Abstract
Previous investigations have revealed sex-specific differences in brain morphometry. The effect of sex on cortical thickness may be influencing cognitive differences between sexes. With this exploratory study, we aimed to investigate the effect of sex in MRI-based cerebral cortex morphometry in healthy young volunteers and how the variability in cortical measures might affect cognitive functioning in men and women. 76 young healthy volunteers (45 men and 31 women) underwent a 1.5 T MR scan and 53 of them completed a comprehensive cognitive battery. Overall no gross significant differences between sexes were found in cortical thickness, surface area and curvature indexes. However, there was a significant group by hemisphere interaction in the total cortical thickness (F(1,72)=5.02; p=0.03). A greater leftward asymmetry was observed in cortical thickness in males. Only females show significant associations between cortical thickness and cognitive functioning (IQ and executive functioning). In conclusion, our findings do not support the notion of sexual dimorphism in cortical mantle morphology. The results also suggest that variability in cortical thickness may affect cognitive functioning in females but not in males.
Keywords: Cognitive functioning, Cortical morphometry, MRI
1. Introduction
Post-mortem and imaging investigations have revealed sex-specific differences in brain morphometry in human beings. Previous structural neuroimaging studies have reported that gray matter (GM), white matter (WM) and brain size are larger in men than in women (Peters and Sikorski, 1998; Raz et al., 2004). Sexual dimorphisms of the brain are more evident in the cortex and seem to be region-specific. Women have larger volumes in frontal and medial paralimbic cortices and men have larger frontomedial, amygdala and hypothalamus volumes (Goldstein et al., 2001). Gur et al. (2002) observed sex differences in regional volumes in the frontal lobes where orbital frontal cortices were relatively larger in women. Region-of-interest (ROI) studies have also reported regional sexual dysmorphism and higher GM/WM ratio in women in specific subregions (Allen et al., 2003; Nopoulos et al., 2000). A nonlinear interaction of sex with age in cortical morphometric measures has been proposed (Giedd et al., 1999) with different neurodevelopmental trajectories (De Bellis et al., 2001). The surface area and the cortical thickness offer relevant information about normal or pathological brain development processes (Bystron et al., 2008; Parent and Carpenter, 1995; Rockel et al., 1980) and may prove a more sensitive measure by which to identify alterations in cortical structure (Eickhoff et al., 2005; Kruggel et al., 2003; Sowell et al., 2003). Cellular shrinkage and reduction in dendritric arborization are likely to account for the normal process of cortical thinning (Morrison and Hof, 1997).
No sex differences in global cortical thickness in adults have been reported (Im et al., 2006; Nopoulos et al., 2000; Rabinowicz et al., 1999; Salat et al., 2004), However, significant greater cortical thickness in women has been described in localized cortical regions (Im et al., 2006; Sowell et al., 2007). Thus, frontal (Im et al., 2006), temporal (Luders et al., 2006b; Sowell et al., 2007) and parietal (Good et al., 2001a; Luders et al., 2006b; Narr et al., 2004) morphological differences have been described between sexes. Hemispheric asymmetries in the thickness of the specific regions of the cortex might be influenced by sex (Im et al., 2006; Luders et al., 2006b). In addition, the effect of sex in total cortical surface area has been reported in postmortem studies (Henery and Mayhew, 1989), although recent imaging investigations do not provide further support to these findings (Barta and Dazzan, 2003).
Cortical thinning might be associated to the normal process of cortical maturation and might influence, at least in part, the efficiency of brain functionality and therefore contribute to a better cognitive functioning (Jernigan et al., 1991; Sowell et al., 2004). Variability in cortical thickness has been associated with differences in general intelligence (Gong et al., 2005; Shaw et al., 2006) and cognitive abilities (Karama et al., 2009). The level of intelligence is associated with the trajectory of cortical development (Shaw et al., 2006) and differences in brain volume measures have been also associated with differences in general intelligence (Pennington et al., 2000). Sex differences in cognitive functioning have been well documented. Whereas women tend to excel on tasks of verbal skills and memory, perceptual speed and accuracy, affect and emotion processing, and fine motor skills, men tend to outperform women on tests of visual memory and mathematical and spatial ability (Halpern, 1992). Therefore, cognitive and behavioral differences between sexes (Kimura, 1996) may be influenced by sex on cortical thickness (Schlaepfer et al., 1995; Yurgelun-Todd et al., 2002). However, only a few studies have investigated this matter. Narr et al. (2007) observed a significant relationship between full-scale intelligence quotients and prefrontal cortical thickness in females. It has been also described that cytoarchitectonic differences in primary visual cortex may underlie observed sex differences in the processing of visuospatial and motion information (Amunts et al., 2007). Amat et al. (2008) found a significant inverse correlation between intellectual abilities and hippocampus volume which was independent of sex.
In the present exploratory study, we aimed to investigate likely sex-specific differences in total and regional cerebral cortex measures in healthy young volunteers. Additionally, we investigated whether variability in cortical measures (total and lobar mean cortical thickness, surface area and gray matter volume) may differentially affect cognitive functioning in men and women. We may hypothesize that differences in specific cognitive domains between sexes could be differentially related to morphological characteristics of the cerebral cortex. Differences in frontal cortical morphometry in females may relate to evidence for sex differences in memory, motor and executive functioning.
2. Materials and methods
2.1. Subjects
From February 2001 to December 2007, and as part of our ongoing investigations on brain morphometry in patients with psychotic disorders, a group of 76 healthy unaffected volunteers (45 men and 31 women) were recruited from the community through advertisements to participate in a study at the University Hospital Marques de Valdecilla, Santander. The unaffected individuals had no current or past history of mental retardation or psychiatric, neurological or general medical illnesses, including substance abuse and significant loss of consciousness, as determined by using an abbreviated version of the Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al., 1992). The absence of psychosis in first-degree relatives was also confirmed by clinical records and family interview. After a detailed description of the study, each subject gave written informed consent to participate, in accordance with the local ethics committee.
2.2. Cognitive assessments
53 subjects completed a comprehensive cognitive battery. For this investigation, we have selected six cognitive tests that comprise 6 cognitive domains, with outcome measures in parenthesis: 1. —executive functions: Trail Making Test B (TMT-B) (Lezak, 1995) (time to complete); 2. — working memory: WAIS-III (Wechsler, 1999) Backward Digits (BD) (total score); 3. — speed of processing: WAIS-III (Wechsler, 1999) Digit Symbol (DS) (standard total score); 4. —attention: Continuous Performance Test Degraded-Stimulus (Cegalis and Bowlin, 1991) (CPT-DS) (total number of correct responses); 5. —visual memory: Rey Complex Figure test (RCFT) (Rey, 1941) (long term recall measure), and 6. — verbal fluency: fluency test (FAS) (Lezak, 1995) (number of words in time limit). The WAIS-III (Wechsler, 1999) subtest of Vocabulary (number of words generated) was used as IQ (Lezak, 1995). Handedness was assessed by the Edinburgh Inventory (Oldfield, 1971).
2.3. MRI acquisition and image processing
All MRI scans were obtained at the University Hospital of Cantabria using a 1.5 T General Electric SIGNA System (GE Medical Systems, Milwaukee, WI). Three different MR sequences were used for each participant. T1-weighted images, using a spoiled grass (SPGR) sequence, were acquired in the coronal plane with the following parameters: echo time (TE)=5 ms, repetition time (TR)=24 ms, number of excitations (NEX)=2, rotation angle=45°, field of view (FOV)=26×19.5 cm, slice thickness=1.5 mm and a matrix of 256×192. The proton density (PD)- and transverse relaxation time (T2)-weighted images were obtained with the following parameters: 3.0 mm thick coronal slices, TR=3000 ms, TE=36 ms (for PD) and 96 ms (for T2), NEX=1, FOV=26×26 cm, and matrix=256×192. The in-plane resolution was 1.016×1.016 mm.
Processing of the images was done by using BRAINS2. Detailed information about the image processing has been previously described (Magnotta et al., 2002). Shortly, the T1-weighted images were spatially normalized and resampled to 1.0-mm3 voxels so that the anterior–posterior axis of the brain was realigned parallel to the anterior commissure/posterior commissure line and the interhemispheric fissure aligned on the other two axes. The T2- and PD-weighted images were aligned to the spatially normalized T1-weighted images using an automated image registration program. These images were then subjected to a linear transformation into standardized stereotaxic Talairach atlas space to generate automated measurements of frontal, temporal, parietal, and occipital lobes. To further classify tissue volumes into gray matter, white matter, and CSF, we used a discriminant analysis method of tissue segmentation based on automated training class selection that utilized data from the T1-weighted, T2-weighted, and proton density sequences (Harris et al., 1999). The discriminant analysis method permits to identify the range of voxel intensity values that characterize GM, WM and CSF. An 8 bit number is assigned to each voxel indicating its partial volume tissue content (10–70 for CSF, 70–190 for GM and 190–250 for WM). In order to define the cortical iso-surface to be used in the posterior analyses, a value of pure GM, or 130, was used as a cut-off. This value represents the parametric center of the GM within the cortex and serves as a useful estimate of its physical center. This triangulated surface was used as the basis for our calculations of cortical area, thickness, and volume. The surface area was calculated as the sum of triangular areas covering this surface of the brain. Several studies have been undertaken to review the reliability and reproducibility of BRAINS (Agartz et al., 2001; Okugawa et al., 2003).
2.4. Measurements
The resulting three-dimensional iso-surface (see Fig. 1) approximates the spatial center of the cortex and is used to provide estimates of values that are direct or indirect quantitative measurements of the cerebral cortex.
Fig. 1.
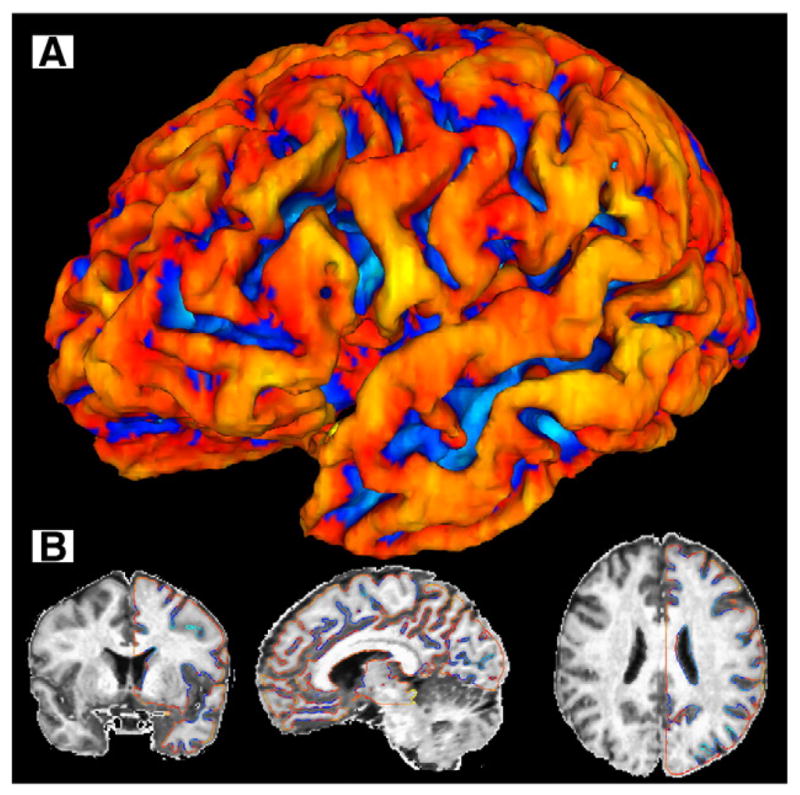
Surface anatomy methodology. The brain surface produced by removing the outer half of the cortex illustrates how sulci are opened up and “buried cortex” is eliminated, with sulci shown in blue and gyri in red (A). The contours demonstrate the location of the “pure” GM iso-surface (B), with sulci shown in blue and gyri in red.
2.4.1. Cortical thickness
This measure is the minimum distance between the 100% gray matter triangle surface and the 50%/50% gray/white matter surface. This measure is an index of cortical thickness; it represents the parametric center of the cortex, or approximately one-half the cortical thickness. Separate measures of total, gyral and sulcal cortical thickness were computed.
2.4.2. Cortical surface area
This value is the straightforward sum of the areas of the triangles making up the surface of the brain.
2.4.3. Curvature index
Both a sulcal and gyral curvature index is calculated by determining the vector angle normal to each triangle surface compared to neighboring vector angles up to four triangle surfaces away. The gyral curvature index measures the degree of convexity with greater absolute values indicating tighter curvatures; thus, higher gyral curvature measures represent gyri with more “peaked” apices while lower gyral curvature indices indicate flatter or broader gyral apices. The sulcal curvature index is a measure of concavity, and absolute numbers of this measure also indicate increasingly tighter curves. High sulcal curvature indices represent sulcal valleys with steep walls, while lower sulcal indices represent sulcal valleys with flatter, looser bases and sloping, rather than steep, walls.
The methods used to quantify these aspects of surface anatomy have been extensively evaluated and validated (Magnotta et al., 1999).
2.5. Statistical analysis
All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS v 15.0, SPSS Inc., Chicago, 2006). Differences in distribution of handedness, tobacco, alcohol and cannabis consumption were assessed with the Chi-square test. Differences in age, intracranial volume and height were evaluated with analysis of variance (ANOVA). Differences in cognitive performance were evaluated with analysis of covariance (ANCOVA) using age and years of education as covariates.
To examine differences in morphological measurements between males and females, a repeated-measures ANCOVA was performed. For the primary analysis, the between subject factor was sex and the within subject was hemisphere (left or right). Mean cortical thickness, surface area, and mean curvature index were used as the dependent variables. Intracranial volume and age were introduced as covariates. Tukey LSD post hoc tests were performed to compare main effects between gender and hemisphere. The group by hemisphere interaction was used to determine differences in asymmetry between sexes. As a secondary analysis, in those cortical measurements in which there were significant group by side interactions, we examined differences in hemispheric asymmetry (100×(L−R)/(L+R)) between the genders with analysis of covariance (ANCOVA). To assess the effect of handedness, we analyzed all the morphological measurements in the sample encompassing only right-handed individuals (43 males; 26 females). Cohen’s d (calculated as (Mean1−Mean2)/sqrt((VAR1+VAR2)/2)) is provided to estimate the magnitude of the differences between groups. According to Cohen (1988) d values of 0.2 are considered to be small effects, values between 0.4 and 0.6 are moderate effects, and values above 0.8 are large effects.
Partial correlations were conducted within each sex to examine associations between morphological measurements and cognition. Age, years of education, and intracranial volume were used as covariates. The slopes of the associations between cognitive test performance and cortical thickness of males and females were compared using Fisher’s Z transformation Throughout, a two-tailed alpha-level of 0.05 was used for statistical testing.
3. Results
3.1. Subject characteristics
Sociodemographic and cognitive characteristics are presented in Tables 1 and 2 respectively. All subjects were Caucasian. Males and females were similar in age, history of drug use, educational level and IQ. Significant differences between sexes were found only for height (F=111.2; p<0.01) and intracranial volume (ICV) (F=62.3; p<0.01). Males have larger total brain volume, although there were no differences in total gray matter volume after controlling for ICV. Males showed a tendency (p=0.08) towards a higher percentage of right handed subjects.
Table 1.
Demographic characteristics of the sample.
| Characteristic | Males (45) | Females (31) | Statistics |
|---|---|---|---|
| Age at initial MRI, mean (SD) [range] years | 26.90 (7.08) [16.00–48.48] | 29.90 (8.24) [15.18–51.62] | F(1,74)=2.89; p=0.10 |
| Height, mean (SD), cm | 176.57 (5.23) | 162.82 (6.02) | F(1,74)=111.16; p<0.01 |
| Intracranial volume, mean (SD), cc | 1439.02 (96.71) | 1277.45 (72.47) | F(1,74)=62.31; p<0.01 |
| Total gray matter volume, mean (SD), cc | 822.74 (62.06) | 735.24 (42.23) | F(1,73)=0.01; p=0.97 |
| Right-handed, N, (%) | 43 (95.6) | 26 (83.87) | χ2=2.99; p=0.08 |
| Parental socioeconomic status, mean (SD)a,b | 3.42 (0.79) | 3.63 (0.72) | Z=−1.23; p=0.22 |
| Years of education, mean (SD)c | 10.57 (2.29) | 11.37 (2.09) | F(1,72)=2.33; p=0.13 |
| Alcohol users, N, (%)d | 28 (63.63) | 18 (62.07) | χ2=0.02; p=0.89 |
| Cannabis users, N, (%)c | 17 (38.64) | 7 (23.33) | χ2=1.91; p=0.17 |
| Tobacco users, N, (%)c | 23 (52.27) | 19 (63.33) | χ2=0.89; p=0.35 |
Based on the Hollingshead–Redlich scale.
Based data from 43 males, 30 females.
Based data from 44 males, 30 females.
Based data from 44 males, 29 females.
Significance threshold=0.05.
Table 2.
Cognitive performance of males and females.
| Test | Males
|
Females
|
F | P | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| WAIS-III Vocabularya | 29 | 9.90 | 2.57 | 23 | 9.48 | 2.29 | 1.28 | 0.27 |
| WAIS-III Backward Digitsa | 30 | 7.67 | 2.20 | 22 | 6.00 | 1.77 | 3.12 | 0.08 |
| Rey Complex Figure Testb | 30 | 24.45 | 6.02 | 23 | 18.50 | 7.02 | 5.87 | 0.02 |
| WAIS-III Digit Symbolb | 30 | 10.13 | 2.58 | 23 | 10.26 | 3.41 | 0.09 | 0.76 |
| Trail Making B Testb | 30 | 58.63 | 13.93 | 23 | 69.22 | 21.79 | 2.46 | 0.12 |
| Continuous Performance Testc | 27 | 78.44 | 1.76 | 19 | 77.26 | 3.71 | 0.32 | 0.57 |
| FASd | 29 | 37.69 | 9.09 | 22 | 37.64 | 9.05 | 0.47 | 0.496 |
Covariates: age, years of education. Significance threshold=0.05.
F(1,48).
F(1,49).
F(1,42).
F(1,47).
Regarding cognitive characteristics, there were only significant differences between sexes in visual memory function (Rey Complex Figure Test) (F=5.87, p=0.02), with males showing better performance than females (see Table 2).
3.2. Total and regional mean cortical measures
Measurements of cerebral cortex variables in both groups are displayed in Tables 3 and 4. The mean cortical thickness across the entire cortex and the four lobes was not significantly different between sexes (all p values>0.28). There were no significant differences between sexes with regard to the total and regional cortical thickness of the gyri (convex regions of the cortex) and sulci (concave regions of the cortex) (Table 3). However, there was a significant group by hemisphere interaction in the total cortical thickness and occipital thickness (total and sulcal region) (Table 3).
Table 3.
Measures of cortical thickness in 45 males and 31 females.
| Males (n=45)
|
Females (n=31)
|
d | Sex
|
Sex×side
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F(1,72) | p | F(1,72) | p | ||
| Total cortical thickness | 4.24 | 0.48 | 4.19 | 0.29 | 0.12 | 0.23 | 0.63 | 5.02 | 0.03 |
| Gyral cortical thickness | 4.60 | 0.58 | 4.56 | 0.37 | 0.10 | 0.58 | 0.45 | 3.30 | 0.07 |
| Sulcal cortical thickness | 3.77 | 0.41 | 3.73 | 0.27 | 0.13 | 0.24 | 0.63 | 2.55 | 0.12 |
| Frontal lobe | |||||||||
| Total cortical thickness | 4.50 | 0.50 | 4.44 | 0.38 | 0.14 | 0.42 | 0.52 | 2.25 | 0.14 |
| Gyral cortical thickness | 4.92 | 0.60 | 4.89 | 0.48 | 0.05 | 0.58 | 0.45 | 1.65 | 0.20 |
| Sulcal cortical thickness | 3.89 | 0.46 | 3.76 | 0.34 | 0.32 | 0.03 | 0.87 | 0.53 | 0.47 |
| Parietal lobe | |||||||||
| Total cortical thickness | 3.93 | 0.45 | 3.88 | 0.29 | 0.13 | 0.36 | 0.55 | 0.16 | 0.69 |
| Gyral cortical thickness | 4.22 | 0.58 | 4.15 | 0.38 | 0.14 | 0.98 | 0.33 | 0.03 | 0.87 |
| Sulcal cortical thickness | 3.59 | 0.43 | 3.58 | 0.31 | 0.03 | 0.43 | 0.51 | 0.08 | 0.78 |
| Temporal lobe | |||||||||
| Total cortical thickness | 4.56 | 0.61 | 4.50 | 0.31 | 0.12 | 0.09 | 0.77 | 2.64 | 0.11 |
| Gyral cortical thickness | 5.17 | 0.78 | 5.08 | 0.41 | 0.15 | 0.33 | 0.57 | 1.45 | 0.23 |
| Sulcal cortical thickness | 3.81 | 0.42 | 3.83 | 0.27 | −0.07 | 1.21 | 0.28 | 2.63 | 0.11 |
| Occipital lobe | |||||||||
| Total cortical thickness | 3.29 | 0.43 | 3.31 | 0.29 | −0.05 | 0.42 | 0.52 | 4.22 | 0.04 |
| Gyral cortical thickness | 3.08 | 0.50 | 3.07 | 0.35 | 0.02 | 0.00 | 0.96 | 0.92 | 0.34 |
| Sulcal cortical thickness | 3.59 | 0.49 | 3.60 | 0.29 | −0.02 | 0.51 | 0.48 | 4.29 | 0.04 |
Covariates: age, intracraneal volume.
Cortical thickness is measured in mm.
Significance threshold=0.05.
The total left cortical thickness was significantly thicker than the right side (p=0.006) for males. In the female group the right occipital thickness was greater than the left side (p=0.02).
Table 4.
Measures of curvature index in 45 males and 31 females.
| Males (n=45)
|
Females (n=31)
|
d | Sex
|
Sex×side
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F(1,72) | p | F(1,72) | p | ||
| Gyral curvature index | 0.068 | 0.004 | 0.069 | 0.003 | −0.36 | 0.42 | 0.52 | 0.02 | 0.88 |
| Sulcal curvature index | −0.088 | 0.011 | −0.089 | 0.008 | 0.13 | 3.31 | 0.07 | 0.86 | 0.36 |
| Frontal lobe | |||||||||
| Gyral curvature index | 0.068 | 0.005 | 0.071 | 0.003 | −0.67 | 0.41 | 0.53 | 0.02 | 0.88 |
| Sulcal curvature index | −0.079 | 0.011 | −0.079 | 0.008 | −0.05 | 1.05 | 0.31 | 0.04 | 0.85 |
| Parietal lobe | |||||||||
| Gyral curvature index | 0.068 | 0.005 | 0.071 | 0.003 | −0.67 | 1.37 | 0.25 | 0.01 | 0.91 |
| Sulcal curvature index | −0.091 | 0.015 | −0.091 | 0.012 | 0.03 | 1.19 | 0.28 | 1.05 | 0.31 |
| Temporal lobe | |||||||||
| Gyral curvature index | 0.074 | 0.004 | 0.074 | 0.004 | 0.13 | 2.61 | 0.11 | 1.76 | 0.19 |
| Sulcal curvature index | −0.087 | 0.010 | −0.090 | 0.011 | 0.33 | 5.21 | 0.03 | 0.01 | 0.94 |
| Occipital lobe | |||||||||
| Gyral curvature index | 0.054 | 0.007 | 0.053 | 0.006 | 0.08 | 3.07 | 0.08 | 0.45 | 0.50 |
| Sulcal curvature index | −0.110 | 0.018 | −0.113 | 0.013 | 0.18 | 4.58 | 0.04 | 0.59 | 0.45 |
Covariates: age, intracraneal volume.
Significance threshold=0.05.
The analysis of right-handed individuals did not show differences between sexes either. This analysis revealed a significant group by hemisphere interaction in total cortical thickness (F(1,65)=5.56; p=0.021) and in total gyral cortical thickness (F(1,65)=4.54; p=0.037). The significant group by hemisphere interaction in the occipital thickness did not maintain significance.
There were no significant differences between sexes in total and regional (four cerebral lobes) surface area measures and GM volumes (all p>0.11). In addition, we did not find significant group by hemisphere interaction in total and regional surface area measures (all p>0.19). The analysis of right-handed individuals did not show meaningful changes from those observed in the entire sample.
Finally, the general shape of the gyri and sulci (curvature indices) did not differ significantly between sexes. Only when we analyzed regional measures of the four cerebral lobes, did we find significant differences in the sulcal curvature index of the temporal lobe (F(1,72)=5.21; p=0.03) and the occipital lobe (F(1,72)=4.85; p=0.04) (Table 4). The mean curvature of the sulci was significantly less concave in males in those two regions. However, the effect size was small in both cases (dCohen < 0.33). The analysis of right-handed individuals did not show meaningful changes from those observed in the whole group. Data are available upon request from the authors.
3.3. Analyses of hemispheric asymmetries
Pairwise tests showed a distinct pattern of hemispheric asymmetries between males and females. The total left cortical thickness was significantly thicker than the right hemisphere (p=0.006) for males and in the female group the right occipital thickness was greater than the left hemisphere (p=0.02).
Group by hemisphere interaction showed also a trend towards significance level in gyral cortical thickness (F(1,72)=3.30, p=0.07).
The secondary analysis of hemispheric asymmetry in these regions showed that males have a higher hemispheric asymmetry (leftward asymmetry) in global cortical thickness (F(1,72)=5.57; p=0.03) than females, but females have a higher rightward asymmetry than males in the occipital cortical thickness (F(1,72)=4.64; p=0.034).
For the right-handed individuals, the secondary analysis of hemispheric asymmetries showed that males have a higher hemispheric asymmetry (leftward asymmetry) in global cortical thickness (F(1,65)= 5.40; p=0.023) and in gyral cortical thickness (F(1,65)=4.10; p=0.047) than females. The data are available upon request.
3.4. Correlations between cortical variables and cognitive measurements
We also sought to determine the relationship between cortical measures and cognitive features in both, male and female sexes. All analyses of correlations are available upon request from the authors.
3.4.1. Cortical thickness and cognitive measurements
Correlations between cortical thickness and cognitive measurements are shown in Table 5. Females had significant positive correlations between executive function (TMT-B) and parietal lobe cortical thickness (r=0.64; p =0.003), temporal lobe cortical thickness (r=0.46; p=0.047), and occipital lobe cortical thickness (r=0.47; p=0.040) and between IQ (WAIS-III Vocabulary subtest) and both total cortical thickness (r=0.47; p=0.041) and frontal lobe cortical thickness (r=0.58; p=0.010). Correlations were close to significance between working memory (WAIS-III Backward Digits) and total cortical thickness (r=−0.44, p=0.067), frontal cortical thickness (r=−0.44, p=0.069), and parietal cortical thickness (r= −0.46, p=0.057). Males did not show any significant correlations.
Table 5.
Correlations of cognitive variables and cortical thickness.
| Cerebrum cortical thickness | Frontal cortical thickness | Parietal cortical thickness | Temporal cortical thickness | Occipital cortical thickness | ||
|---|---|---|---|---|---|---|
| WAIS-III Vocabulary | M | 0.10 (0.624) | 0.07 (0.739) | −0.07 (0.750) | 0.19 (0.373) | 0.17 (0.410) |
| F | 0.47 (0.041) | 0.58 (0.010) | 0.28 (0.237) | 0.40 (0.089) | −0.04 (0.872) | |
| WAIS-III Backward Digits | M | −0.10 (0.628) | −0.15 (0.460) | −0.20 (0.337) | 0.09 (0.653) | 0.08 (0.691) |
| F | −0.44 (0.067) | −0.44 (0.069) | −0.46 (0.057) | −0.19 (0.454) | −0.18 (0.467) | |
| Rey Complex Figure Test | M | 0.11 (0.576) | 0.16 (0.437) | 0.06 (0.753) | 0.07 (0.716) | −0.06 (0.764) |
| F | 0.04 (0.877) | 0.32 (0.178) | −0.33 (0.172) | 0.07 (0.790) | −0.18 (0.472) | |
| WAIS-III Digit Symbol | M | 0.10 (0.621) | 0.06 (0.755) | 0.09 (0.664) | 0.17 (0.398) | 0.08 (0.685) |
| F | 0.21 (0.383) | 0.17 (0.488) | 0.10 (0.694) | 0.33 (0.163) | 0.09 (0.714) | |
| Trail Making B Test | M | <0.01 (0.685) | 0.01(0.950) | 0.09 (0.950) | −0.16 (0.950) | 0.12 (0.560) |
| F | 0.44 (0.062) | 0.17 (0.499) | 0.64 (0.003) | 0.46 (0.047) | 0.47 (0.040) | |
| Continuous Performance Test | M | −0.10 (0.661) | −0.02 (0.938) | −0.15 (0.501) | −0.12 (0.571) | 0.02 (0.936) |
| F | 0.16 (0.564) | 0.13 (0.646) | 0.03 (0.925) | 0.28 (0.925) | <0.01 (0.925) | |
| FAS Verbal Fluency Test | M | −0.14 (0.495) | 0.03 (0.893) | −0.31 (0.124) | −0.22 (0.272) | 0.03 (0.876) |
| F | 0.17 (0.485) | 0.15 (0.534) | 0.04 (0.871) | 0.40 (0.087) | −0.23 (0.350) |
Control variables: age, years of education, ICV. Data presented are r (p); M=Males; F=females.
Significance threshold=0.05.
The slopes of the associations between cognitive test performance and cortical thickness were significantly different for the correlations between WAIS-III Vocabulary and frontal cortical thickness (z =−1.38, p =0.046), between TMT-B and parietal cortical thickness (z= −2.26, p=0.023) and temporal cortical thickness (z=−2.23, p =0.026), and between FAS and temporal cortical thickness (z=−2.14, p =0.032).
3.4.2. Surface area and cognitive measurements
Females had significant negative correlations between parietal lobe area and speed of processing (WAIS-III Digit Symbol) (r= −0.46; p = 0.050) and attention function (CPT) (r = −0.61; p=0.017); and also between frontal lobe area and executive function (TMT-B) (r=−0.64; p=0.003). It is noteworthy that males did not show any significant correlations.
3.4.3. Gyrification indices and cognitive measurements
Overall, the global shape of the gyri and sulci (curvature indices) was not significantly associated with any cognitive dimension. Only when we analyzed regional measures of the four cerebral lobes, we found significant correlations. Thus, females had significant negative correlations between speed of processing (DS) and temporal gyral and sulcal indices (r=−0.45 and r=−0.50; p<0.05) and between attention and frontal gyral index (r=−0.56; p=0.025). In males, occipital gyral index was associated with attention functioning (r=0.48; p<0.017) and there was also a significant negative correlation between working memory and temporal sulcal index (r=−0.46; p<0.015).
4. Discussion
We used image analysis tools to quantify the gross morphometry of the cerebral cortex in a well-matched sample of young, healthy adults to investigate the impact of sex. Overall, the findings here indicate that total and regional cerebral cortex thickness appeared to be remarkably similar in both sexes. Handedness may affect measures of cortical thickness asymmetry (higher leftward asymmetry in right-handed) in males. A greater curvature (increased concavity) of the temporal and occipital sulci was found in women. No significant differences between sexes were found in either gray matter or surface area measures. Interestingly, only females showed significant relationships between cognitive and cortical thickness measures.
Previous studies, but not all, have observed a significant regional greater thickness in women. Increased cortical thickness in temporal (Luders et al., 2006b; Sowell et al., 2007) and parietal (Good et al., 2001a; Luders et al., 2006b; Narr et al., 2004) cortices has been described in women. Accordingly, Im et al. (2006) found greater cortical thickness in the frontal, parietal and occipital regions in women, with less significant increase in temporal regions. In this study, no differences in mean cortical thickness were found in native space and the mean cortical thickness across the entire cortex was not significantly different between men and women. Some other investigations have failed to demonstrate sex differences in total cortical thickness (Im et al., 2006; Nopoulos et al., 2000; Rabinowicz et al., 1999; Salat et al., 2004). Our results did not reveal significant differences in total and regional cortical thickness between sexes. Consistent with our results, previous imaging studies utilizing the same methodologies had not found sex differences in cortical morphometry in healthy young adults (Magnotta et al., 1999) and adolescents (White et al., 2003). In our study the cortical thickness values, ranging from 3.29–4.66 mm in males and from 3.29–4.59 mm in females, are consistent with previous reports of average thickness (Fischl and Dale, 2000; Narr et al., 2004).
Age has been found to influence cortical thickness (Good et al., 2001b; Sowell et al., 2003) and brain volume (Coffey et al., 1998). Males seem to have more pronounced age-related cortical thinning (Good et al., 2001b) and cortical atrophy (Coffey et al., 1998) than females. The fact that we selected a sample of young adults with a somewhat narrow range of age compared to the ages studied in other investigations might explain, at least partly, the discrepancies between findings. In addition, differences in the methodology to assess cortical characteristics may also account for inconsistencies between investigations. Reliability in MRI-derived automated morphometric measures can be influenced by several sources of variance (Jovicich et al., 2009). Thus, reliability can be affected by subject-related factors, such as hydration status (Walters et al., 2001), instrument related factors, such as field strength, scanner manufacturer, imaging magnetic gradients (Jovicich et al., 2006), pulse sequence, and data processing-related factors, including software package and version and the parameters used in the analysis (Senjem et al., 2005; Han et al., 2006).
Brain hemispheric asymmetries have been identified in postmortem and imaging investigations although a great variability in the magnitude of this asymmetry is found among individuals (Allen et al., 2002). Sex differences appear to modulate asymmetries (Amunts et al., 2000; Ide et al., 1996; Zilles et al., 1996). Overall, most studies seem to indicate that female structures are more bilaterally symmetric. We have observed a greater effect of sex in hemispheric asymmetries in total and occipital cortical thickness. However, the small effect size of the total and occipital cortical thickness differences (d=0.19 and d=0.25 respectively) suggests that the differences between sexes are slight. Consistent with our findings, earlier investigations have reported no differences in hemispheric asymmetries in cortical thickness (Im et al., 2006; Luders et al., 2006a) and in cortical shape (Narr et al., 2007) in men and women. No significant differences between sexes were found in any of the other cortical measures assessed (curvature indexes, surface areas and gray matter volumes). Due to the fact that handedness is associated with hemispheric asymmetries in human beings (Jancke et al., 1994; White et al., 1994), we investigated the influence of handedness in asymmetries of cortical measures by analyzing only the subsample of right-handed individuals (N=43 males and N=26 females). Our results have shown that right-handed males have higher hemispheric asymmetries (leftward asymmetry) in total cortical thickness and total gyral cortical thickness than right-handed females. No other significant effects in total or regional measures of hemispheric asymmetries were associated with handedness.
The cytoarchitecture of gyri and sulci differs considerably in the normal human brain. Cellular studies have demonstrated when comparing to the cortex found in sulci that the cortex in the gyral crown is thicker (Welker, 1990) and previous imaging studies have observed thicker cortex in gyral crowns (White et al., 2003). Our results here have consistently demonstrated the difference in total cortical thickness of gyri and sulci [4.58 (0.50) mm and 3.75 (0.36) mm, respectively], but no sex-related differences were found. The degree of cortical gyrification, a quantitative measure of developmental cortical organization, may differ between sexes and may account for behavioral and cognitive sex differences (Luders et al., 2004). A greater gyrification in females than males in frontal and parietal regions has been described (Luders et al., 2004). We observed that men and women differed in temporal and occipital sulcal curvature indices, with a greater curvature (increased concavity) of the sulci in women, but no significant differences were found in measures of gyral curvature indexes and hemispheric gyrification pattern between sexes.
No significant difference between sexes in cortical gray matter and cortical surface area measures was observed in our sample. Previous literature exploring whether there is a sex effect on total or regional gray matter volume has yielded to inconsistent results. No sex differences (Filipek et al., 1994; Good et al., 2001b), in increased (Goldstein et al., 2001; Gur et al., 1999) or decreased (Resnick et al., 2000; Sullivan et al., 2004) total gray matter volume have been described in women relative to men after controlling for the overall increase in male brain size. Regional gray matter volumetric studies have shown greater parietal gray matter in women (Allen et al., 2003; Nopoulos et al., 2000). Voxel-based morphometry (VBM) studies have also shown widespread higher gray matter concentrations in women (Good et al., 2001a; Luders et al., 2005; Verchinski et al., 2000). In our sample of young healthy individuals there were no significant differences in gray matter volume in any of the four lobes analyzed between sexes after controlling for total brain volume. As expected, males showed a relative increase in total brain volume when compared to females. Our results herein are also consistent with imaging studies in young healthy volunteers which found no evidence for sex differences or hemispheric asymmetry in surface area measures (Barta and Dazzan, 2003).
Cortical thinning might be associated to the normal process of cortical maturation that is thought to improve the efficiency of brain functionality and therefore contribute to a better cognitive functioning (Jernigan et al., 1991; Sowell et al., 2004). Studies in rats have revealed that disruption of neurogenesis late during gestation is associated with reduced cortical thickness and with deficits in a wide variety of cognitive functions (Flagstad et al., 2005). Variability in cortical thickness has been associated with differences in general intelligence (Shaw et al., 2006) and cognitive abilities (Karama et al., 2009). Therefore, sex-related changes in cortical thickness might lead to differences in cognitive functioning between men and women (Schlaepfer et al., 1995). Accordingly, Narr et al. (2007) observed that only females showed a significant relationship between full-scale intelligence quotients and prefrontal cortical thickness. Consistently, our results have shown a positive strong correlation between cortical thickness, primarily in the frontal cortex and IQ (r=0.58), specifically in healthy adult females (n=23). Moreover, females showed significant correlations between cortical thickness and executive functioning (TMT-B performance), with the greatest associations in parietal areas (r=0.64). Interestingly, imaging studies seem to point out that the posterior parietal cortex is a crucial brain region to executive processing requirements. The lack of association between frontal cortical thickness and executive functioning is contrary to the expected results considering that numerous lines of evidence converge to implicate frontal regions (anterior cingulate, and dorsolateral and ventrolateral prefrontal regions) in neural network that mediates executive function (Minzenberg et al., 2009). On the contrary, there were no significant associations between cortical thickness and cognitive measures in males. Despite this differential pattern of associations, in our sample men and women are, on average, similar in intelligence and overall cognitive performance, although as expected men had a better performance in visual memory than women (Gallagher and Burke, 2007). It is of note that we failed to observe gender differences in the pattern of associations between visual memory performance and gross cortical morphometry.
In this line of research, Im et al. (2008) observed that a wider and shallower sulcal shape was associated with mild cognitive impairment. We have observed that the pattern of correlations between cognitive dimensions and sulcal and gyral curvature indices differed between sexes. To our knowledge no previous investigation has explored the effect of sex on the relationship between surface morphology and cognition. We found no clear explanation for the differential pattern of associations between sexes and any conceivable explanation is going to be highly speculative. Further research is warranted to confirm our results and to set up and rational theories about the influence of the general shape of the gyri and sulci on cognitive functioning.
There were a few limitations to this study. First, the sample size may limit the statistical power to detect significant effects. Therefore, we might fail to observe “medium” effects when they do actually exist. Further investigations with larger sample sizes are warranted to confirm our findings. Second, one of the limitations of this report may be the fact that multiple comparisons were made to explore clinical and cognitive correlations. However, we decided not to correct for multiple testing and thus, some of the significant associations we found could have been type I errors. Third, the fact that a high proportion of individuals in our sample used cannabis (males: 38.64%; females: 23.33%) and alcohol (males: 63.63%; females: 62.07%) may limit the generalizability of our findings and should be taken into account when comparing to the results of other studies. Although the effect of cannabis in brain morphology is still under debate, our group has recently observed that cannabis use in adolescence and early-adulthood might involve a premature alteration in cortical gyrification (Mata et al., 2010). Alcohol consumption has been also related to changes in brain morphometry (Gazdzinski et al., 2000). Nonetheless, when we repeated the analyses only with non-user individuals the results did not change significantly (data available from the authors under request). Finally, our findings do not rule out the possibility that sex-related structural cortical differences exist on more specific cortical regions or on a finer microstructural level, cellular architecture and connectivity.
In summary, the present study revealed no gross significant differences between young healthy females and males in cortical thickness, surface area and curvature indexes. These results may not support the hypothesis of sexual dimorphism in cortical gross morphology. However, the asymmetry profile seems to be influenced by sex, with a greater leftward asymmetry observed in cortical thickness in males. It is also of note that the results suggest that sex might be influencing the pattern of correlations between cortical thickness and cognitive abilities. Women show significant associations between cortical thickness and IQ and executive functioning. Further studies are warranted to fully address whether differences in cortical structure and organization may account for the sex-related differences in cognitive abilities and/or behavioral between sexes.
Acknowledgments
The present study was performed at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant support: Instituto de Salud Carlos III PI020499, PI050427, and PI060507, SENY Fundació Research Grant CI 2005-0308007 and Fundación Marqués de Valdecilla API07/011.
No pharmaceutical company supplied any financial support towards it. The study, designed and directed by B C-F and JL V-B, conformed to international standards for research ethics and was approved by the local institutional review board.
We wish to thank the PAFIP researchers who helped with data collection and specially acknowledge Cesar Gonzalez-Blanch, Obdulia Martinez and Mrs. Gema Pardo for data collection and David Torrellas for their assistance in imaging analysis. In addition, we acknowledge the participants and their families for enrolling in this study.
Abbreviations
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- BD
backward digits
- CPT-DS
continuous performance test degraded-stimulus
- CSF
cerebrospinal fluid
- DPP
duration of prodromic period
- DUI
duration of untreated illness
- DS
digit symbol
- DUP
duration of untreated psychosis
- F
F-test
- FOV
field of view
- GM
gray matter
- ICV
intracranial volume
- MRI
magnetic resonance imaging
- NEX
number of excitations
- PD
proton density
- RCFT
Rey Complex Figure Test
- ROI
region of interest
- TE
echo time
- TMT-B
Train Making Test B
- T1
spin–lattice relaxation time
- T2
spin–spin relaxation time
- TR
repetition time
- VBM
voxel based morphometry
- WM
white matter
References
- Agartz I, Okuguwa G, Nordstrom M, Greitz D, Magnotta V, Sedvall G. Reliability and reproducibility of brain tissue volumetry from segmented MR scans. Eur Arch Psychiatry Clin Neurosci. 2001;251(6):255–61. doi: 10.1007/pl00007542. [DOI] [PubMed] [Google Scholar]
- Allen JS, Damasio H, Grabowski TJ. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am J Phys Anthropol. 2002;118(4):341–58. doi: 10.1002/ajpa.10092. [DOI] [PubMed] [Google Scholar]
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray–white composition of the human cerebrum. Neuroimage. 2003;18(4):880–94. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Amat JA, Bansal R, Whiteman R, Haggerty R, Royal J, Peterson BS. Correlates of intellectual ability with morphology of the hippocampus and amygdala in healthy adults. Brain Cogn. 2008;66(2):105–14. doi: 10.1016/j.bandc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Jancke L, Mohlberg H, Steinmetz H, Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000;38(3):304–12. doi: 10.1016/s0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Amunts K, Armstrong E, Malikovic A, Hömke L, Mohlberg H, Schleicher A, et al. Gender-specific left–right asymmetries in human visual cortex. J Neurosci. 2007;27(6):1356–64. doi: 10.1523/JNEUROSCI.4753-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49(8):615–23. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Barta P, Dazzan P. Hemispheric surface area: sex, laterality and age effects. Cereb Cortex. 2003;13(4):364–70. doi: 10.1093/cercor/13.4.364. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9(2):110–22. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Cegalis J, Bowlin J. Vigil: software for the assessment of attention. Nashua, NH: Forthought; 1991. [Google Scholar]
- Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, et al. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55(2):169–79. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NY: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11(6):552–7. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Walters NB, Schleicher A, Kril J, Egan GF, Zilles K, et al. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum Brain Mapp. 2005;24(3):206–15. doi: 10.1002/hbm.20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4(4):344–60. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagstad P, Glenthoj BY, Didriksen M. Cognitive deficits caused by late gestational disruption of neurogenesis in rats: a preclinical model of schizophrenia. Neuropsychopharmacology. 2005;30(2):250–60. doi: 10.1038/sj.npp.1300625. [DOI] [PubMed] [Google Scholar]
- Gallagher C, Burke T. Age, gender and IQ effects on the Rey–Osterrieth Complex Figure Test. Br J Clin Psychol. 2007;46(Pt. 1):35–45. doi: 10.1348/014466506x106047. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volume. Alcohol Clin Exp Res. 2000;29 (8):1484–95. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11(6):490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gong QY, Sluming V, Mayes A, Keller S, Barrick T, Cezayirli E, et al. Voxel-based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. Neuroimage. 2005;25(4):1175–86. doi: 10.1016/j.neuroimage.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001a;14 (3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001b;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–72. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12(9):998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Halpern DF. Sex differences in cognitive abilities. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, et al. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23(1):144–54. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Henery CC, Mayhew TM. The cerebrum and cerebellum of the fixed human brain: efficient and unbiased estimates of volumes and cortical surface areas. J Anat. 1989;167:167–80. [PMC free article] [PubMed] [Google Scholar]
- Ide A, Rodriguez E, Zaidel E, Aboitiz F. Bifurcation patterns in the human sylvian fissure: hemispheric and sex differences. Cereb Cortex. 1996;6(5):717–25. doi: 10.1093/cercor/6.5.717. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31(1):31–8. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Won Seo S, Hyung Kim S, Kim SI, Na DL. Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2008;43(1):103–13. doi: 10.1016/j.neuroimage.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Jancke L, Schlaug G, Huang Y, Steinmetz H. Asymmetry of the planum parietale. NeuroReport. 1994;5(9):1161–3. doi: 10.1097/00001756-199405000-00035. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(Pt. 5):2037–49. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46(1):177–92. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Ad-Dab’bagha Y, Haierb RJ, Dearyc IJ, Lytteltona OC, Lepagea C, et al. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–55. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Sex, sexual orientation and sex hormones influence human cognitive function. Curr Opin Neurobiol. 1996;6(2):259–63. doi: 10.1016/s0959-4388(96)80081-x. [DOI] [PubMed] [Google Scholar]
- Kruggel F, Bruckner MK, Arendt T, Wiggins CJ, von Cramon DY. Analyzing the neocortical fine-structure. Med Image Anal. 2003;7(3):251–64. doi: 10.1016/s1361-8415(03)00006-9. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, et al. Gender differences in cortical complexity. Nat Neurosci. 2004;7(8):799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Woods RP, Rex DE, Jancke L, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26(2):493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. Hemispheric asymmetries in cortical thickness. Cereb Cortex. 2006a;16(8):1232–8. doi: 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, Deluca H, et al. Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp. 2006b;27(4):314–24. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, et al. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex. 1999;9(2):151–60. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26 (4):251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santiañez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, et al. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278 (5337):412–9. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Kim S, Thompson PM, Szeszko P, Robinson D, et al. Abnormal gyral complexity in first-episode schizophrenia. Biol Psychiatry. 2004;55(8):859–67. doi: 10.1016/j.biopsych.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Luders E, Thompson PM, Woods RP, Robinson D, et al. Asymmetries of cortical shape: effects of handedness, sex and schizophrenia. Neuroimage. 2007;34(3):939–48. doi: 10.1016/j.neuroimage.2006.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98(1):1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Takase K, Nobuhara K, Yoshida T, Minami T, Tamagaki C, et al. Inter- and intraoperator reliability of brain tissue measures using magnetic resonance imaging. Eur Arch Psychiatry Clin Neurosci. 2003;253(6):301–6. doi: 10.1007/s00406-003-0444-3. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parent A, Carpenter MB. Carpenter’s human neuroatonomy. Williams & Wilkins; 1995. [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DN, Simon JH, et al. A twin MRI study of size variations in human brain. J Cogn Neurosci. 2000;12(1):223–32. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- Peters R, Sikorski R. Imaging. The art of bubble making. Science. 1998;282(5388):432–3. doi: 10.1126/science.282.5388.432b. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Dean DE, Petetot JM, de Courten-Myers GM. Gender differences in the human cerebral cortex: more neurons in males; more processes in females. J Child Neurol. 1999;14(2):98–107. doi: 10.1177/088307389901400207. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25(3):377–96. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10(5):464–72. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Rey A. L’exam psychologique dans les cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TP. The basic uniformity in structure of the neocortex. Brain. 1980;103(2):221–44. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Res. 1995;61(3):129–35. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–8. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362(9397):1699–707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550–60. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25(2):185–92. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Verchinski B, Meyer-Lindenberg A, Japee S, Kohn P, Egan M, Bigelow L, et al. Gender differences in gray matter density: a study of structural MRI images using voxel-based morphometry. Neuroimage. 2000;11(5):S228. [Google Scholar]
- Walters RJC, Fox NC, Crum WR, Taube D, Thomas D. Hemodialysis and cerebral edema. Nephron. 2001;87:143–7. doi: 10.1159/000045903. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Weschsler adult intelligence scale-III. Madrid: TEA ediciones; 1999. [Google Scholar]
- Welker WI. The significance of foliation and fissuration of cerebellar cortex. The cerebellar folium as a fundamental unit of sensorimotor integration. Arch Ital Biol. 1990;128(2–4):87–109. [PubMed] [Google Scholar]
- White LE, Lucas G, Richards A, Purves D. Cerebral asymmetry and handedness. Nature. 1994;368(6468):197–8. doi: 10.1038/368197a0. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54(4):418–26. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD, Young AD. Sex differences in cerebral tissue volume and cognitive performance during adolescence. Psychol Re. 2002;91(3 Pt 1):743–57. doi: 10.2466/pr0.2002.91.3.743. [DOI] [PubMed] [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, et al. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neurosci Biobehav Rev. 1996;20(4):593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]


