
| NYHA class III | 284 (32%) | 64 (31%) | 220 (32%) |
| Six-minute walk distance***, mean (SD), (feet) | 821 (465) | 718 (449) | 852 (465) |
| Hypertension* | 675 (75%) | 168 (81%) | 507 (74%) |
| Diabetes Mellitus | 361 (40%) | 89 (43%) | 272 (39%) |
| Comorbid conditions**, mean (SD) | 3.2 (1.7) | 3.5 (1.6) | 3.1 (1.7) |
| Total number of medications, mean (SD) | 6.8 (3.0) | 6.7 (2.9) | 6.8 (3.0) |
| ACE inhibitor or ARB use*** | 772 (86%) | 163 (78%) | 609 (88%) |
| Beta blocker use*** | 635 (71%) | 111 (53%) | 524 (76%) |
| Major depressive symptoms | 264 (29%) | 66 (32%) | 198 (29%) |
| Social support-emotional, mean (SD) | 75.2 (22.2) | 75.6 (22.5) | 75.0 (22.1) |
| Purpose in life, mean (SD) | 4.5 (0.8) | 4.4 (0.8) | 4.5 (0.8) |
| Quality of life, mean (SD) | |||
| SF-36 | |||
| Physical function*** | 48.2 (24.9) | 43.2 (22.7) | 49.7 (25.4) |
| Energy and vitality, | 46.5 (23.7) | 44.4 (23.8) | 47.1 (23.6) |
| Quality of Life Index – Cardiac | |||
| Satisfaction with health and function | 4.3 (1.0) | 4.2 (1.1) | 4.3 (1.0) |
| Satisfaction with psychological/spiritual function | 4.7 (1.1) | 4.7 (1.1) | 4.8 (1.0) |
| Non-adherence to drug therapy | 273 (37%) | 60 (36%) | 213 (37%) |
| Sodium intake, median (IQR), (mg/day) | 3696 (1577) | 3753 (1621) | 3513 (1417) |
| Current smoker | 85 (9.5) | 20 (9.6) | 65 (9.4) |
| Body mass index (kg/m2)***, mean (SD) | 31.0 (7.7) | 32.9 (8.4) | 30.5 (7.4) |
| Self-efficacy at self-management, mean (SD) | 7.6 (1.7) | 7.4 (1.8) | 7.7 (1.7) |
p < 0.05,
p < 0.01,
p < 0.001
Sample sizes in any particular comparison may be slightly different due to missing data.
ACE- Angiotensin Converting Enzyme; ARB- Angiotensin Receptor Blocker, HART- Heart Failure Adherence and Retention Trial, HFpEF- Heart Failure with Preserved Ejection Fraction; HFrEF- Heart Failure with Reduced Ejection Fraction; NYHA- New York Heart Association; SD- Standard Deviation; NYHA- New York Heart Association; SF36- Short form 36.
TABLE 2.
Univariate odds of death or heart failure hospitalization by baseline characteristics in patients having HFpEF and HFrEF
| Risk Factor | HFpEF Odds Ratio (95% CI) |
HFrEF Odds Ratio (95% CI) |
|---|---|---|
| NYHA Class III symptoms | 3.47*** (1.82, 6.63) | 2.43*** (1.72, 3.42) |
| Male | 2.63** (1.40, 4.76) | 0.85 (0.62, 1.17) |
| Distance covered on 6-MWT of < 620 (feet) | 2.22* (1.20, 4.11) | 2.88*** (2.00, 4.15) |
| Diabetes Mellitus | 2.03* (1.12, 3.68) | 1.94*** (1.40, 2.69) |
| Medication adherence < 80% | 2.15* (1.08, 4.28) | 1.81*** (1.25, 2.60) |
| ≥ 3 Co-morbidities | 1.90 (0.94, 3.84) | 2.63* (1.87, 3.71) |
| Self-Management Treatment Arm | 1.67 (0.93, 3.02) | 0.82 (0.59, 1.12) |
| Hypertension | 1.74 (0.78, 3.87) | 1.28 (0.89, 1.85) |
| On Diuretics | 2.64 (0.55, 12.80) | 3.30***(1.83, 5.94) |
| Coronary Artery Disease | 1.45 (0.80, 2.63) | 1.37 (0.98, 1.91) |
| On ACE-I or ARB | 0.68 (0.33, 1.39) | 0.48** (0.29, 0.78) |
| Glomerular Filtration Rate ≤ 30 (ml/min/m2) | 1.55 (0.60, 4.0) | 0.53* (0.30, 0.95) |
| Geriatric Depression Scale score ≥ 10 | 1.32 (0.71, 2.46) | 1.79*** (1.26, 2.54) |
| Age > 65 (years) | 1.28 (0.68, 2.39) | 2.00*** (1.45, 2.76) |
| On Beta-Blocker | 0.80 (0.45, 1.43) | 0.61** (0.42, 0.88) |
| Education ≤ High School | 1.24 (0.68, 2.25) | 1.39* (1.01, 1.91) |
| Atrial Fibrillation | 1.24 (0.68, 2.27) | 1.38 (0.98, 1.94) |
| Sleep Apnea | 1.28 (0.63, 2.57) | 1.30 (0.84, 2.03) |
| Ever Smoker | 0.84 (0.46, 1.51) | 0.89 (0.64, 1.25) |
| Income < $ 30,000.00/year | 1.17 (0.63, 2.15) | 1.19 (0.85, 1.66) |
| Diabetes and blood pressure at goal | 0.87 (0.46, 1.64) | 0.74 (0.50, 1.09) |
| Asthma | 0.90 (0.44, 1.84) | 1.29 (0.80, 2.09) |
| Minority race/ethnicity | 0.95 (0.52, 1.74) | 1.21 (0.88, 1.68) |
| Kidney Disease and blood pressure at goal | 0.97 (0.53, 1.79) | 0.66* (0.46,0.94) |
p=<0.05,
p=<0.01,
p=<0.001
ACE-I- Angiotensin Converting Enzyme- Inhibitor; ARB- Angiotensin Receptor Blocker, CI- Confidence Interval, HFpEF- Heart Failure with preserved Ejection Fraction, HFrEF- Heart Failure with reduced Ejection Fraction, NYHA- New York Heart Association, 6-MWT- Six Minute Walk Test.
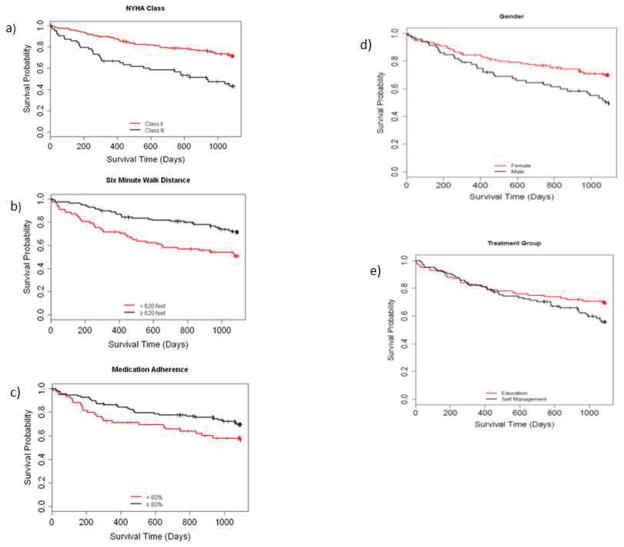
Table 3 presents the results of the multivariate model aimed at identifying independent predictors of death or HF hospitalization in patients with HFpEF and HFrEF. The Likelihood-ratio test was significant (p < 0.0001) in models for both groups, while the Hosmer-Lemeshow test was insignificant (p=0.4211 for HFpEF and p=0.0533 for HFrEF patients) indicating a good model fit. Predictors common to both subgroups included indicators of functional status i.e. NYHA class and distance covered on 6-MWT. Unique predictors for patients having HFpEF were male sex and medication non-adherence; and for patients having HFrEF were being on diuretics, having ≥ 3 comorbidities and age > 65 years. Figure 1 presents unadjusted time-to-event Kaplan-Meier curves portraying these associations in the subgroup having HFpEF.
TABLE 3.
Results of multivariate model identifying independent predictors of death or heart failure hospitalization in patients having HFpEF and HFrEF.
| Characteristic | HFpEF OR (95% CI) |
HFrEF OR (95% CI) |
|---|---|---|
| Male vs. Female | 3.45** (1.47, 8.07) | |
| NYHA Class III vs. Class II | 3.05** (1.33, 6.98) | 1.90** (1.28, 2.82) |
| Baseline medication adherence < 80% vs. ≥ 80% | 2.61* (1.18, 5.76) | |
| Distance covered on 6-MWT < 620 vs. ≥ 620 (ft.) | 2.81* (1.24, 6.40) | 1.94** (1.30, 2.90) |
| Self-Management vs. Enhanced Education arm | 2.16 (0.98, 4.76) | |
| ≥ 3 Co-morbidities | 2.11*** (1.44, 3.10) | |
| On Diuretics | 3.06*** (1.57, 5.97) | |
| Age > 65 years | 1.63** (1.11, 2.40) | |
| Minority race/ethnicity | 1.34 (0.91, 1.97) | |
| CKD with BP ≤ 130/80; no CKD with BP ≤ 140/90 | 0.69 (0.46, 1.05) |
p<0.05,
p<0.01,
p<0.001.
All factors retained on multivariate model at final iteration are displayed. P-values are listed where significant. Factors retained on final model were different for the 2 subtypes of HF, hence the blanks.
BP- Blood Pressure, CKD- Chronic Kidney Disease, CI- Confidence Interval, ft.- Feet, HFpEF- Heart Failure with Preserved Ejection Fraction, HFrEF- Heart Failure with Reduced Ejection Fraction, NYHA- New York Heart Association, OR- Odds Ratio, 6-MWT- Six Minute Walk Test, vs.- Versus.
Figure 1.
Kaplan-Maier curves for the five independent predictors of death or heart failure hospitalization in patients with HFpEF. Unadjusted log-rank p-values for subgroup differences in time to primary event over the study duration are: NYHA Class, p < 0.0001; Six-Minute Walk distance, p = 0.002; Medication Adherence, p = 0.05; Gender, p = 0.006 and treatment group, p = 0.13.
Of the 208 patients with HFpEF in this subgroup analysis, only 146 were available for the planned multivariate model analyses due to missing data; the majority of missingness was due to two variables: 40 (19%) patients were missing data on adherence to medication doses and 16 (8%) on distance covered during 6-MWT. Of the 692 patients having HFrEF, 577 were available in the final iteration of model selection; most missingness was the result of missing data on distance covered during 6-MWT (8%). To assess the sensitivity of the final models to missing data, two additional models were run. One assumed a “best case” scenario in which all missing covariate values were set to the “protective” value indicated by the final multivariate model. Thus, missing values on 6-MWT were set to > 620 feet and missing medication adherence values were set to ≥80%. Next, a “worst case” scenario was assumed in which missing covariate values were set to the “non-protective” value (missing values on 6-MWT set to < 620 feet, missing medication adherence set to <80%). For the HFrEF model only the best case/worst case sensitivity analysis was done for the 6-MWT variable as medication adherence was not a significant predictor. Results under these two scenarios yielded models almost identical to those described above, indicating that the missing data did not significantly affect the results.
Discussion
Our interest was to determine the predictors of death or HF hospitalization in the subgroup of patients with HFpEF. Comparing the patients having HFpEF and HFrEF helped us in understanding how the two populations are different. We considered multiple demographic and clinical factors and identified male gender, higher NYHA class, shorter distance covered on 6-MWT and non-adherence to prescribed medications as independent predictors for patients having HFpEF. This is in contrast to patients having HFrEF in whom higher NYHA class, shorter distance covered on 6-MWT, having ≥ 3 comorbidities, being on diuretics and age > 65 years were the predictors. By using these predictors as a guide, physicians could possibly stratify patients having HFpEF more appropriately and dedicate more time and resources through more frequent follow-up visits with closer monitoring to insure compliance with diet, salt intake, exercise, and medications.
NYHA class and distance covered on 6-MWT were predictive of adverse outcome in both HF subtypes. These 2 predictors can be used to stratify patients with HFpEF by disease severity, monitor disease progression and being modifiable, can be used to assess response to treatment. NYHA classification is a well-established system of classifying and assessing the severity of HFrEF3–6. Current guidelines for HFrEF recommend treatment based on patient’s NYHA class7,8 since a higher NYHA class indicates a poor functional status and port ends a poorer prognosis. Few studies have examined the utility of NYHA class in patients having HFpEF 9 and as with patients having HFrEF; higher NYHA class is associated with worse prognosis in patients having HFpEF. Since our study enrolled only NYHA Class II and III patients, we were only able to compare prognosis across these 2 categories. We noted that patients having NYHA class III symptoms had a worse prognosis than those with NYHA class II, indicating that NYHA classification may have a role in disease staging and management even in patients having HFpEF. Thus, our study is consistent with what some of the larger studies have shown.
The 6-MWT is an underutilized tool for assessing chronic cardiopulmonary conditions10. It is a simple test requiring a patient to walk for 6 minutes and measuring the distance walked in that time period2. We noted that patients who were unable to walk > 620 feet on 6-MWT had an increased risk of death or HF related hospitalization. This supports the value of the 6-MWT as an easy and objective method of assessing the functional capacity in patients with HF in an ambulatory setting. The 6-MWT has been studied more extensively in pulmonary diseases and HFrEF10–13, but to the best of our knowledge, studies specifically utilizing 6-MWT to assess functional status in patients having HFpEF are lacking. Our analysis supports the use of the 6-MWT in prognostication and management of HFpEF.
Effective management of patients having HFpEF is limited by a lack of evidence-based therapies. Typical treatment involves treating comorbid conditions. In our study, non-adherence to prescribed medications in patients having HFpEF predicted death or HF related hospitalization on both univariate and multivariate analyses. This could be due to several reasons. It could relate to poor control of comorbid conditions in non-adherent patients or it could be a more general index of non-adherence to a healthy lifestyle. In both cases, providers should identify non-adherent patients and focus management strategies on increasing adherence. Alternatively, since the specific monitored medications were antihypertensive agents i.e. ACE-I/ARBs, diuretics and beta blockers, and these medications have been shown to have survival benefits in patents having HFrEF, it is possible that these benefits may extend to patients having HFpEF as well. Studies in HFpEF patients using drugs that inhibit the renin angiotensin system14–17 and beta blockers 18 have yielded mixed results and further studies may be warranted. At the same time, these medications are also antihypertensive agents. Poorly controlled blood pressure is a precipitating factor for HF exacerbation. Thus, adherent patients were more likely to have better control of their blood pressure leading to lower incidence of HF related hospitalization or death.
Although women had a higher prevalence of HFpEF, an observation observed by others 19,20, men had a poorer prognosis. When comparing men and women for adverse outcomes, women were noted to have lower income, lesser education, were more likely to be unmarried, walked shorter distance on 6 MWT and were less likely to be able to walk > 620 feet in on 6 MWT. Men, on the other hand, were more likely to have a history of smoking, coronary artery disease, renal disease and sleep apnea. Thus, men seem to have a higher comorbidity burden compared to women, which may partially explain increased incidence of adverse outcomes in this population. The exact reasoning still remains unclear and merits further investigation but implies that clinicians should be extra vigilant when managing males with HFpEF.
Except for diabetes on univariate analysis, none of the comorbid conditions were noted to be predictive of death or HF hospitalization on univariate or multivariate analysis for patients having HFpEF. This could be due to appropriate control or lower incidence of these conditions in our study population. Nonetheless, comorbidities should be aggressively managed in patients having HFpEF. In patients having HFrEF, having more co-morbidities was predictive of death or HF hospitalization, substantiating the need to aggressively manage the co-morbidities and prevent their development when feasible. Similarly, patients aged > 65 years had an increased incidence of death or HF hospitalization if they had HFrEF, while this was not true for patients having HFpEF. This could be due to the fact that the cohort of patients having HFpEF was older (mean age 67.3 years) compared to those having HFrEF, thus negating the impact of age to some extent.
A limitation of this analysis study includes the fact that it is an analysis of a subgroup of a larger randomized controlled trial and thus was not powered to detect associations in this smaller sample size. However, the direction of bias for underpowered studies is toward the null hypothesis. Therefore, those associations for which we found significance are likely to be robust. Second, medication adherence was measured only at baseline, over the ensuing 30 days post-randomization. Since it was not monitored continuously throughout the study, there is a possibility that initially non-adherent patients may have become adherent during the study or vice versa. Third, we do not have echocardiographic findings to fully assess grade of diastolic dysfunction among the patients studied. Higher grade of diastolic dysfunction indicate a more severe compromise in cardiac function. It is possible that the patients who had adverse outcome may have had higher grade of diastolic dysfunction on echocardiogram. Fourth, missing data limited the sample size in the multivariate modeling. Despite the fact that we employed a reasonable sensitivity analysis to confirm the stability of our results, larger prospective studies may provide better estimates of risk.
Footnotes
- Principal Site: Rush University Medical Center, Chicago, IL
- Other hospitals: John H. Stroger Hospital of Cook County, University of Illinois College of Medicine at Chicago, Advocate Lutheran General Hospital, Evanston Hospital, Rush North Shore Medical Center, Glenbrook Hospital, Advocate Christ Medical Center and South Suburban Hospital.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powell L, Calvin J, Jr, Mendes de Leon C, Richardson D, Grady K, Flynn K, Rucker-Whitaker C, Janssen I, Kravitz G, Eaton C Heart Failure ART, Investigators. The Heart Failure Adherence and Retention Trial (HART): design and rationale. Am Heart J. 2008;156:452–460. doi: 10.1016/j.ahj.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell L, Calvin J, Jr, Richardson D, Janssen I, Mendes de Leon C, Flynn K. Self-management Counseling in Patients With Heart Failure: The Heart Failure Adherence and Retention Randomized Behavioral Trial. JAMA. 2010;304:1331–1338. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scrutenid D, Lagioia R, Ricci A, Clemente M, Boni L, Rizzon P. Prediction of mortality in mild to moderately symptomatic patients with left ventricular dysfunction: The role of the New York Heart Association classification, cardiopulmonary exercise testing, two-dimensional echocardiography and Holter Monitoring. Eur Heart J. 1994;15:1089–1095. doi: 10.1093/oxfordjournals.eurheartj.a060633. [DOI] [PubMed] [Google Scholar]
- 4.Madsen B, Hansen J, Stokholm K, Brons J, Husum D, Mortensen L. Chrome congestive heart failure: Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J. 1994;15:303–310. doi: 10.1093/oxfordjournals.eurheartj.a060495. [DOI] [PubMed] [Google Scholar]
- 5.Muntwyler J, Abetel G, Gruner C, Follath F. One-year mortality among unselected outpatients with heart failure. Eur Heart J. 2002;23:1861–1866. doi: 10.1053/euhj.2002.3282. [DOI] [PubMed] [Google Scholar]
- 6.Bouvy M, Heerdink E, Leufkens H, Hoes A. Predicting mortality in patients with heart failure: a pragmatic approach. Heart. 2003;89:605–609. doi: 10.1136/heart.89.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 8.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed A, Aronow W, Fleg J. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444–450. doi: 10.1016/j.ahj.2005.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bittner V, Weiner D, Yusuf S, Rogers W, McIntyre K, Bangdiwala S, Kroneberg M, Kostis J, Kohn R, Guillotte M, Greenberg B, Woods P, Bourassa M. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 11.ATS Statement: Guidelines for six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 12.Rostagno C, Olivo G, Comeglio M, Boddi V, Banchelli M, Galanti G, Gensini G. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: comparison with other methods of functional evaluation. Eur J Heart Fail. 2003;5:247–252. doi: 10.1016/s1388-9842(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 13.Arslan S, Erol M, Gundogdu F, Sevimli S, Aksakal E, Senocak H, Alp N. Prognostic Value of 6-Minute Walk Test in Stable Outpatients with Heart Failure. Tex Heart Inst J. 2007;24:166–169. [PMC free article] [PubMed] [Google Scholar]
- 14.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. The Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 15.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 16.Cleland J, Tendera M, Adamus J, Freemantle N, Polonski L, JT Perindopril in elderly people with chronic heart failure. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 17.Yip J, Wang M, Wang T, Chan S, Fung J, Yeung L, Yip T, Lau S, Lau C, Tang M, Yu C, Sanderson J. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart. 2008;94:573–580. doi: 10.1136/hrt.2007.117978. [DOI] [PubMed] [Google Scholar]
- 18.Flather M, Shibata M, Coats A, Van Veldhuisen D, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker S, Thompson S, Poole-Wilson P. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 19.Yancy C, Lopatin M, Stevenson L, De Marco T, Fonarow G Adhere Scientific Advisory Committee Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure A Report From the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]