Abstract
Purpose
To develop and validate an associative model using pupillography that best discriminates those with and without glaucoma.
Design
A prospective case-control study.
Methods
148 patients with glaucoma (mean age 67±11) and 71 controls (mean age 60±10) were enrolled in a clinical setting. This prototype pupillometer is designed to record and analyze pupillary responses at multiple, controlled stimulus intensities, while using varied stimulus patterns and colors. We evaluated three approaches: 1) comparing the responses between the two eyes, 2) comparing responses to stimuli between the superonasal and inferonasal fields within each eye, and 3) calculating the absolute pupil response of each individual eye. Associative models were developed using stepwise regression or forward selection with Akaike information criterion and validated with 5-fold cross validation. We assessed the associative model using sensitivity, specificity and the area under the receiver operating characteristic curve (AUROC).
Results
Persons with glaucoma had a more asymmetric pupil response between the two eyes (p<0.001), between superonasal and inferonasal visual field within the same eye (p=0.014), and also had a smaller amplitude, slower velocity and longer latency of pupil response compared to controls (all p<0.001). A model including age and these three components resulted in an AUROC of 0.87 (95% CI 0.83 to 0.92) with 80% sensitivity and specificity in detecting glaucoma. This result remained robust after cross-validation.
Conclusions
Using pupillography, we were able to discriminate persons with glaucoma from those with normal eye exams. With refinement, pupil testing may provide a simple approach for glaucoma screening.
INTRODUCTION
Glaucoma is the second leading cause of blindness worldwide, affecting 60 million people, 8.4 million of whom are blind.1,2 Population based surveys indicate that glaucoma remains undiagnosed in 90% of affected people globally and in 50% of those in developed countries.1,3 There is a need to improve on screening and case detection, especially to develop methods that can be applied worldwide and in less developed countries.4
Unlike visual field testing, the assessment of pupillary response to light does not require a subjective patient response. Abnormalities in the pupillary light reflex can be detected by alternately illuminating each eye while comparing the change in pupil size.5 Asymmetry in this response is referred to as a relative afferent pupillary defect (RAPD) and indicates asymmetric disease of the anterior visual system. As glaucoma is frequently more severe in one eye, an RAPD is often clinically detectable in persons with glaucoma.6,7
An RAPD can be quantified by sequentially placing optical filters of increasing density in front of the normal eye as a light source alternatively illuminates each eye.5 By using this technique, an RAPD can be detected when there is approximately 25%–50% unilateral loss of retinal ganglion cells in monkeys.8 Compared to this method, high-resolution infrared pupillography allows for more precise quantification of the pupillary response using controlled stimulus intensities and this has improved our ability to detect and objectively quantify subtle RAPDs. 9,10
Glaucoma does not result in uniform loss of ganglion cells across the retina.11 This asymmetry is detectable by comparing the pupil response between superior versus inferior quadrants or peripheral versus central parts of the visual field.12,13 This is similar to the glaucoma hemifield test, which also tests for asymmetry between the superior and inferior visual fields.14 In addition, it is likely that pupil responses in glaucoma may be generally diminished when compared to normal subjects,15 which can also help with detection.
The purpose of this study was to use pupillography to compare how pupils respond to stimuli of varied patterns, colors, and intensities in normal subjects and glaucoma patients. Further, we developed and validated an associative model that best discriminates those with and without glaucoma by combined use of 1) between-eye pupil response 2) pupil responses in different parts of the visual field within the same eye, and 3) the absolute response of individual eyes.
METHODS
Subjects and Eye Examinations
In this prospective case-control study, we enrolled a total of 243 participants, including 165 glaucoma patients and 78 normal subjects. All participants were enrolled between March 2011 and June 2012. To be eligible for participation, participants had to be 40 years or older at enrollment, have presenting visual acuity better than 20/100 in both eyes and have not had ocular surgery within 3 months. All subjects provided informed consent to participate in the study and the Institutional Review Board of the Johns Hopkins University School of Medicine approved the protocol prospectively.
We enrolled patients with glaucoma of any cause in at least one eye, defined by having both optic disc or retinal nerve fiber layer structural abnormalities and a visual field abnormality consistent with glaucomatous damage. We included a full spectrum of glaucoma disease severity and excluded patients with other macular or retinal comorbidities. All glaucoma patients had visual acuity assessed with habitual correction, intraocular pressure measured by Goldman applanation tonometry, fundus examination by slit lamp biomicroscopy, visual field testing with standard automated perimetry (Humphrey Field Analyzer, Carl Zeiss Meditec, Inc., Dublin, CA) using the Swedish Interactive Threshold Algorithm (SITA) standard strategy and 24-2 pattern,16 and retinal nerve fiber layer thickness (RNFL) measured by a circumpapillary scan at 3.45 mm from the center of the disc using Spectral Domain Optical Coherence Tomography (SD-OCT, Spectralis, Heidelberg Engineering, Heidelberg, Germany).
We enrolled normal subjects who were at the Wilmer Eye Institute accompanying patients attending examinations. All normal subjects underwent an undilated fundus exam, visual acuity testing, intraocular pressure measurement using the Icare tonometer (Icare, Finland), and RNFL measurement with SD-OCT. We also assessed their visual field using frequency-doubling technology (FDT - Humphrey Matrix Perimeter, Carl Zeiss Meditec, Dublin, CA). We excluded normal subjects with a cup to disc ratio greater than 0.7, signs of retinal or optic nerve structural abnormalities, or abnormal visual field results in either eye. When analyzing the FDT results, we minimized the false negative rate by using the N-30-5 screening protocol.17 The test was considered reliable if false positives and fixation losses were less than 30%. A subject was considered eligible if there was no abnormal point in the central locations and no more than 2 abnormal points at any probability level (p<5%, 2%, 1% or <0.5%) at any of the peripheral locations.18
Pupillometer and waveform analysis
We used a prototype automated pupillometer (prototype of RAPDx, Konan Medical USA, Inc., Irvine, CA) to record and analyze the pupillary light reflex. Each subject was dark-adapted for one minute before the test. While the patient was viewing binocularly, monocular stimuli were presented on the LCD panel, alternating between eyes (similar to the swinging flashlight test). We applied stimuli of varied colors: white, red, green, blue and yellow, of varied patterns: full field, peripheral (28° with 10.5° macular sparing), central (2.9°), and superior and inferior nasal quadrant arcs (21° with 11.7° macular spacing), and of varied intensities: 35 lux (bright) and 25 lux (dim) (Figure 1). All stimulus cycles add up to 2.1 seconds, with full field stimulations of 200ms long followed by 1900ms dark and patterned stimulations of 600 ms long followed by 1500ms dark. The entire exam lasted approximately 7 minutes.
Figure 1.
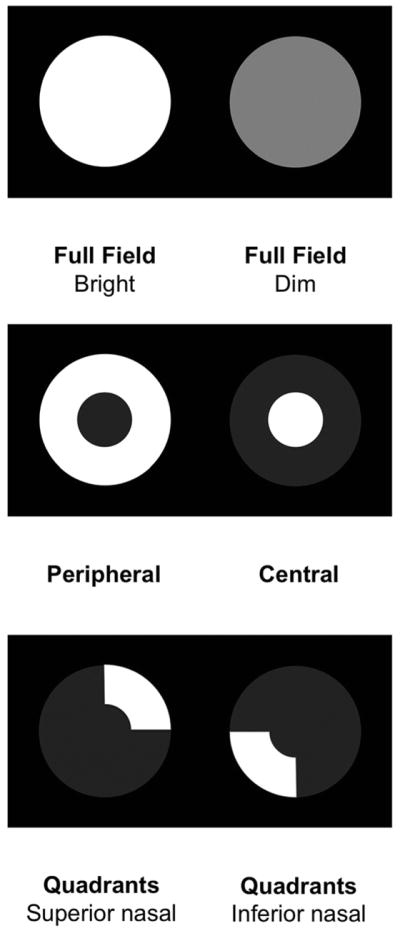
The pupillography provided light stimuli of varied patterns, colors, and intensities.
Patterns: Full field (F), peripheral (P), central (C), superionasal (Snq) and inferonasal (Inq) quadrant arcs.
Intensities: Bright (Br) and dim (Di).
Colors: White (W), red (R), green (G), yellow (Y) and blue (B)
Nine stimuli sequences used in this study:
1. FBrW→ FBrW→FBrW→FBrW→FBrW→FBrW→ FBrW
2. FBrW→FBrR →FBrG→FBrB→FBrY
3. FBrW→FBrR →FBrG→FBrB→FBrY
4. PDiW→ PDiR→ PDiG→ PDiB→ PDiY
5. CDiW→ CDiR→ CDiG→ CDiB→ CDiY
6. PBrW→ PBrR→ PBrG→ PBrB→ PBrY
7. CBrW→ CBrR→ CBrG→ CBrB→ CBrY
8. SnqBrW→ SnqBrR→SnqBrG→SnqBrB→SnqBrY
9. InqBrW→InqBrR→InqBrG→InqBrB→InqBrY
The device records pupil diameter over time and calculates six metrics (response amplitude, latency, maximum constriction velocity, maximum dilation velocity, and time to peak constriction and dilation). The amplitude of pupil constriction is calculated by the percentage change in pupil diameter (PD) between constriction onset and peak constriction in response to each stimulus [(PDresting – PDconstricted)/PDresting]. By using the first-order and second-order derivatives of the pupil diameter over time, two additional metrics, maximum constriction velocity and maximum dilation velocity, were calculated. We defined the onset of pupil constriction when the velocity first crosses a threshold of 50% of the maximum contraction velocity and then calculated the latency using the time from the onset of stimulation to the onset of pupil constriction. We also calculated the time from stimulus onset to the time of these maximal velocities.
The between-eye score is defined as the log of the relative ratio of a given pupil metric between the right and left eye multiplied by 10. Similarly, the within-eye score was defined by taking the log of the relative ratio of the given pupil metric between the superonasal and inferonasal field and multiplying by 10.
Statistical analysis
Differences in characteristics and pupil metrics between glaucoma patients and normal subjects were compared using Student’s t test and the Chi-square test. We used Pearson correlation to assess the asymmetry of pupil response between the two eyes or between superior and inferior stimuli within each eye. For analyses performed at the level of individual eyes, we used the eye with the higher within-eye score or eyes with lower response amplitude. To avoid multi-collinearity in a regression model, we assessed the correlation between responses under different colors, between responses under different patterns, and between pupil metrics of the same pupil response. We then built models only with variables that are less correlated with each other (R<0.6).
We used univariate and multivariate logistic regression models to assess the association between each pupil metric and glaucoma. The five models included 1) a univariate model with the between-eye score of response amplitude as the sole predictor 2) a multivariable model using four variables: age, between-eye score, within-eye score, and the absolute value of response amplitude 3) a model with variables selected using stepwise forward regression (p-value<0.1 to be included to the model and p-value>0.2 to be excluded) 4) a model with variables determined using forward selection using Akaike information criterion and 5) a model using all variables.
We determined the ability of the model to discriminate glaucoma from normal by calculating the area under the receiver operating characteristic curve (AUROC) with a non-parametric approach. We used bootstrap resampling to calculate pointwise confidence intervals for the ROC curve at the false positive rate of 0.2 and the true positive rate of 0.2. The resampling was done separately for case and control strata with 1,000 replications. We also assessed the performance of each model among patients who had a visual field mean deviation (MD) worse than −5 dB and those who had an MD of −5 dB or better. Finally, we validated the results by spiting the data randomly into quintiles, with model development in four of the five and testing in one of the five, which was repeated five times (five-fold cross validation). Statistical analyses were performed using STATA 12.0 (StataCorp LP, College Station, TX).
RESULTS
A total of 236 eligible participants were enrolled in our study and 219 (93%) of them completed the pupillographic examination. Of the 17 incomplete examinations, 4 were due to frequent blinking, 3 were due to software errors and the pupil waveforms of the other 10 were unanalyzable. Among 219 subjects who successfully completed the pupillographic test, 148 cases have glaucoma in at least one eye (93% primary open angle glaucoma, 3% primary angle closure glaucoma, and 4% secondary glaucoma) and 71 controls don’t have it in either eye. Compared to controls, glaucoma patients were significantly older (67±11 versus 60±10 years old) and were more likely to be male (51% versus 32% - Table 1). Glaucoma patients had greater between-eye differences in intraocular pressure and RNFL thickness but had similar baseline pupil diameters when compared to controls.
Table 1.
Baseline characteristics of study population in glaucoma detection using pupillography.
| Glaucoma (n=148) | Control (n=71) | |
|---|---|---|
|
| ||
| Age, year* | 67.4 ± 10.7 | 60.4 ± 9.6 |
|
| ||
| Female, no. (%)* | 72 (49) | 48 (68) |
|
| ||
| Race, no. (%) | ||
| Non-Hispanic white | 121 (81.8) | 51 (71.8) |
| Black | 20 (13.5) | 12 (16.9) |
| Asian | 7 (4.7) | 7 (9.9) |
| Hispanic | 0 (0) | 1 (1.4) |
|
| ||
| Baseline pupil diameter, mm | ||
| Average between the two eyes | 4.10 ± 0.81 | 4.17 ± 0.71 |
| Absolute difference between the two eye | 0.05 ± 0.04 | 0.05 ± 0.04 |
|
| ||
| Intraocular pressure, mmHg | ||
| Average between the two eyes | 14.1 ± 3.5 | 13.6 ± 3.9 |
| Absolute difference between the two eye* | 2.8 ± 3.2 | 1.1 ± 0.9 |
|
| ||
| Visual field mean deviation (dB) | ||
| Average between the two eyes* | −7.35 (6.24) | −0.72 (0.78) |
| Absolute differences between the two eyes* | 5.81 (5.69) | 0.69 (0.53) |
|
| ||
| Retinal nerve fiber layer thickness, μm | ||
| Average between the two eyes* | 65.8 ± 15.7 | 93.6 ± 9.6 |
| Absolute difference between the two eye* | 13.4 ± 12.3 | 4.2 ± 4.9 |
Plus-minus values are means ± standard deviation.
p-value <0.05.
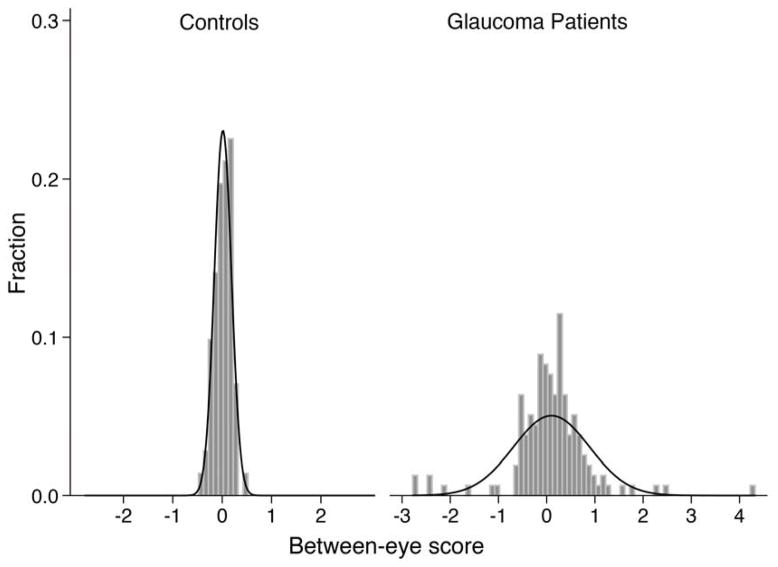
The correlation of each pupil metric was lower between eyes of glaucoma patients than controls across different stimuli (Table 2). The absolute value of the between-eye scores was generally higher in glaucoma patients, suggesting greater between-eye asymmetry (Table 2). However, many patients with glaucoma also had a between-eye score close to zero (Figure 2). When looking at within-eye response to stimuli, the correlation between superonasal and inferonasal stimuli was lower in glaucoma patients and their absolute within-eye scores were higher, suggesting greater within-eye asymmetry (Table 3). We summarize the absolute values of each pupil metric under varied stimuli for each individual eye in Table 4. Eyes with glaucoma had smaller response amplitude, slower velocities and longer latency than control eyes (all p < 0.05).
Table 2.
Between eye symmetry of pupillary light reflex measured by pupillography in patients with glaucoma (N=148) and controls (N=71)
| Correlation coefficient | Absolute between-eye score Mean ± SD | |||
|---|---|---|---|---|
| Glaucoma | Control | Glaucoma | Control | |
| White, Full field, Bright | ||||
| Amplitude* | 0.76 | 0.96 | 0.47 ± 0.51 | 0.14 ± 0.10 |
| Latency* | 0.83 | 0.93 | 0.23 ± 0.21 | 0.15 ± 0.14 |
| Max constriction velocity* | 0.89 | 0.98 | 0.36 ± 0.36 | 0.12 ± 0.11 |
| Time to max constriction velocity* | 0.86 | 0.92 | 0.16 ± 0.13 | 0.11 ± 0.08 |
| Max dilation velocity | 0.76 | 0.77 | 0.42 ± 0.53 | 0.30 ± 0.49 |
| Time to max dilation velocity* | 0.74 | 0.88 | 0.24 ± 0.19 | 0.12 ± 0.11 |
| Red, Full field, Bright | ||||
| Amplitude* | 0.66 | 0.91 | 0.68 ± 0.72 | 0.28 ± 0.25 |
| Latency | 0.73 | 0.85 | 0.31 ± 0.26 | 0.25 ± 0.23 |
| Max constriction velocity* | 0.79 | 0.90 | 0.50 ± 0.50 | 0.36 ± 0.28 |
| Time to max constriction velocity* | 0.80 | 0.81 | 0.23 ± 0.23 | 0.17 ± 0.13 |
| Max dilation velocity | 0.73 | 0.83 | 0.55 ± 0.49 | 0.47 ± 0.52 |
| Time to max dilation velocity* | 0.65 | 0.74 | 0.30 ± 0.25 | 0.20 ± 0.19 |
| Green, Full field, Bright | ||||
| Amplitude* | 0.74 | 0.94 | 0.57 ± 0.57 | 0.22 ± 0.19 |
| Latency | 0.82 | 0.87 | 0.24 ± 0.25 | 0.24 ± 0.20 |
| Max constriction velocity* | 0.83 | 0.96 | 0.49 ± 0.44 | 0.21 ± 0.18 |
| Time to max constriction velocity | 0.73 | 0.79 | 0.22 ± 0.20 | 0.19 ± 0.14 |
| Max dilation velocity | 0.66 | 0.48 | 0.55 ± 0.63 | 0.51 ± 0.68 |
| Time to max dilation velocity | 0.66 | 0.59 | 0.29 ± 0.24 | 0.25 ± 0.20 |
| Blue, Full field, Bright | ||||
| Amplitude* | 0.76 | 0.92 | 0.60 ± 0.58 | 0.28 ± 0.20 |
| Latency* | 0.74 | 0.92 | 0.33 ± 0.27 | 0.18 ± 0.14 |
| Max constriction velocity* | 0.84 | 0.92 | 0.45 ± 0.43 | 0.33 ± 0.23 |
| Time to max constriction velocity | 0.77 | 0.77 | 0.23 ± 0.22 | 0.19 ± 0.15 |
| Max dilation velocity | 0.68 | 0.69 | 0.59 ± 0.64 | 0.46 ± 0.52 |
| Time to max dilation velocity* | 0.61 | 0.68 | 0.34 ± 0.24 | 0.22 ± 0.19 |
| Yellow, Full Field, Bright | ||||
| Amplitude* | 0.75 | 0.93 | 0.59 ± 0.55 | 0.23 ± 0.19 |
| Latency* | 0.85 | 0.91 | 0.27 ± 0.19 | 0.20 ± 0.17 |
| Max constriction velocity* | 0.86 | 0.96 | 0.46 ± 0.41 | 0.23 ± 0.17 |
| Time to max constriction velocity* | 0.66 | 0.86 | 0.25 ± 0.24 | 0.16 ± 0.15 |
| Max dilation velocity | 0.75 | 0.79 | 0.48 ± 0.57 | 0.40 ± 0.42 |
| Time to max dilation velocity* | 0.60 | 0.69 | 0.31 ± 0.25 | 0.23 ± 0.21 |
| White, Peripheral, Bright | ||||
| Amplitude* | 0.62 | 0.85 | 0.81 ± 0.63 | 0.46 ± 0.33 |
| Latency* | 0.61 | 0.75 | 0.41 ± 0.36 | 0.27 ± 0.26 |
| Max constriction velocity* | 0.75 | 0.88 | 0.73 ± 0.53 | 0.47 ± 0.43 |
| Time to max constriction velocity* | 0.58 | 0.59 | 0.37 ± 0.29 | 0.28 ± 0.22 |
| Max dilation velocity* | 0.60 | 0.65 | 0.75 ± 0.78 | 0.52 ± 0.68 |
| Time to max dilation velocity | 0.57 | 0.43 | 0.32 ± 0.28 | 0.31 ± 0.24 |
| White, Peripheral, Dim | ||||
| Amplitude* | 0.68 | 0.75 | 0.77 ± 0.63 | 0.54 ± 0.45 |
| Latency | 0.61 | 0.75 | 0.45 ± 0.39 | 0.42 ± 0.31 |
| Max constriction velocity* | 0.78 | 0.85 | 0.71 ± 0.54 | 0.58 ± 0.49 |
| Time to max constriction velocity | 0.38 | 0.55 | 0.42 ± 0.43 | 0.33 ± 0.28 |
| Max dilation velocity | 0.48 | 0.39 | 0.71 ± 0.83 | 0.67 ± 0.85 |
| Time to max dilation velocity | 0.50 | 0.26 | 0.33 ± 0.30 | 0.28 ± 0.22 |
| White, Central, Bright | ||||
| Amplitude* | 0.54 | 0.57 | 1.52 ± 1.42 | 1.04 ± 0.80 |
| Latency | 0.35 | 0.32 | 0.73 ± 0.67 | 0.67 ± 0.60 |
| Max constriction velocity | 0.70 | 0.66 | 0.99 ± 0.98 | 0.78 ± 0.64 |
| Time to max constriction velocity | 0.27 | 0.49 | 0.54 ± 0.64 | 0.40 ± 0.32 |
| Max dilation velocity | 0.10 | 0.19 | 1.18 ± 1.53 | 1.30 ± 1.80 |
| Time to max dilation velocity | 0.40 | 0.26 | 0.51 ± 0.42 | 0.46 ± 0.38 |
| White, Central, Dim | ||||
| Amplitude* | 0.61 | 0.62 | 1.54 ± 1.72 | 1.00 ± 0.89 |
| Latency | 0.31 | 0.11 | 0.91 ± 0.81 | 0.88 ± 0.84 |
| Max constriction velocity | 0.70 | 0.75 | 1.02 ± 0.89 | 0.81 ± 0.64 |
| Time to max constriction velocity | 0.29 | 0.49 | 0.60 ± 1.16 | 0.45 ± 0.42 |
| Max dilation velocity | 0.42 | 0.46 | 0.93 ± 0.97 | 0.91 ± 0.84 |
| Time to max dilation velocity | 0.45 | 0.25 | 0.46 ± 0.35 | 0.50 ± 0.40 |
SD, standard deviation.
p-value<0.05 calculated with Student’s t-test.
Figure 2.
Distribution of the between-eye score of the pupillography in glaucoma patients and controls.
Table 3.
Within-eye symmetry of pupil response to superonasal versus inferonasal stimulation measured by pupillography in patients with glaucoma (N=148) and controls (N=71).
| Correlation coefficient | Absolute within-eye score Mean ± SD | |||
|---|---|---|---|---|
| Glaucoma | Normal | Glaucoma | Normal | |
| White | ||||
| Amplitude* | 0.62 | 0.64 | 1.63 ± 1.23 | 1.20 ± 0.78 |
| Latency | 0.30 | 0.43 | 0.93 ± 0.70 | 0.78 ± 0.60 |
| Max constriction velocity | 0.72 | 0.70 | 1.17 ± 0.75 | 1.01 ± 0.56 |
| Time to max constriction velocity | 0.08 | 0.26 | 0.71 ± 0.96 | 0.51 ± 0.47 |
| Max dilation velocity | 0.13 | 0.52 | 1.29 ± 1.29 | 0.97 ± 0.72 |
| Time to max dilation velocity* | 0.26 | 0.24 | 0.54 ± 0.35 | 0.41± 0.26 |
| Red | ||||
| Amplitude* | 0.41 | 0.54 | 2.97 ± 2.48 | 2.28 ± 1.63 |
| Latency | 0.10 | 0.32 | 1.11 ± 0.80 | 0.99 ± 0.64 |
| Max constriction velocity* | 0.45 | 0.63 | 1.90 ± 1.39 | 1.47 ± 0.92 |
| Time to max constriction velocity | 0.22 | 0.39 | 0.72 ± 0.50 | 0.60 ± 0.39 |
| Max dilation velocity | 0.02 | 0.05 | 1.97 ± 1.50 | 2.19 ± 2.00 |
| Time to max dilation velocity | 0.07 | 0.08 | 0.82 ± 0.47 | 0.85 ± 0.52 |
| Green | ||||
| Amplitude* | 0.63 | 0.59 | 1.70 ± 1.14 | 1.31 ± 0.92 |
| Latency | 0.40 | 0.50 | 0.90 ± 0.63 | 0.78 ± 0.45 |
| Max constriction velocity | 0.73 | 0.69 | 1.18 ± 0.82 | 1.05 ± 0.66 |
| Time to max constriction velocity | 0.31 | 0.27 | 0.67 ± 0.86 | 0.53 ± 0.35 |
| Max dilation velocity | 0.07 | 0.05 | 1.57 ± 1.25 | 1.66 ± 1.69 |
| Time to max dilation velocity* | 0.26 | 0.07 | 0.61 ± 0.37 | 0.74 ± 0.42 |
| Blue | ||||
| Amplitude | 0.62 | 0.60 | 1.99 ± 1.91 | 1.51 ± 1.10 |
| Latency* | 0.20 | 0.53 | 0.98 ± 0.74 | 0.74 ± 0.46 |
| Max constriction velocity | 0.70 | 0.67 | 1.37 ± 1.05 | 1.16 ± 0.87 |
| Time to max constriction velocity* | 0.25 | 0.47 | 0.64 ± 0.48 | 0.50 ± 0.36 |
| Max dilation velocity | 0.06 | 0.38 | 1.75 ± 1.62 | 1.47 ± 1.16 |
| Time to max dilation velocity | 0.28 | 0.13 | 0.68 ± 0.38 | 0.79 ± 0.43 |
| Yellow | ||||
| Amplitude* | 0.62 | 0.58 | 1.87 ± 1.37 | 1.51 ± 1.16 |
| Latency* | 0.31 | 0.55 | 0.88 ± 0.62 | 0.71 ± 0.48 |
| Max constriction velocity | 0.63 | 0.68 | 1.35 ± 1.01 | 1.14 ± 0.76 |
| Time to max constriction velocity* | 0.21 | 0.38 | 0.70 ± 0.58 | 0.46 ± 0.43 |
| Max dilation velocity | 0.03 | 0.30 | 1.72 ± 1.59 | 1.57 ± 1.42 |
| Time to max dilation velocity | 0.29 | 0.03 | 0.61 ± 0.36 | 0.61 ± 0.37 |
SD, standard deviation.
The eye with greater within-eye asymmetry of each pupil metric was selected for each individual.
p-value<0.05 calculated with Student’s t-test.
Table 4.
Pupil response of each individual eye to various stimulation measured by pupillography in patients with glaucoma (N=148) and controls (N=71). All values expressed as Mean ± standard deviation.
| Amplitude (Ratio) | Latency (second) | Max Contraction Velocity (mm/ms) | Time to Max Contraction (second) | Max Dilation Velocity (mm/ms) | Time to Max Dilation (second) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glaucoma | Control | Glaucoma | Control | Glaucoma | Control | Glaucoma | Control | Glaucoma | Control | Glaucoma | Control | |
| Full, bright | ||||||||||||
| White | 0.25 ± 0.05 | 0.29 ± 0.04 | 0.20 ± 0.03 | 0.18 ± 0.02 | 3.28 ± 0.79 | 3.81 ± 0. 74 | 0.42 ± 0.04 | 0.40 ± 0.03 | 2.02 ± 0.47 | 2.18 ± 0.43 | 1.20 ± 0.11 | 1.16 ± 0.09 |
| Red | 0.21 ± 0.05 | 0.25 ± 0.05 | 0.22 ± 0.03 | 0.20 ± 0.03 | 2.95 ± 0.74 | 3.39 ± 0.76 | 0.43 ± 0.04 | 0.41 ± 0.03 | 1.79 ± 0.42 | 2.03 ± 0.60 | 1.16 ± 0.11 | 1.10 ± 0.09 |
| Green | 0.24 ± 0.06 | 0.28 ± 0.05 | 0.21 ± 0.03 | 0.19 ± 0.03 | 3.19 ± 0.81 | 3.75 ± 0.78 | 0.42 ± 0.04 | 0.40 ± 0.03 | 1.95 ± 0.47 | 2.11 ± 0.41 | 1.18 ± 0.11 | 1.13 ± 0.09 |
| Blue | 0.22 ± 0.06 | 0.26 ± 0.05 | 0.22 ± 0.03 | 0.19 ± 0.03 | 3.10 ± 0.79 | 3.57 ± 0.78 | 0.43 ± 0.04 | 0.41 ± 0.04 | 1.86 ± 0.46 | 2.03 ± 0.40 | 1.17 ± 0.11 | 1.12 ± 0.09 |
| Yellow | 0.24 ± 0.06 | 0.29 ± 0.05 | 0.20 ± 0.03 | 0.18 ± 0.03 | 3.27 ± 0.83 | 3.80 ± 0.78 | 0.42 ± 0.04 | 0.40 ± 0.03 | 1.97 ± 0.51 | 2.18 ± 0.42 | 1.18 ± 0.11 | 1.14 ± 0.10 |
| Peripheral, Bright | ||||||||||||
| White | 0.26 ± 0.06 | 0.31± 0.05 | 0.22 ± 0.03 | 0.19 ± 0.03 | 3.11 ± 0.81 | 3.69 ± 0.88 | 0.44 ± 0.05 | 0.41 ± 0.03 | 1.82 ± 0.49 | 2.12 ± 0.52 | 1.38 ± 0.12* | 1.37 ± 0.09* |
| Red | 0.19 ± 0.06 | 0.23 ± 0.05 | 0.23 ± 0.05 | 0.20 ± 0.03 | 2.53 ± 0.78 | 3.03 ± 0.75 | 0.45 ± 0.07 | 0.42 ± 0.04 | 1.64 ± 0.45 | 1.80 ± 0.41 | 1.29 ± 0.15* | 1.26 ± 0.12* |
| Green | 0.23 ± 0.06 | 0.28 ± 0.05 | 0.22 ± 0.04 | 0.19 ± 0.03 | 3.02 ± 0.84 | 3.55 ± 0.85 | 0.44 ± 0.05 | 0.41 ± 0.04 | 1.86 ± 0.51 | 2.02 ± 0.49 | 1.33 ± 0.12* | 1.31 ± 0.10* |
| Blue | 0.21 ± 0.06 | 0.26 ± 0.06 | 0.23 ± 0.03 | 0.20 ± 0.04 | 2.85 ± 0.80 | 3.43 ± 0.90 | 0.44 ± 0.05 | 0.41 ± 0.04 | 1.77 ± 0.48 | 1.94 ± 0.52 | 1.29 ± 0.13* | 1.25 ± 0.13* |
| Yellow | 0.24 ± 0.06 | 0.29 ± 0.05 | 0.22 ± 0.03 | 0.19 ± 0.03 | 3.08 ± 0.81 | 3.58 ± 0.82 | 0.44 ± 0.05 | 0.41 ± 0.04 | 1.90 ± 0.50 | 2.06 ± 0.45 | 1.32 ± 0.12* | 1.32 ± 0.09* |
| Peripheral, Dim | ||||||||||||
| White | 0.24 ± 0.06 | 0.27 ± 0.05 | 0.23 ± 0.03 | 0.20 ± 0.03 | 2.87 ± 0.79 | 3.38 ± 0.89 | 0.45 ± 0.04 | 0.42 ± 0.04 | 1.77 ± 0.46 | 1.91 ± 0.41 | 1.37 ± 0.12* | 1.36 ± 0.08* |
| Red | 0.16 ± 0.05 | 0.19 ± 0.05 | 0.24 ± 0.04 | 0.22 ± 0.04 | 2.23 ± 0.65 | 2.61 ± 0.71 | 0.46 ± 0.05 | 0.43 ± 0.04 | 1.56 ± 0.45* | 1.61 ± 0.35* | 1.32 ± 0.12 | 1.28 ± 0.12 |
| Green | 0.21 ± 0.06 | 0.25 ± 0.06 | 0.23 ± 0.03 | 0.20 ± 0.03 | 2.73 ± 0.83 | 3.25 ± 0.79 | 0.45 ± 0.05 | 0.42 ± 0.04 | 1.76 ± 0.46* | 1.88 ± 0.54* | 1.33 ± 0.13 | 1.29 ± 0.09 |
| Blue | 0.19 ± 0.06 | 0.23 ± 0.06 | 0.24 ±0.04 | 0.21 ± 0.04 | 2.59 ± 0.79 | 3.06 ± 0.76 | 0.45 ± 0.05 | 0.42 ± 0.04 | 1.66 ± 0.47 | 1.82 ± 0.46 | 1.30 ± 0.14 | 1.26 ± 0.10 |
| Yellow | 0.22 ± 0.06 | 0.26 ± 0.05 | 0.23 ± 0.04 | 0.20 ± 0.03 | 2.85 ± 0.79 | 3.39 ± 0.85 | 0.44 ± 0.05 | 0.41 ± 0.04 | 1.82 ± 0.51* | 1.95 ± 0.38* | 1.32 ± 0.10* | 1.29 ± 0.12* |
The eye with smaller amplitude, slower velocities, and longer time to max contraction/dilation was selected for each individual.
Pupil metrics between glaucoma patients and normal subjects were compared using Student’s t test. All p-value<0.05 except those marked in *.
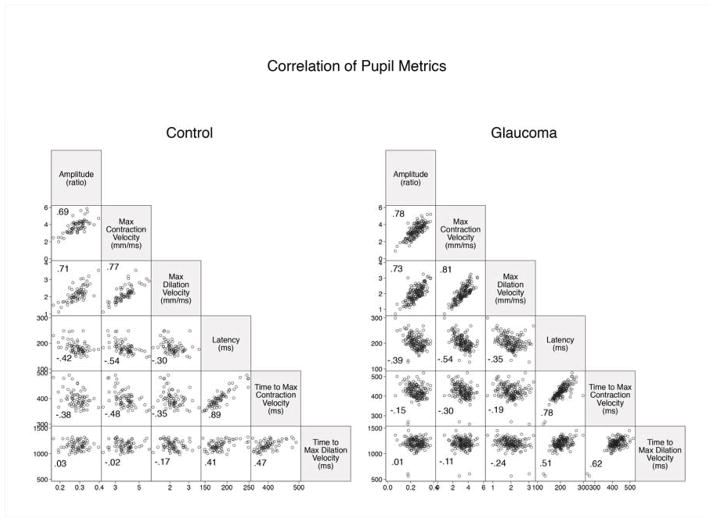
We found strong correlations between response amplitude and constriction and dilation velocities (R=0.69–0.81). Latency and time to maximum contraction velocity were also strongly correlated (R=0.89 in normal subjects and R=0.78 in glaucoma patients), Figure 3. To avoid multi-colinearity, we selected three out of six pupil metrics that were less correlated (R<0.6, amplitude, latency and time to maximum dilation velocity) to develop the model. In addition, pupil metrics using different stimulation colors and patterns were also correlated with each other (Supplemental Table 1–2). We therefore only selected pupil metrics under white light and full field stimulation to develop the final model. The final list of nine variables included was age and the between-eye, within-eye, and individual-eye value of the amplitude, latency, and time to maximum dilation (Table 5).
Figure 3.
Correlation of pupil metrics for full field white stimulation of the pupillography between glaucoma patients and controls.
Table 5.
Associative models of detecting glaucoma using pupillography developed by different methods and their sensitivity, specificity, and area under the receiver operating characteristics curve (AUROC)
| Odds ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Univariate | Combined model | Stepwise forward | Forward selection (Akaike information criterion) | Full model | |
| Age (per 1 year older) | 1.06 (1.03 to 1.10) | 1.04 (1.01 to 1.08) | 1.04 (1.00 to 1.08) | 1.03 (0.99 to 1.07) | 1.04 (1.00 to 1.08) |
| Between-eye | |||||
| Amplitude (per 0.1 unit) | 1.97 (1.55 to 2.51) | 1.72 (1.35 to 2.19) | 1.71 (1.32 to 2.20) | 1.64 (1.26 to 2.14) | 1.67 (1.27 to 2.19) |
| Latency (per 0.1 unit) | 1.35 (1.10 to 1.65) | - | - | - | 0.97 (0.73 to 1.28) |
| Time to Max Dilation (per 0.1 unit) | 1.87 (1.42 to 2.46) | - | 1.61 (1.15 to 2.26) | 1.62 (1.15 to 2.28) | 1.53 (1.07 to 2.19) |
| Within-eye | |||||
| Amplitude (per 0.1 unit) | 1.05 (1.01 to 1.08) | 1.03 (0.99 to 1.07) | - | - | 1.02 (0.98 to 1.07) |
| Latency (per 0.1 unit) | 1.04 (0.99 to 1.09) | - | - | - | 0.99 (0.93 to 1.06) |
| Time to Max Dilation (per 0.1 unit) | 1.17 (1.05 to 1.30) | - | 1.15 (1.01 to 1.30) | 1.16 (1.02 to 1.32) | 1.17 (1.02 to 1.34) |
| Individual-eye | |||||
| Amplitude (per 0.1 ratio decrease) | 5.36 (2.77 to 10.38) | 2.10 (0.99 to 4.44) | - | 1.89 (0.80 to 4.44) | 2.35 (0.95 to 5.84) |
| Latency (per 0.01 sec) | 1.42 (1.23 to 1.64) | - | 1.22 (1.03 to 1.44) | 1.19 (1.00 to 1.42) | 1.07 (0.86 to 1.34) |
| Time to Max Dilation (per 0.1 sec) | 1.43 (1.06 to 1.93) | - | - | - | 1.42 (0.89 to 2.27) |
| Overall | |||||
| AUROC | 0.78 (0.73 to 0.84) | 0.84 (0.79 to 0.89) | 0.86 (0.82 to 0.91) | 0.87 (0.82 to 0.91) | 0.87 (0.83 to 0.92) |
| Sensitivity (at specificity=0.80) | 0.68 (0.61 to 0.77) | 0.80 (0.68 to 0.86) | 0.74 (0.66 to 0.85) | 0.78 (0.65 to 0.86) | 0.80 (0.65 to 0.88) |
| Specificity (at sensitivity=0.80) | 0.47 (0.32 to 0.66) | 0.77 (0.45 to 0.90) | 0.71 (0.58 to 0.87) | 0.75 (0.57 to 0.88) | 0.80 (0.62 to 0.93) |
| 5-fold cross validation | |||||
| AUROC | 0.78 (0.72 to 0.84) | 0.83 (0.77 to 0.88) | 0.85 (0.80 to 0.90) | 0.85 (0.80 to 0.90) | 0.84 (0.78 to 0.89) |
| Sensitivity (at specificity=0.80) | 0.70 (0.60 to 0.78) | 0.76 (0.60 to 0.85) | 0.72 (0.66 to 0.82) | 0.76 (0.62 to 0.85) | 0.74 (0.57 to 0.86) |
| Specificity (at sensitivity=0.80) | 0.44 (0.30 to 0.65) | 0.71 (0.51 to 0.87) | 0.70 (0.66 to 0.84) | 0.71 (0.52 to 0.88) | 0.75 (0.54 to 0.85) |
| Visual field MD< −5 dB | |||||
| AUROC | 0.82 (0.76 to 0.87) | 0.88 (0.83 to 0.93) | 0.89 (0.84 to 0.94) | 0.89 (0.84 to 0.94) | 0.90 (0.86 to 0.95) |
| Sensitivity (at specificity=0.80) | 0.74 (0.65 to 0.84) | 0.87 (0.75 to 0.93) | 0.81 (0.70 to 0.90) | 0.84 (0.70 to 0.91) | 0.84 (0.74 to 0.92) |
| Specificity (at sensitivity=0.80) | 0.59 (0.38 to 0.89) | 0.87 (0.70 to 0.94) | 0.81 (0.62 to 0.94) | 0.81 (0.67 to 0.94) | 0.84 (0.69 to 0.96) |
| Visual field MD≥ −5 dB | |||||
| AUROC | 0.71 (0.61 to 0.81) | 0.76 (0.67 to 0.85) | 0.81 (0.73 to 0.89) | 0.81 (0.73 to 0.89) | 0.82 (0.74 to 0.90) |
| Sensitivity (at specificity=0.80) | 0.56 (0.44 to 0.74) | 0.66 (0.50 to 0.78) | 0.62 (0.50 to 0.80) | 0.68 (0.48 to 0.80) | 0.72 (0.41 to 0.86) |
| Specificity (at sensitivity=0.80) | 0.32 (0.21 to 0.56) | 0.45 (0.32 to 0.83) | 0.68 (0.38 to 0.81) | 0.61 (0.45 to 0.84) | 0.68 (0.49 to 0.87) |
Using univariate logistic regression, age, between-eye scores, within-eye scores, and pupillary light reflexes of each individual eye were all significant predictors for glaucoma. Using the between-eye score of response amplitude alone, the AUROC was 0.78 (95% confidence interval [CI]: 0.73–0.84) with 68% sensitivity and 80% specificity in predicting glaucoma (Table 5). By including three additional variables (age, the amplitude of within-eye score and the amplitude of each individual eye), the AUROC of this combined model increased to 0.84 (95% CI: 0.79–0.91) with higher sensitivity and specificity. Using forward stepwise selection, five variables were selected and the AUROC increased to 0.86 (95% CI: 0.82 to 0.91). Forward selection with Akaike information criterion produced a model that included 6 variables and resulted in an AUROC of 0.87 (95% CI: 0.82–0.91). When including all 10 variables, the AUROC of this full model was the highest (0.87, 95% CI: 0.83 to 0.92).
We stratified glaucoma cases into 2 groups based on the severity of visual field defect (cut off at mean deviation of −5 dB) and compared the ability of each model in separating them from controls. All five predictive models performed better in detecting patients with more severe visual field loss (MD<−5dB) from controls (Table 5). The AUROC of the full model increased to 0.90 with 84% sensitivity and 80% specificity in detecting glaucoma patients with greater visual field loss. We validated these five models by five-fold cross validation and the results remained robust (Table 5).
The combined, stepwise, Akaike information criterion and full models had significantly higher AUROC values than the univariate model (p<0.001). The full model was also significantly better than the combined model (Chi-square=4.39 with 1 degree of freedom, p=0.04) but it was not significantly different from the stepwise model (Chi-square=1.45 with 1 degree of freedom, p=0.23) or the Akaike information criterion model (Chi-square=1.36 with 1 degree of freedom, p=0.25).
DISCUSSION
The asymmetry of the pupillary light reflex between eyes (a surrogate for an RAPD) differed significantly when comparing glaucoma patients to those without evidence of retinal or optic nerve disease. However, nearly half of those with glaucoma were missed when just comparing the between-eye responses. Noting this limitation, we compared the pupil responses for corresponding superonasal versus inferonasal fields and observed greater within-eye asymmetry of those patients who did not have an RAPD. These patients also had a smaller and slower pupil contraction, accompanied by a slower recovery from stimulation at different intensities, colors and patterns. By combing all information, the pupillary light reflex assessed by pupillography discriminates those with glaucoma from controls with 80% sensitivity and 80% specificity.
Based on previous systematic reviews, the pooled estimate of the sensitivity of the tests in detecting glaucoma ranged from 46% (Goldmann Applanation Tonometry, GAT) to 92% (Frequency doubling technology perimetry, FDT C20-1), while specificity ranged from 75% (FDT C20-5) to 95% (GAT). 4,19 As compared to those screening devices, the pupillometer has good accuracy in detecting glaucoma. The detection rate may be improved by refining the cutoff values and algorithms or by combining other screening modalities such as FDT or OCT. However, the accuracies were estimated from a clinical-based study and the performance of the pupillometer may be reduced in a community setting where there are more glaucoma suspects and mild glaucoma patients. We attempted to reduce this bias by enrolling patients with a full spectrum of disease severities but larger scale population based studies are needed for better estimation. Also, external validation and calibration of this associative model would be an important next step before applying in any clinical settings. The seven-minute stimulation sequence was developed for research purposes and it may be further reduced to a 2-minute test based on the selective stimulations included in this associative model. The shorter stimulation test will be more suitable for a community based screening strategy.
Glaucoma is most often bilateral, and on average there is asymmetric damage between eyes.20 Due to this asymmetry, an RAPD can be detected using the swinging flashlight test in 9–34% of glaucoma patients.21–24 With the introduction of infrared video pupillography, studies have generally reported a detection rate of 29–68% overall, and of 82% for asymmetric glaucoma.25–28 In our study, the mean absolute between-eye score for controls was 0.14, and 0.34 was the cutoff at 2 standard deviations. At this cutoff, an RAPD was present in 47% of glaucoma patients and 3% of controls, suggesting that the amount of asymmetry needed to segregate the two groups may be too large and therefore using just an RAPD between eyes will miss nearly half the individuals with glaucoma.
We also assessed the relative pupillary responses comparing superonasal to inferonasal field stimulation within the same eye. The pupillary light reflex measured when stimulating the superior visual field was greater than that measured from the inferior field stimulation in normal subjects. This normal asymmetry may limit the interpretation of a subtle reduction in pupillary light reflex in the inferior field (false negative). We conducted a sensitivity analysis by centering the within-eye score value at the mean of normal subjects (positive if higher than the normal mean, negative if lower than the normal mean). This approach did not improve the accuracy significantly (Supplemental Table 3). Despite this, we still observed a significantly higher within-eye asymmetry among glaucoma patients who had an RAPD (1.73 units versus 1.36 in controls, p=0.02) and among those who did not have an RAPD (1.72 units versus 1.36 in controls, p=0.03). Therefore, sensitivity was improved by adding within-eye score to the model without reducing specificity.
We analyzed the waveform of the pupillary light reflex using six metrics. Glaucoma patients had significantly smaller amplitude, longer latency, slower constriction and dilation velocities, and longer time to maximum constriction and dilation as compared to controls (all p < 0.05). However, the measurements of amplitude, constriction velocity, and dilation velocity are highly correlated. This relationship is unaffected by disease status. In other words, the pupil is expected to constrict and dilate slowly whenever the response is small. Similarly, the latency of pupil responses is also highly correlated with time to maximum contraction velocity. Collinearity limited our ability to develop stable regression coefficients when using correlated variables, so we reduced the model from six metrics to three less correlated ones (amplitude, latency, time to maximum dilation velocity).
It has been reported that a blue-and-yellow pattern visual evoked potentials test is more sensitive than black-and-white stimulation in detecting early glaucoma. 29,30 Short-wavelength automated perimetry has been claimed to predict conversion to glaucoma 3 to 4 years before standard automated perimetry defects occur.31–33 These techniques used blue light to stimulate short wavelength sensitive cones preferentially and a yellow background light to adapt the medium and long wavelength sensitive cones as well as simultaneously to saturate the rods. However, using stimuli of different colors did not improve the discrimination of the pupillary light reflex testing in our population. Other studies reported reduced sustained pupil contraction (>30 seconds) to blue light in glaucoma, suggesting a dysfunction of the intrinsically photosensitive retinal ganglion cells. We did not test this phenomenon and focused on pupil response to short duration (0.2–0.6 seconds) stimuli to avoid noise introduced by blinking.
One limitation for pupillography is that abnormal pupillary light reflexes may be present in other ocular or neurological diseases such as macular degeneration and diabetic retinopathy.34–36 This may lead to “false positive” test results in persons with other eye diseases. However, the ability to detect multiple eye diseases may be an advantage for a screening tool. In any case, the definitive diagnosis of glaucoma still requires detailed examination of optic nerve appearance and visual field testing. Another limitation of this study is the age difference between the glaucoma and control groups. We adjusted for age in all models even if it was not significant in the multivariate analysis for this reason. It is possible that residual confounding due to age may have affected our outcomes. In addition, we explored possible interaction between age and pupillary light reflex but the odds ratios were similar between strata with age older and younger than 65 years old (data not shown). Finally, certain systemic medications (e.g. alpha-blockers), glaucoma medications (e.g. brimonidine), ocular surgery (e.g. previous cataract surgery, iridotomy) or comorbidities (e.g. diabetes) may affect pupillary response. This may hamper the generalizability of our results and it is important to externally validate this model and assess its performance in an independent cohort in community settings.
In summary, we assessed pupillary response in a large group of subjects in a clinical setting and found that an automated testing device can detect glaucoma patients using the pupillary response. Glaucoma patients had more asymmetric pupil response between the two eyes and more asymmetric response between superior and inferior stimulation within the same eye. In addition, they also had a smaller and slower pupil contraction and a delayed recovery time when looking at individual eyes with glaucoma. By combining those findings, we developed an associative model that separated glaucoma patients from control subjects. This may provide a simple and inexpensive approach for glaucoma screening but additional study is warranted to validate these findings in a community-based population and to determine if further refinements can be made to improve performance.
Supplementary Material
Acknowledgments
Funding/support:
Dolly Chang received a training grant from the National Institute on Aging 2T32AG000247-16.
David Friedman received the RAPiD pupillometry device from Konan Medical, Inc on loan.
Financial disclosures:
David Friedman is a consultant to Alcon, Allergan, Bausch & Lomb, Merck, Pfizer, QLT, Inc, and Epidemiology International, Inc. The other coauthors have no financial disclosures.
Biography

Dolly S. Chang, MD, MPH, PhD, received her medical degree from National Taiwan University and subsequently earned her MPH and PhD in epidemiology from Johns Hopkins University Bloomberg School of Public Health. Her areas of research interest include the screening, prevention, and interventions of eye diseases, the methodology and conduct of clinical trials and systematic reviews, and pupil pathophysiology.
Footnotes
All authors agreed with each of the changes made to the revision.
Contributions to Authors:
Design and conduct of the study (DC, KA, MB, WS, DF)
Collection (DC, KA, WS)
Management (DC, MB, DF)
Analysis (DC)
Interpretation of the data (DC, MB, DF)
Preparation and review of the manuscript (DC, KA, MB, DF)
Approval of the manuscript (DC, KA, MB, WS, DF)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 3.The Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ervin A-M, Boland MV, Myrowitz EH, et al. Screening for Glaucoma: Comparative Effectiveness. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. [PubMed] [Google Scholar]
- 5.Thompson HS, Corbett JJ, Cox TA. How to measure the relative afferent pupillary defect. Surv Ophthalmol. 1981;26(1):39–42. doi: 10.1016/0039-6257(81)90124-7. [DOI] [PubMed] [Google Scholar]
- 6.Lankaranian D, Altangerel U, Spaeth GL, Leavitt JA, Steinmann WC. The usefulness of a new method of testing for a relative afferent pupillary defect in patients with ocular hypertension and glaucoma. Trans Am Ophthalmol Soc. 2005;103:200–7. discussion 207–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Ichhpujani P, Rome JE, Jindal A, et al. Comparative study of 3 techniques to detect a relative afferent pupillary defect. J Glaucoma. 2011;20(9):535–539. doi: 10.1097/IJG.0b013e3181f464e8. [DOI] [PubMed] [Google Scholar]
- 8.Kerrison JB, Buchanan K, Rosenberg ML, et al. Quantification of optic nerve axon loss associated with a relative afferent pupillary defect in the monkey. Arch Ophthalmol. 2001;119(9):1333–1341. doi: 10.1001/archopht.119.9.1333. [DOI] [PubMed] [Google Scholar]
- 9.Lowenstein O, Loewenfeld IE. Electronic pupillography; a new instrument and some clinical applications. AMA Arch Ophthalmol. 1958;59(3):352–363. [PubMed] [Google Scholar]
- 10.Fison PN, Garlick DJ, Smith SE. Assessment of unilateral afferent pupillary defects by pupillography. Br J Ophthalmol. 1979;63(3):195–199. doi: 10.1136/bjo.63.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakobs TC, Libby RT, Ben Y, John SWM, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171(2):313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Wyatt HJ, Swanson WH, Dul MW. Rapid pupil-based assessment of glaucomatous damage. Optom Vis Sci. 2008;85(6):471–481. doi: 10.1097/OPX.0b013e318177ec02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalaboukhova L, Fridhammar V, Lindblom B. Relative afferent pupillary defect in glaucoma: a pupillometric study. Acta Ophthalmol Scand. 2007;85(5):519–525. doi: 10.1111/j.1600-0420.2006.00863.x. [DOI] [PubMed] [Google Scholar]
- 14.Asman P, Heijl A. Glaucoma Hemifield Test. Automated visual field evaluation. Arch Ophthalmol. 1992;110(6):812–819. doi: 10.1001/archopht.1992.01080180084033. [DOI] [PubMed] [Google Scholar]
- 15.Link B, Junemann A, Rix R, et al. Pupillographic Measurements with Pattern Stimulation: The Pupil’s Response in Normal Subjects and First Measurements in Glaucoma Patients. Invest Ophthalmol Vis Sci. 2006;47(11):4947–4955. doi: 10.1167/iovs.06-0021. [DOI] [PubMed] [Google Scholar]
- 16.Inazumi K, Tsuji A, Yamamoto T, Kitazawa Y. Evaluation of the Swedish interactive thresholding algorithm, a new thresholding algorithm, of the Humphrey Field Analyzer in glaucoma patients. Nippon Ganka Gakkai Zasshi. 1998;102(10):667–672. [PubMed] [Google Scholar]
- 17.Chauhan BC, Johnson CA. Test-retest variability of frequency-doubling perimetry and conventional perimetry in glaucoma patients and normal subjects. Invest Ophthalmol Vis Sci. 1999;40(3):648–656. [PubMed] [Google Scholar]
- 18.Paczka JA, Friedman DS, Quigley HA, Barron Y, Vitale S. Diagnostic capabilities of frequency-doubling technology, scanning laser polarimetry, and nerve fiber layer photographs to distinguish glaucomatous damage. Am J Ophthalmol. 2001;131(2):188–197. doi: 10.1016/s0002-9394(00)00644-9. [DOI] [PubMed] [Google Scholar]
- 19.Burr JM, Mowatt G, Hernández R, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess. 2007;11:iii–iv. ix–x, 1–190. doi: 10.3310/hta11410. [DOI] [PubMed] [Google Scholar]
- 20.Broman AT, Quigley HA, West SK, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49(1):66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page CJ, Merritt JC, Evans B. Relative afferent pupillary defects in primary open-angle glaucoma-five years’ experience. J Natl Med Assoc. 1985;77(12):979–984. [PMC free article] [PubMed] [Google Scholar]
- 22.Skorkovská K, Wilhelm H, Lüdtke H, Wilhelm B. Relative afferent pupillary defect in glaucoma. Klin Monbl Augenheilkd. 2011;228(11):979–983. doi: 10.1055/s-0031-1273356. [DOI] [PubMed] [Google Scholar]
- 23.Schiefer U, Dietzsch J, Dietz K, et al. Associating the magnitude of relative afferent pupillary defect (RAPD) with visual field indices in glaucoma patients. Br J Ophthalmol. 2012;96(5):629–633. doi: 10.1136/bjophthalmol-2011-300776. [DOI] [PubMed] [Google Scholar]
- 24.Hennessy AL, Katz J, Ramakrishnan R, et al. The utility of relative afferent pupillary defect as a screening tool for glaucoma: prospective examination of a large population-based study in a south Indian population. Br J Ophthalmol. 2011;95(9):1203–6. doi: 10.1136/bjo.2010.194217. [DOI] [PubMed] [Google Scholar]
- 25.Jonas JB, Zach FM, Naumann GO. Quantitative pupillometry of relative afferent defects in glaucoma. Arch Ophthalmol. 1990;108(4):479–480. doi: 10.1001/archopht.1990.01070060025009. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto E, Hagiwara T, Hashimoto T, Namba K, Utsumi T, Azuma I. A follow-up study of pupillary dynamics in patients with ocular hypertension and primary open angle glaucoma. Nippon Ganka Gakkai Zasshi. 1991;95(3):265–272. [PubMed] [Google Scholar]
- 27.Hashimoto-Takahashi E. Pupillary dynamics in patients with primary open angle glaucoma. Bull Osaka Med Coll. 1990;36(1–2):71–77. [PubMed] [Google Scholar]
- 28.Kalaboukhova L, Fridhammar V, Lindblom B. An objective method for measuring relative afferent pupillary defect in glaucomatous optic neuropathy-stimulus optimization. Neuro-Ophthalmology. 2006;30(1):7–15. [Google Scholar]
- 29.Horn FK, Bergua A, Junemann A, Korth M. Visual evoked potentials under luminance contrast and color contrast stimulation in glaucoma diagnosis. J Glaucoma. 2000;9(6):428–437. doi: 10.1097/00061198-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Korth M, Nguyen NX, Junemann A, Martus P, Jonas JB. VEP test of the blue-sensitive pathway in glaucoma. Invest Ophthalmol Vis Sci. 1994;35(5):2599–2610. [PubMed] [Google Scholar]
- 31.Johnson CA, Adams AJ, Casson EJ, Brandt JD. Blue-on-yellow perimetry can predict the development of glaucomatous visual field loss. Arch Ophthalmol. 1993;111(5):645–650. doi: 10.1001/archopht.1993.01090050079034. [DOI] [PubMed] [Google Scholar]
- 32.Sample PA, Weinreb RN. Color perimetry for assessment of primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1990;31(9):1869–1875. [PubMed] [Google Scholar]
- 33.Polo V, Larrosa JM, Pinilla I, Perez S, Gonzalvo F, Honrubia FM. Predictive value of short-wavelength automated perimetry: a 3-year follow-up study. Ophthalmology. 2002;109(4):761–765. doi: 10.1016/s0161-6420(01)01014-4. [DOI] [PubMed] [Google Scholar]
- 34.Sabeti F, Maddess T, Essex RW, James AC. Multifocal pupillographic assessment of age-related macular degeneration. Optom Vis Sci. 2011;88(12):1477–1485. doi: 10.1097/OPX.0b013e318235af61. [DOI] [PubMed] [Google Scholar]
- 35.Rosli Y, Bedford SM, James AC, Maddess T. Photopic and scotopic multifocal pupillographic responses in age-related macular degeneration. Vision Res. 2012;69:42–48. doi: 10.1016/j.visres.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Bell A, James AC, Kolic M, Essex RW, Maddess T. Dichoptic Multifocal Pupillography Reveals Afferent Visual Field Defects in Early Type 2 Diabetes. Invest Ophthalmol Vis Sci. 2010;51(1):602–608. doi: 10.1167/iovs.09-3659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.