Abstract
Background
Large cohort studies have reported no relationship between dietary fat and nonmelanoma skin cancer (NMSC), although a low-fat diet intervention reduced NMSC risk in a small clinical trial. In animal studies, skin tumor development has been reduced by low-fat diet. We evaluated the effect of a low-fat dietary pattern on NMSC and melanoma in the Women’s Health Initiative Dietary Modification trial.
Methods
Postmenopausal women aged 50 to 79 years (N=48,835) were randomly assigned to the low-fat dietary pattern intervention (N=19,541) or comparison group (N=29,294). The intervention goals included decreasing fat intake to ≤20% of calories, increasing vegetable and fruit intake, and increasing grain intake. Self-reported incident NMSC (N=4,907) and physician-adjudicated incident melanoma (N=279) were ascertained every 6 months.
Results
Over 8.1 years of follow-up, the low-fat diet intervention did not affect overall incidence of NMSC (hazard ratio [HR] 0.98, 95% confidence interval [CI]: 0.92–1.04) or melanoma (HR 1.04, 95% CI: 0.82–1.32). In subgroup analyses of melanoma risk, baseline fat intake interacted significantly with group assignment (Pinteraction=0.006). Among women with higher baseline fat intake, the dietary intervention significantly increased risk (HR 1.48; 95% CI: 1.06–2.07), whereas, among women with lower baseline fat intake, the intervention tended to reduce melanoma risk (HR 0.72, 95% CI: 0.50–1.02).
Conclusions
In this large randomized trial, a low-fat dietary pattern did not affect overall incidence of NMSC or melanoma.
Impact
A low-fat diet does not reduce incidence of NMSC, but an interaction between baseline fat intake and dietary intervention on melanoma risk warrants further investigation.
Keywords: Melanoma, nonmelanoma, skin cancer, diet, low-fat
INTRODUCTION
Skin cancer is the most common malignancy in the United States, affecting more than 2 million individuals in 2006, which is double the incidence reported in 1994(1, 2). The American Cancer Society estimated that Americans would develop 3.5 million new cases of nonmelanoma skin cancer (i.e., basal cell and squamous cell carcinomas) and 76,250 new cases of melanoma in 2012(1, 3). Sun exposure is the main established risk factor for skin cancer; however, only part of the variation in skin cancer incidence is explained by variables related to sun exposure(4–7). Despite public health campaigns emphasizing sunscreen use and sun avoidance, nonmelanoma skin cancer (NMSC) and melanoma incidences continue to rise, especially among women(8–10). Consequently, there is a great need to identify other modifiable risk factors for skin cancer and new approaches for skin cancer prevention.
Clinical studies of dietary fat and NMSC are inconsistent(11–18). One large prospective cohort found no relationship between dietary fat intake and NMSC risk in women, while another noted lower NMSC risk with higher dietary fat intake in men(16, 18). On the other hand, a 2-year randomized trial of subjects with a history of NMSC reported that a low-fat diet intervention reduced NMSCs in the last 8 months of the study, but not in the first 16 months(11). The clinical data on dietary fat and melanoma are also inconsistent(19–24).
There is a biologic rationale for an association between fat intake and risk of skin cancer. Laboratory studies have shown that a high-fat diet contributes to oxidative stress and DNA damage(25), increasing inflammatory cytokines in the skin while decreasing cell apoptosis(26). One study found a nearly linear relationship between increasing lipid level and development of actinic tumors in mice, noting both shorter time to tumor formation and greater number of tumors(27). Another study noted that low-fat diet was associated with slower melanoma tumor growth(28).
The Women’s Health Initiative (WHI) Dietary Modification (DM) Trial of 48,835 postmenopausal women, designed to evaluate whether a low-fat dietary pattern intervention would decrease the incidence of breast and colorectal cancers (primary outcomes) and/or coronary heart disease (secondary outcome)(29–32), provides an opportunity to investigate whether a low-fat dietary pattern reduces the risk of NMSC or melanoma within a large randomized controlled trial.
MATERIALS & METHODS
Study Population
The WHI DM Trial (NCT00000611) design has been described previously, as have eligibility criteria and recruitment methods(29, 33, 34). Briefly, postmenopausal women, aged 50 to 79 years, were recruited at 40 Clinical Centers throughout the US between 1993 and 1998. Major exclusions included prior breast cancer, colorectal cancer, or history of any cancer other than nonmelanoma skin cancer in the past 10 years; predicted survival of less than 3 years; type 1 diabetes mellitus; and other conditions that posed adherence and retention concerns (e.g., alcoholism, dementia)(33). Participants had to have a baseline fat intake ≥32% of total energy, as estimated by the WHI Food Frequency Questionnaire (FFQ).
Study Design
Eligible women (N = 48,835) were randomly assigned by permuted block algorithm to either the dietary intervention (40%, N = 19,541) or the comparison group (60%, N = 29,294). The dietary goals for the intervention group included decreasing total fat intake to ≤20% of energy and consuming five or more servings per day of vegetables and fruits and six or more servings per day of grains. The women received an individualized dietary plan with a daily fat gram goal based on their expected energy intake(29). The intervention did not include weight-loss or total energy reduction goals. Women in the intervention group participated in nutritionist-facilitated small group sessions (18 sessions during year 1, quarterly sessions each year thereafter). Women assigned to the comparison group received a copy of Nutrition and Your Health: Dietary Guidelines for Americans but were not asked to change their diet(30, 35). The trial design for comparison-intervention difference in percent of energy from total fat was 13% at year 1 and 11.75% at year 6. Among all participants, the actual Comparison-Intervention difference at year 1 was 10.7% and at year 6 was 8.1%(30).
Demographic information, medical history, and other characteristics were obtained by questionnaire or physical measurement at study entry (baseline). This analysis included all women enrolled in the DM Trial, except participants with missing data for body mass index (BMI) at baseline. All procedures and protocols were approved by the Institutional Review Boards at each participating institution and all participants provided written informed consent.
Follow-up and Data Collection
On average, women in the DM Trial were followed for 8.1 years (SD 1.6 years). As of March 2005, the percent of women still actively participating in the trial was similar between the two groups, with 17,674 women in the dietary intervention group (90.4%) and 26,677 in the comparison group (91.1%)(30). Participants’ clinical measures (such as weight) and self-reported measures (such as physical activity) were collected during annual clinic visits(29).
The WHI FFQ was used to assess dietary intake in both groups. All participants completed the FFQ at baseline (before randomization) and year 1. After year 1, a rotating sample of 33% of participants was surveyed each year, such that each participant completed a FFQ every 3 years. A detailed description of the FFQ validation has been published(36). The response rate to the FFQ was 100% at baseline and approximately 81% in subsequent years(32). The nutrient database was derived from the University of Minnesota Nutrition Coordinating Center nutrient database (NDSR, Minneapolis, Minnesota)(36).
Outcome Ascertainment
Participants completed questionnaires every 6 months to report medical outcomes, including NMSC and melanoma(37). Melanoma cases were confirmed by adjudication of pathology reports, and coded as invasive or in-situ following the ICD-O-2 coding scheme(38). NMSC cases were not adjudicated.
Statistical Analysis
Baseline descriptive characteristics, potential skin cancer risk factors, and dietary intake were compared in the intervention and comparison groups. Differences in each category were evaluated using Chi-square tests for categorical variables and t-tests for continuous variables.
In post-hoc analyses, incidence of NMSC and melanoma were compared between the groups using hazard ratios (HRs) with 95% confidence intervals (CIs) and Wald statistic P values from Cox proportional hazards models. The proportionality assumption was confirmed by running a proportional hazards model that modeled each outcome as a function of the interaction between the low-fat dietary pattern effect and the log survival time. Modeling analyses used time-to-event methods according to the intention-to-treat principle. Kaplan-Meier estimates were provided to describe event rates over time. As in prior analyses of the DM Trial(30), sensitivity analyses were performed by censoring intervention participants who missed an annual clinic visit, failed to participate in 9 or more of the 18 first year group sessions, or failed to participate in 2 or more of the 4 group sessions in subsequent years; or comparison participants who missed an annual clinic visit. With these criteria, the intervention group adherence rates were 57% at year 3, 31% at year 6, and 19% at year 9, while the comparison group adherence rates were 87%, 75%, and 65%(30). All proportional hazards models were stratified by age groups at recruitment (50–54, 55–59, 60–69, 70–79) and randomized treatment assignment in the other WHI clinical trials(34), i.e., the Hormone Therapy Trials of combined estrogen and progestin(39) or estrogen only(40) and the Calcium/Vitamin D Trial(41).
To assess whether the effect of low-fat dietary pattern on NMSC or melanoma risk varied according to baseline risk factors for both types of skin cancer, Cox proportional hazards models were extended to include the variable of interest and interaction with group assignment. Hazard ratios for intervention versus comparison within each subgroup are presented along with the P value for interaction. Twelve predefined subgroup analyses were performed for NMSC and melanoma to assess possible statistical interactions between a low-fat dietary pattern and the following known or potential skin cancer risk factors at baseline: 1) age, 2) BMI, 3) regional solar radiation, 4) history of NMSC, 5) smoking status, 6) nonsteroidal anti-inflammatory drug (NSAID) use, 7) vitamin D intake, 8) total energy intake, 9) percent energy from total fat intake, 10) total fat intake, 11) vegetable and fruit servings, and 12) grain servings. Cut points for age, BMI, and regional solar radiation were previously defined in the WHI clinical trials. For NMSC, baseline dietary intake was evaluated by quartiles determined by the natural distribution of participants’ intake at baseline. For melanoma, as there were fewer cases, baseline dietary intake is presented by two methods: the intake groups for energy and fat are divided into an upper and lower half determined by the mean intake of the cohort, while the intake groups for vegetable and fruit servings and grain servings are presented by the upper quartile versus the lower three quartiles to reflect current intake recommendations for cancer prevention(42, 43). All statistical analyses were completed using SAS 9.2 (SAS Institute Inc, Cary, NC.). All statistical tests were two-sided.
RESULTS
Baseline characteristics of participants
Table 1 shows baseline characteristics of participants in the dietary intervention and comparison groups. Participants had an average age of 62.3 years (SD 6.9 years) and an average BMI of 29.1 kg/m2 (SD 5.9 kg/m2). The demographics, health behaviors, skin cancer risk factors (i.e., sun exposure [measured via regional solar radiation and total outdoor walking], history of NMSC and melanoma), and medical history were comparable between the randomization groups.
Table 1.
Baseline characteristics of participantsa
| Intervention (N=19,541) | Comparison (N=29,294) | |
|---|---|---|
| Number (%) | Number (%) | |
| Age | ||
| 50–59 | 7206 (36.9) | 10792 (36.8) |
| 60–69 | 9083 (46.5) | 13632 (46.5) |
| 70–79 | 3252 (16.6) | 4870 (16.6) |
| Race/ethnicity | ||
| White | 15871 (81.2) | 23891 (81.6) |
| Black | 2135 (10.9) | 3127 (10.7) |
| Hispanic | 751 (3.8) | 1094 (3.7) |
| American Indian | 88 (0.5) | 114 (0.4) |
| Asian/Pacific Islander | 431 (2.2) | 674 (2.3) |
| Unknown | 265 (1.4) | 394 (1.3) |
| Education | ||
| Less than high school diploma or GED | 4267 (21.8) | 6468 (22.1) |
| Some school after high school diploma | 7712 (39.5) | 11597 (39.6) |
| College degree or higher | 7446 (38.1) | 11044 (37.7) |
| Body-mass index (kg/m2) | ||
| <25 | 5072 (26.0) | 7587 (25.9) |
| 25 to <30 | 6944 (35.5) | 10452 (35.7) |
| ≥30 | 7442 (38.1) | 11125 (38.0) |
| Smoking status | ||
| Never | 9918 (50.8) | 15029 (51.3) |
| Past | 8121 (41.6) | 11979 (40.9) |
| Current | 1273 (6.5) | 1977 (6.7) |
| NSAID use | ||
| Yes | 6316 (32.3) | 9796 (33.4) |
| No | 13224 (67.7) | 19498 (66.6) |
| Total vitamin D intake, IUb | ||
| <200 IU | 7763 (39.7) | 11892 (40.6) |
| 200 to <400 IU | 3986 (20.4) | 5787 (19.8) |
| 400 to <600 IU | 4454 (22.8) | 6602 (22.5) |
| ≥600 IU | 3226 (16.5) | 4892 (16.7) |
| Total energy intake, kcal | ||
| <1296 | 4820 (24.7) | 7327 (25.0) |
| 1296 to <1677 | 4924 (25.2) | 7226 (24.7) |
| 1677 to <2150 | 4879 (25.0) | 7267 (24.8) |
| ≥2150 | 4807 (24.6) | 7353 (25.1) |
| Percent energy from total fat, % | ||
| <33.8 | 4892 (25.0) | 7109 (24.3) |
| 33.8 to <36.9 | 4885 (25.0) | 7536 (25.7) |
| 36.9 to <40.8 | 4752 (24.3) | 7285 (24.9) |
| ≥40.8 | 4901 (25.1) | 7243 (24.7) |
| Total fat Intake, grams | ||
| <52.4 | 4828 (24.7) | 7322 (25.0) |
| 52.4 to <69.0 | 4897 (25.1) | 7265 (24.8) |
| 69.0 to <91.2 | 4893 (25.0) | 7243 (24.7) |
| ≥91.2 | 4812 (24.6) | 7343 (25.1) |
| Total vegetable and fruit servings/day | ||
| <2.3 | 5013 (25.7) | 7500 (25.6) |
| 2.3 to <3.3 | 4696 (24.0) | 7090 (24.2) |
| 3.3 to <4.6 | 4755 (24.3) | 7104 (24.3) |
| ≥4.6 | 4966 (25.4) | 7479 (25.5) |
| Total grain servings/day | ||
| <3.0 | 4789 (24.5) | 7250 (24.7) |
| 3.0 to <4.3 | 5096 (26.1) | 7407 (25.3) |
| 4.3 to <5.9 | 4727 (24.2) | 7029 (24.0) |
| ≥5.9 | 4818 (24.7) | 7487 (25.6) |
| Regional solar radiation, langleysc | ||
| 300–325 | 5661 (29.0) | 8512 (29.1) |
| 350 | 3801 (19.5) | 5701 (19.5) |
| 375–380 | 2292 (11.7) | 3435 (11.7) |
| 400–430 | 3398 (17.4) | 5088 (17.4) |
| 475–500 | 4381 (22.4) | 6548 (22.4) |
| Total outdoor walking energy expenditure, METs/week | ||
| 0 | 6714 (34.4) | 9817 (33.5) |
| ≤3.5 | 3791 (19.4) | 5790 (19.8) |
| 3.6–7.0 | 3341 (17.1) | 5114 (17.5) |
| >7. | 3661 (18.7) | 5532 (18.9) |
| History of cancerd | ||
| Yes | 853 (4.4) | 1286 (4.4) |
| No | 18688 (95.6) | 28008 (95.6) |
| History of melanoma | ||
| Yes | 122 (0.6) | 170 (0.6) |
| No | 19419 (99.4) | 29124 (99.4) |
| History of nonmelanoma skin cancer | ||
| Yes | 1264 (6.5) | 1996 (6.8) |
| No | 18277 (93.5) | 27298 (93.2) |
| Hormone therapy use | ||
| Never used | 8072 (41.3) | 12102 (41.3) |
| Past user | 2813 (14.4) | 4181 (14.3) |
| Current user | 8639 (44.2) | 12979 (44.3) |
| Hormone Therapy intervention assignment | ||
| Not randomly assigned | 16359 (83.7) | 24426 (83.4) |
| Active | 1587 (8.1) | 2496 (8.5) |
| Placebo | 1595 (8.2) | 2372 (8.1) |
| Calcium/Vitamin D intervention assignment | ||
| Not randomly assigned | 9896 (50.6) | 13729 (46.9) |
| Active | 4767 (24.4) | 7827 (26.7) |
| Placebo | 4878 (25.0) | 7738 (26.4) |
Percentages may not total 100% because of missing data.
From diet and supplements
Based on the mean annual amount of sunlight reaching the clinic site as measured by the US Weather Bureau; 1 langley = 1 g-cal/cm2
History of cancer (cancers diagnosed more than 10 years before enrollment) is defined as any cancer except nonmelanoma skin cancer
GED = general equivalency diploma; MET = metabolic equivalent tasks
Nonmelanoma skin cancer
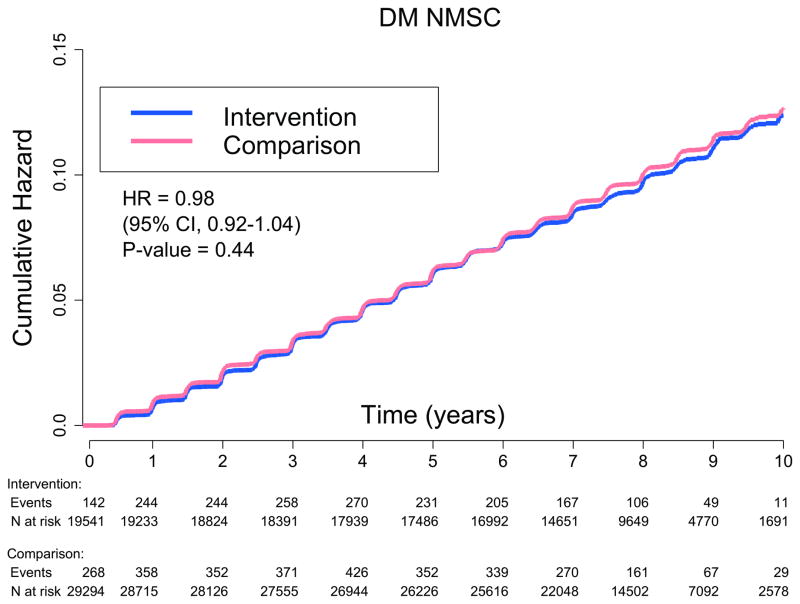
Incidence of self-reported NMSC was similar between randomization groups over an average follow-up of 8.1 years, with 1,923 NMSC cases in the dietary intervention group and 2,984 cases in the comparison group (annualized percentage of 1.28% versus 1.32%, HR 0.98, 95% CI: 0.92–1.04; Table 2, Figure 1). Even when participants with a history of NMSC were excluded, NMSC incidence did not differ between the groups (HR 0.97, 95% CI: 0.91–1.04), nor did NMSC outcomes differ by group assignment within any of the predefined subgroups (i.e., age, BMI, regional solar radiation [langleys], history of NMSC, smoking, NSAID use, vitamin D intake, total energy or fat intake, or vegetable and fruit servings) (Table 4).
Table 2.
Number of nonmelanoma skin cancer and melanoma events by overall trial and sensitivity analysis
| Overall trial | ||||
|---|---|---|---|---|
| Number of cases (annualized %) | HR (95% CI)a | Pb | ||
| Intervention (N=19,541) | Comparison (N=29,294) | |||
| NMSC | 1923 (1.28) | 2984 (1.32) | 0.98 (0.92, 1.04) | 0.44 |
| Melanoma | 114 (0.07) | 165 (0.07) | 1.04 (0.82, 1.32) | 0.78 |
| Sensitivity analysisc | ||||
| Number of cases (Annualized %) | HR (95% CI)a | Pb | ||
| Intervention | Comparison | |||
| NMSC | 977 (1.27) | 2470 (1.34) | 0.99 (0.92, 1.07) | 0.76 |
| Melanoma | 54 (0.07) | 136 (0.07) | 1.04 (0.75, 1.43) | 0.83 |
All models were adjusted for age, assignment in the Hormone Therapy trial, and assignment in the Calcium/Vitamin D trial.
Two-sided (from Cox proportional hazards model).
Sensitivity analysis censored women who did not comply with trial requirements (e.g., intervention participants who missed an annual clinic visit, failed to participate in ≥9 of the 18 first year group sessions, or failed to participate in ≥2 of the 4 group sessions in subsequent years; or comparison participants who missed an annual clinic visit)
Figure 1.
Kaplan-Meier estimate of cumulative hazards for nonmelanoma skin cancer events (N=4,907)
Cox proportional hazards, Wald statistic P value (interaction). CI = confidence interval; DM = dietary modification; HR = hazard ratio; NMSC = nonmelanoma skin cancer
Table 4.
Estimated effect of dietary modification on risk of nonmelanoma skin cancer NMSC), according to selected baseline characteristics.
| Subgroup | Intervention | Comparison | HR (95% CI)a | P | ||
|---|---|---|---|---|---|---|
| Events | Ann% | Events | Ann% | |||
| Overall | 1923 | 1.28 | 2984 | 1.32 | 0.98 (0.92, 1.04) | |
|
| ||||||
| Age | 0.17 | |||||
| 50–59 | 505 | 0.86 | 824 | 0.93 | 0.93 (0.83, 1.04) | |
| 60–69 | 953 | 1.39 | 1472 | 1.44 | 0.98 (0.91, 1.07) | |
| 70–79 | 465 | 2.00 | 688 | 1.97 | 1.03 (0.91, 1.15) | |
|
| ||||||
| BMI, kg/m2 | 0.26 | |||||
| <25 | 641 | 1.65 | 932 | 1.59 | 1.06 (0.96, 1.17) | |
| 25 to <30 | 687 | 1.28 | 1145 | 1.42 | 0.92 (0.84, 1.01) | |
| ≥30 | 589 | 1.03 | 896 | 1.05 | 0.97 (0.88, 1.08) | |
|
| ||||||
| Langley exposureb | 0.74 | |||||
| ≤375 | 908 | 1.23 | 1382 | 1.24 | 0.98 (0.90, 1.07) | |
| >375 | 1015 | 1.33 | 1602 | 1.40 | 0.98 (0.90, 1.06) | |
|
| ||||||
| History of NMSC | 0.64 | |||||
| Yes | 471 | 5.96 | 754 | 5.99 | 1.01 (0.90, 1.13) | |
| No | 1452 | 1.02 | 2230 | 1.05 | 0.97 (0.91, 1.04) | |
|
| ||||||
| Smoking | 0.93 | |||||
| Never | 956 | 1.25 | 1517 | 1.30 | 0.97 (0.89, 1.05) | |
| Past | 842 | 1.35 | 1287 | 1.40 | 0.98 (0.90, 1.07) | |
| Current | 103 | 1.07 | 156 | 1.03 | 1.02 (0.80, 1.31) | |
|
| ||||||
| NSAID use | 0.14 | |||||
| Yes | 634 | 1.31 | 1063 | 1.42 | 0.92 (0.84, 1.02) | |
| No | 1289 | 1.27 | 1921 | 1.28 | 1.01 (0.94, 1.08) | |
|
| ||||||
| Total vitamin D intake, IUc | 0.43 | |||||
| <400 | 1053 | 1.15 | 1643 | 1.19 | 0.97 (0.90, 1.05) | |
| ≥400 | 863 | 1.48 | 1330 | 1.53 | 0.99 (0.91, 1.08) | |
|
| ||||||
| Total energy intake, kcal | 0.62 | |||||
| <1296 | 397 | 1.07 | 672 | 1.20 | 0.92 (0.81, 1.04) | |
| 1296 to <1677 | 525 | 1.39 | 737 | 1.32 | 1.06 (0.95, 1.19) | |
| 1677 to <2150 | 512 | 1.37 | 824 | 1.47 | 0.93 (0.83, 1.04) | |
| ≥2150 | 482 | 1.30 | 740 | 1.31 | 1.01 (0.90, 1.13) | |
|
| ||||||
| Percent energy from total fat, % | 0.20 | |||||
| <33.8 | 512 | 1.36 | 803 | 1.47 | 0.95 (0.85, 1.06) | |
| 33.8 to <36.9 | 489 | 1.30 | 783 | 1.35 | 0.96 (0.86, 1.07) | |
| 36.9 to <40.8 | 488 | 1.34 | 753 | 1.34 | 1.01 (0.90, 1.13) | |
| ≥40.8 | 427 | 1.14 | 634 | 1.14 | 1.01 (0.90, 1.15) | |
|
| ||||||
| Total fat intake, grams | 0.44 | |||||
| <52.4 | 412 | 1.11 | 696 | 1.24 | 0.92 (0.81, 1.04) | |
| 52.4 to <69.0 | 504 | 1.34 | 762 | 1.36 | 0.99 (0.89, 1.11) | |
| 69.0 to <91.2 | 516 | 1.37 | 784 | 1.41 | 0.98 (0.88, 1.10) | |
| ≥91.2 | 484 | 1.31 | 731 | 1.29 | 1.02 (0.91, 1.14) | |
|
| ||||||
| Vegetable and fruit servings/day | 0.67 | |||||
| <2.3 | 391 | 1.01 | 621 | 1.07 | 0.94 (0.83, 1.07) | |
| 2.3 to <3.3 | 455 | 1.25 | 727 | 1.33 | 0.95 (0.85, 1.07) | |
| 3.3 to <4.6 | 507 | 1.39 | 738 | 1.35 | 1.04 (0.93, 1.16) | |
| ≥4.6 | 563 | 1.49 | 887 | 1.55 | 0.98 (0.88, 1.09) | |
|
| ||||||
| Grain servings/day | 0.95 | |||||
| <3.0 | 435 | 1.19 | 690 | 1.25 | 0.99 (0.87, 1.11) | |
| 3.0 to <4.3 | 515 | 1.31 | 797 | 1.40 | 0.96 (0.86, 1.07) | |
| 4.3 to <5.9 | 526 | 1.45 | 766 | 1.42 | 1.00 (0.90, 1.12) | |
| ≥5.9 | 440 | 1.18 | 720 | 1.24 | 0.97 (0.86, 1.09) | |
All models were adjusted for age, assignment in the Hormone Therapy trial, and assignment in the Calcium/Vitamin D trial
Based on the mean annual amount of sunlight reaching the clinic site as measured by the US Weather Bureau; 1 langley = 1 g-cal/cm2
From diet and supplements
Melanoma
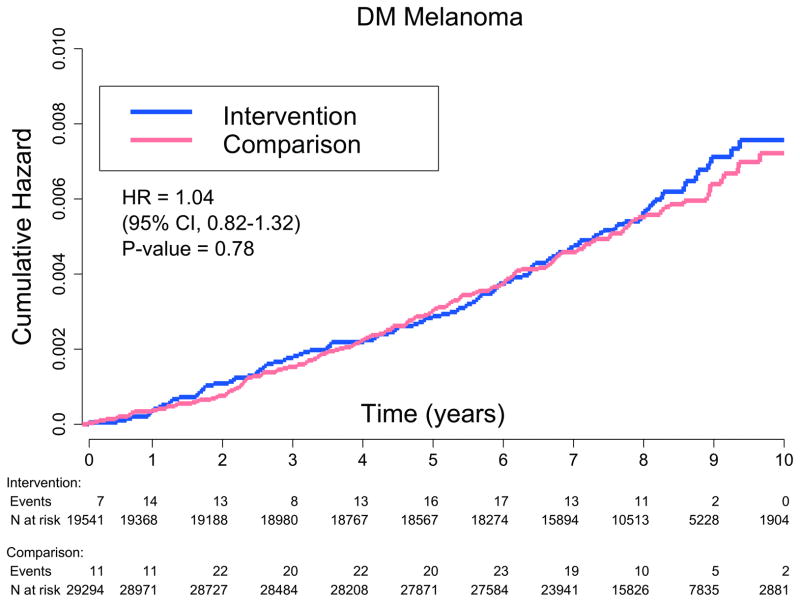
Incidence of physician-adjudicated melanoma was similar between randomization groups, with 114 melanoma cases in the dietary intervention group and 165 cases in the comparison group (annualized percentage of 0.07% versus 0.07%, HR 1.04, 95% CI: 0.82–1.32; Table 2, PFig. 2). Melanoma incidence was similar between the groups even when participants with a history of melanoma were excluded (HR 1.04, 95% CI: 0.82–1.32); however, in subgroup analysis, there was a significant differential effect of low-fat dietary intervention on risk of melanoma by baseline fat intake (interaction= 0.006). Specifically, women with higher baseline fat intake (≥36.9% of total energy, or the upper half of baseline intake as determined by the participants’ mean) had a significantly higher melanoma risk (HR 1.48, 95% CI: 1.06–2.07), while women with lower baseline fat intake (<36.9% of total energy) trended towards lower melanoma risk (HR 0.72, 95% CI: 0.50–1.02) when assigned to dietary intervention versus comparison (Table 3, Figure 3). A significant differential effect of the low-fat dietary intervention on melanoma risk was also found by baseline vegetable and fruit intake (interactionP= 0.037), with women in the lower three quartiles of vegetable and fruit servings per day at baseline (≤ 4.6) trended towards higher risk of melanoma (HR 1.26, 95% CI: 0.95–1.67), while women in the upper quartile tended to have lower melanoma risk (HR 0.65, 95% CI: 0.41–1.03) when assigned to dietary intervention versus comparison. Dietary intervention did not affect melanoma within any other subgroups.
Figure 2.
Kaplan-Meier estimate of cumulative hazards for melanoma events (N=279)
Cox proportional hazards, Wald statistic P value (interaction). CI = confidence interval; DM = dietary modification; HR = hazard ratio
Table 3.
Estimated effect of dietary modification on risk of melanoma, according to selected baseline characteristics.
| Subgroup | Intervention | Comparison | HR (95% CI)a | P | ||
|---|---|---|---|---|---|---|
| Events | Ann% | Events | Ann% | |||
| Overall | 114 | 0.07 | 165 | 0.07 | 1.04 (0.82, 1.32) | |
|
| ||||||
| Age | 0.25 | |||||
| 50–59 | 41 | 0.07 | 62 | 0.07 | 0.99 (0.67, 1.47) | |
| 60–69 | 54 | 0.08 | 78 | 0.07 | 1.04 (0.73, 1.46) | |
| 70–79 | 19 | 0.08 | 25 | 0.07 | 1.15 (0.63, 2.09) | |
|
| ||||||
| BMI, kg/m2 | 0.13 | |||||
| <25 | 28 | 0.07 | 46 | 0.07 | 0.91 (0.57, 1.46) | |
| 25 to <30 | 36 | 0.06 | 66 | 0.08 | 0.83 (0.55, 1.24) | |
| ≥30 | 50 | 0.08 | 52 | 0.06 | 1.43 (0.97, 2.11) | |
|
| ||||||
| Langley exposureb | 0.64 | |||||
| ≤375 | 451 | 1.05 | 85 | 0.07 | 1.03 (0.73, 1.43) | |
| >375 | 449 | 1.11 | 80 | 0.07 | 1.05 (0.74, 1.47) | |
|
| ||||||
| History of NMSC | 0.31 | |||||
| Yes | 22 | 0.22 | 26 | 0.16 | 1.37 (0.77, 2.41) | |
| No | 92 | 0.06 | 139 | 0.06 | 0.99 (0.76, 1.29) | |
|
| ||||||
| Smoking | 0.59 | |||||
| Never | 62 | 0.08 | 84 | 0.07 | 1.12 (0.80, 1.55) | |
| Past | 46 | 0.07 | 73 | 0.08 | 0.93 (0.64, 1.34) | |
| Current | 6 | 0.06 | 6 | 0.04 | 1.57 (0.51, 4.87) | |
|
| ||||||
| NSAID use | 0.42 | |||||
| Yes | 44 | 0.09 | 58 | 0.07 | 0.96 (0.71, 1.30) | |
| No | 70 | 0.07 | 107 | 0.07 | 1.18 (0.80, 1.75) | |
|
| ||||||
| Total vitamin D intake, IUc | 0.89 | |||||
| <400 | 64 | 0.07 | 97 | 0.07 | 0.99 (0.72, 1.36) | |
| ≥400 | 49 | 0.08 | 67 | 0.07 | 1.10 (0.76, 1.58) | |
|
| ||||||
| Total energy intake, kcal | 0.66 | |||||
| <1677 | 67 | 0.09 | 91 | 0.08 | 1.11 (0.81, 1.52) | |
| ≥1677 | 47 | 0.06 | 73 | 0.06 | 0.97 (0.67, 1.39) | |
|
| ||||||
| Percent energy from total fat, % | 0.006 | |||||
| <36.9 | 45 | 0.06 | 94 | 0.08 | 0.72 (0.50, 1.02) | |
| ≥36.9 | 69 | 0.09 | 70 | 0.06 | 1.48 (1.06, 2.07) | |
|
| ||||||
| Total fat intake, grams | 0.18 | |||||
| <69.0 | 60 | 0.08 | 95 | 0.08 | 0.95 (0.69, 1.32) | |
| ≥69.0 | 54 | 0.07 | 69 | 0.06 | 1.17 (0.82, 1.67) | |
|
| ||||||
| Vegetable and fruit servings/day | 0.037 | |||||
| <4.6 | 89 | 0.08 | 106 | 0.06 | 1.26 (0.95, 1.67) | |
| ≥4.6 | 25 | 0.06 | 58 | 0.10 | 0.65 (0.41, 1.03) | |
|
| ||||||
| Grain servings/day | 0.43 | |||||
| <5.9 | 96 | 0.08 | 124 | 0.07 | 1.15 (0.88, 1.50) | |
| ≥5.9 | 18 | 0.05 | 40 | 0.07 | 0.69 (0.40, 1.21) | |
All models were adjusted for age, assignment in the Hormone Therapy trial, and assignment in the Calcium/Vitamin D trial.
Based on the mean annual amount of sunlight reaching the clinic site as measured by the US Weather Bureau; the 1 langley = 1 g=cal/cm2
From diet and supplements
Figure 3.
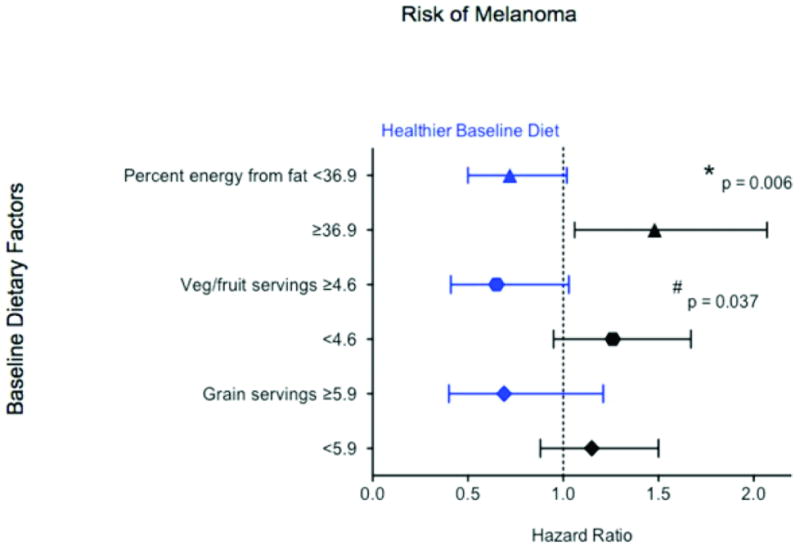
Effect of dietary modification on risk of melanoma, according 580 to baseline 581 dietary factors targeted by intervention (N=279)
Cox proportional hazards, Wald statistic P value (interaction). CI = confidence interval; DM = dietary modification; HR = hazard ratio
* Models were adjusted for age, assignment in the Hormone Therapy trial, and assignment in the Calcium/Vitamin D trial
Sensitivity Analysis
In analyses in which participants who did not fully comply with the trial requirements were censored (e.g., intervention participants who missed an annual clinic visit, failed to participate in ≥ 9 of the 18 first year group sessions, or failed to participate in ≥ 2 of the 4 group sessions in subsequent years; or comparison participants who missed an annual clinic visit)(30), incidences of NMSC and melanoma were similar in the intervention and comparison groups (NMSC: HR 0.99, 95% CI: 0.92–1.07; melanoma: HR 1.04, 95% CI: 0.75–1.43; Table 2). Limiting the primary analysis to Caucasian women showed similar results, with no significant difference in incidence of NMSC or melanoma. A time-dependent analysis of weight loss did not alter the relationship between diet and skin cancer risk (data not shown).
DISCUSSION
The WHI Dietary Modification Trial is the largest randomized controlled trial to evaluate whether a low-fat dietary pattern, with decreased fat intake and increased vegetable, fruit, and grain intake, reduces cancer risk in postmenopausal women. A low-fat dietary pattern did not affect the overall incidence of nonmelanoma skin cancer or melanoma over an average 8.1-years of follow-up.
While the comparison-intervention difference in percent of energy from total fat was lower than anticipated, the intervention group maintained a significant long-term difference in the percent energy from total fat versus the comparison group (−8.1%, P<0.001)(30, 44), as well as vegetable and fruit intake (1.1 servings per day, P<0.001) and grain intake (0.4 servings per day, P<0.001)(30, 37). While these differences were small, this dietary intervention was shown to reduce breast cancer risk significantly among participants in the upper quartile of dietary fat at baseline, hence suggesting that biologically meaningful changes in diet were achieved in the WHI DM Trial(30). Of note, the small but significant decrease in polyunsaturated fat in the WHI intervention versus comparison group (−1.5% at year 6, p<0.001)(32) was not associated with risk of skin cancers, consistent with several studies of NMSC(11, 12, 16, 18) and melanoma(22–24).
Our overall null results are consistent with several observational studies that found no association between fat intake(16, 17) or vegetable and fruit intake(15, 17, 18) and risk of NMSCs (i.e., basal cell and/or squamous cell carcinomas). A 4-year prospective study of 73,366 women without history of skin cancer in the Nurses’ Health Study found no overall association between dietary fat intake and incidence of basal cell carcinoma(16), while an 8-year prospective study of 43,217 men without history of skin cancer in the Health Professionals Follow-up Study found that higher percent energy from total fat was associated with lower risk of basal cell carcinoma(18). An 11-year prospective cohort within a randomized trial of beta-carotene and daily sunblock also found no overall association between dietary fat intake and incidences of basal cell and squamous cell carcinoma(12).
In a previous 2-year trial(11) of low-fat diet, the average number of NMSCs among 101 participants with a history of skin cancer was only lower in the last 8 months of the intervention, suggesting that greater follow-up might be required to determine the effect of diet on skin cancer. However, in our 8-year trial we saw no effect of dietary intervention on NMSC among participants with a history of skin cancer. Of note, the Black et al(11) trial achieved a greater decrease in percent energy from total fat than in the DM Trial(30).
Our results are the first to evaluate melanoma risk within a randomized trial of low-fat diet. One prospective cohort(19) and several case-control studies(21–24) showed no association between total fat intake(19, 21–24) or vegetable and fruit intake(24) and melanoma. Other studies found lower risk of melanoma with higher fat intake(20) and vegetable and fruit intake(21). In subgroup analysis, we found a significant, differential effect of low-fat diet on melanoma risk depending on participants’ baseline fat, and also vegetable and fruit, intake. Women with higher baseline fat intake (≥36.9% of total energy) assigned to dietary intervention had a higher risk of melanoma, while women with lower baseline fat intake (32 to 36.9%) trended towards lower melanoma risk. A similar differential effect was seen among intervention participants by baseline vegetable and fruit intake. Thus, perhaps women with unhealthier baseline diets (e.g., high fat intake and low vegetable and fruit intake at baseline) assigned to dietary intervention became more health-conscious during the trial, and underwent skin examination leading to increased diagnosis of melanoma. Other studies have shown that healthy habits, such as physical activity and healthy diet, may be associated with cancer screening(45, 46). In contrast, intervention participants with healthier baseline diets (e.g., moderate-fat intake and greater vegetable and fruit intake at baseline) may have achieved an even lower fat diet than their counterparts in the intervention group, leading to a trend towards lower melanoma risk. Further investigations on the effect of greater dietary modification than that achieved in the WHI DM Trial on risk of melanoma may be merited. Of note, these results should be interpreted with caution given the multiple subgroups tested.
The strengths of this study include the randomized dietary intervention, the large diverse study population, and the long follow-up time. This analysis is the first to assess melanoma risk within a randomized controlled trial of low-fat diet. This study has several limitations. The primary limitation is that the intervention group did not achieve the comparison-intervention goal, and the percentage of participants who adhered to the trial requirements decreased as the trial progressed. However, a sensitivity analysis that was restricted to those who complied did not show an effect of low-fat dietary pattern on risk of skin cancers. Furthermore, these adherent participants achieved a comparison-intervention difference that was closer to the trial goal (at year 1, 12.1%, and at year 6, 11.1%)(30). Like many prior studies of diet and skin cancer, recorded dietary intake was dependent on self-report. Greater underreporting has been associated with higher BMI(47), but Food Frequency Questionnaire (FFQ) self-reported diet has been shown to correlate with nutritional biomarkers and chronic disease measures in some studies but not all(30, 48). However, the validity of FFQ may be less critical given the randomized treatment assignment and large sample size. Also, as the WHI DM Trial was designed to examine the effect of a low-fat dietary pattern on breast and colorectal cancer as well as cardiovascular disease, post-hoc analyses of melanoma risk may lack statistical power to detect differences. Finally, this study was dependent upon self-report of NMSCs with no indication of type (e.g., basal cell or squamous cell carcinoma), but others have found self report of skin cancer to be reliable(49, 50).
In conclusion, our results do not support a role for reducing dietary fat to prevent skin cancer in postmenopausal women. However, further investigations of lower fat diets in women who are consuming a baseline moderate-fat diet, or of larger dietary change, are relevant areas for future research in melanoma prevention.
Acknowledgments
Financial Support: This study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number 1K23AR056736-01); and the Damon Runyon Clinical Investigator Award to JT. CG held a Stanford Medical Scholars Fellowship and Selected Professions Fellowship from the American Association of University Women.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
GRANT SUPPORT: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Footnotes
Conflict of Interest: Jean Tang has a consultant relationship with Genentech.
References
- 1.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–8. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 4.Epstein JH. Photocarcinogenesis, skin cancer, and aging. J Am Acad Dermatol. 1983;9:487–502. doi: 10.1016/s0190-9622(83)70160-x. [DOI] [PubMed] [Google Scholar]
- 5.Rosso S, Zanetti R, Pippione M, Sancho-Garnier H. Parallel risk assessment of melanoma and basal cell carcinoma: skin characteristics and sun exposure. Melanoma Res. 1998;8:573–83. doi: 10.1097/00008390-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–30. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 8.Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128:2905–8. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–74. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–90. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 11.Black HS, Thornby JI, Wolf JE, Jr, Goldberg LH, Herd JA, Rosen T, et al. Evidence that a low-fat diet reduces the occurrence of non-melanoma skin cancer. Int J Cancer. 1995;62:165–9. doi: 10.1002/ijc.2910620210. [DOI] [PubMed] [Google Scholar]
- 12.Ibiebele TI, van der Pols JC, Hughes MC, Marks GC, Green AC. Dietary fat intake and risk of skin cancer: a prospective study in Australian adults. Int J Cancer. 2009;125:1678–84. doi: 10.1002/ijc.24481. [DOI] [PubMed] [Google Scholar]
- 13.Ibiebele TI, van der Pols JC, Hughes MC, Marks GC, Williams GM, Green AC. Dietary pattern in association with squamous cell carcinoma of the skin: a prospective study. Am J Clin Nutr. 2007;85:1401–8. doi: 10.1093/ajcn/85.5.1401. [DOI] [PubMed] [Google Scholar]
- 14.Hughes MC, van der Pols JC, Marks GC, Green AC. Food intake and risk of squamous cell carcinoma of the skin in a community: the Nambour skin cancer cohort study. Int J Cancer. 2006;119:1953–60. doi: 10.1002/ijc.22061. [DOI] [PubMed] [Google Scholar]
- 15.van der Pols JC, Hughes MC, Ibiebele TI, Marks GC, Green AC. Food intake and risk of basal cell carcinoma in an 11-year prospective study of Australian adults. Eur J Clin Nutr. 2011;65:39–46. doi: 10.1038/ejcn.2010.229. [DOI] [PubMed] [Google Scholar]
- 16.Hunter DJ, Colditz GA, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of basal cell carcinoma of the skin in a prospective cohort of women. Ann Epidemiol. 1992;2:231–9. doi: 10.1016/1047-2797(92)90055-u. [DOI] [PubMed] [Google Scholar]
- 17.Davies TW, Treasure FP, Welch AA, Day NE. Diet and basal cell skin cancer: results from the EPIC-Norfolk cohort. Br J Dermatol. 2002;146:1017–22. doi: 10.1046/j.1365-2133.2002.04763.x. [DOI] [PubMed] [Google Scholar]
- 18.van Dam RM, Huang Z, Giovannucci E, Rimm EB, Hunter DJ, Colditz GA, et al. Diet and basal cell carcinoma of the skin in a prospective cohort of men. Am J Clin Nutr. 2000;71:135–41. doi: 10.1093/ajcn/71.1.135. [DOI] [PubMed] [Google Scholar]
- 19.Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. Int J Cancer. 1997;71:600–4. doi: 10.1002/(sici)1097-0215(19970516)71:4<600::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Bain C, Green A, Siskind V, Alexander J, Harvey P. Diet and melanoma. An exploratory case-control study. Ann Epidemiol. 1993;3:235–8. doi: 10.1016/1047-2797(93)90024-x. [DOI] [PubMed] [Google Scholar]
- 21.Millen AE, Tucker MA, Hartge P, Halpern A, Elder DE, Guerry Dt, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042–51. [PubMed] [Google Scholar]
- 22.Stryker WS, Stampfer MJ, Stein EA, Kaplan L, Louis TA, Sober A, et al. Diet, plasma levels of beta-carotene and alpha-tocopherol, and risk of malignant melanoma. Am J Epidemiol. 1990;131:597–611. doi: 10.1093/oxfordjournals.aje.a115544. [DOI] [PubMed] [Google Scholar]
- 23.Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. Diet, alcohol, and obesity. Am J Epidemiol. 1994;139:869–80. doi: 10.1093/oxfordjournals.aje.a117093. [DOI] [PubMed] [Google Scholar]
- 24.Le Marchand L, Saltzman BS, Hankin JH, Wilkens LR, Franke AA, Morris SJ, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232–45. doi: 10.1093/aje/kwj115. [DOI] [PubMed] [Google Scholar]
- 25.Djuric Z, Lewis SM, Lu MH, Mayhugh M, Tang N, Hart RW. Effect of varying dietary fat levels on rat growth and oxidative DNA damage. Nutr Cancer. 2001;39:214–9. doi: 10.1207/S15327914nc392_9. [DOI] [PubMed] [Google Scholar]
- 26.Meeran SM, Singh T, Nagy TR, Katiyar SK. High-fat diet exacerbates inflammation and cell survival signals in the skin of ultraviolet B-irradiated C57BL/6 mice. Toxicol Appl Pharmacol. 2009;241:303–10. doi: 10.1016/j.taap.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Black HS, Lenger WA, Gerguis J, Thornby JI. Relation of antioxidants and level of dietary lipid to epidermal lipid peroxidation and ultraviolet carcinogenesis. Cancer Res. 1985;45:6254–9. [PubMed] [Google Scholar]
- 28.Erickson KL. Dietary fat influences on murine melanoma growth and lymphocyte-mediated cytotoxicity. J Natl Cancer Inst. 1984;72:115–20. doi: 10.1093/jnci/72.1.115. [DOI] [PubMed] [Google Scholar]
- 29.Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels-Tinker L, Howard B, et al. The women’s health initiative dietary modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S87–S97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 30.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–42. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 31.Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:643–54. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 32.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–66. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 33.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 34.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 35.US Department of Health and Human Services. Nutrition and Your Health: Dietary Guidelines for Americans. 3. Washington, DC: US Department of Health and Human Services; 1990. [Google Scholar]
- 36.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 37.Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, et al. Low-fat dietary pattern and cancer incidence in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99:1534–43. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 39.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 40.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 41.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 42.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: WCRF/AICR; 2007. [Google Scholar]
- 43.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–81. doi: 10.3322/canjclin.56.5.254. quiz 313–4. [DOI] [PubMed] [Google Scholar]
- 44.Women’s Health Initiative Study Group. Dietary adherence in the Women’s Health Initiative Dietary Modification Trial. J Am Diet Assoc. 2004;104:654–8. doi: 10.1016/j.jada.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Martin-Lopez R, Hernandez-Barrera V, De Andres AL, Garrido PC, De Miguel AG, Garcia RJ. Breast and cervical cancer screening in Spain and predictors of adherence. Eur J Cancer Prev. 2010;19:239–45. doi: 10.1097/CEJ.0b013e3283372125. [DOI] [PubMed] [Google Scholar]
- 46.Saraiya M, Hall HI, Thompson T, Hartman A, Glanz K, Rimer B, et al. Skin cancer screening among U.S. adults from 1992, 1998, and 2000 National Health Interview Surveys. Prev Med. 2004;39:308–14. doi: 10.1016/j.ypmed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 47.Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. Am J Epidemiol. 2008;167:1247–59. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 48.Willett W. Nutritional epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 49.Ming ME, Levy RM, Hoffstad OJ, Filip J, Gimotty PA, Margolis DJ. Validity of patient self-reported history of skin cancer. Arch Dermatol. 2004;140:730–5. doi: 10.1001/archderm.140.6.730. [DOI] [PubMed] [Google Scholar]
- 50.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]