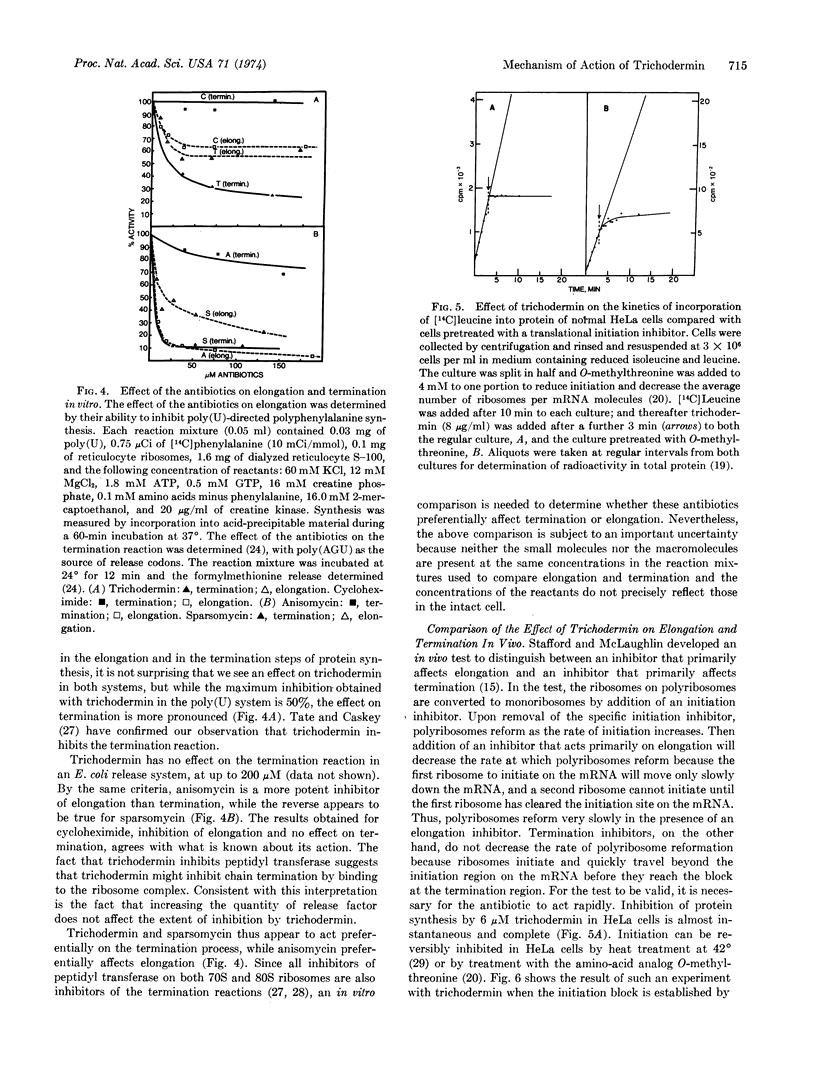
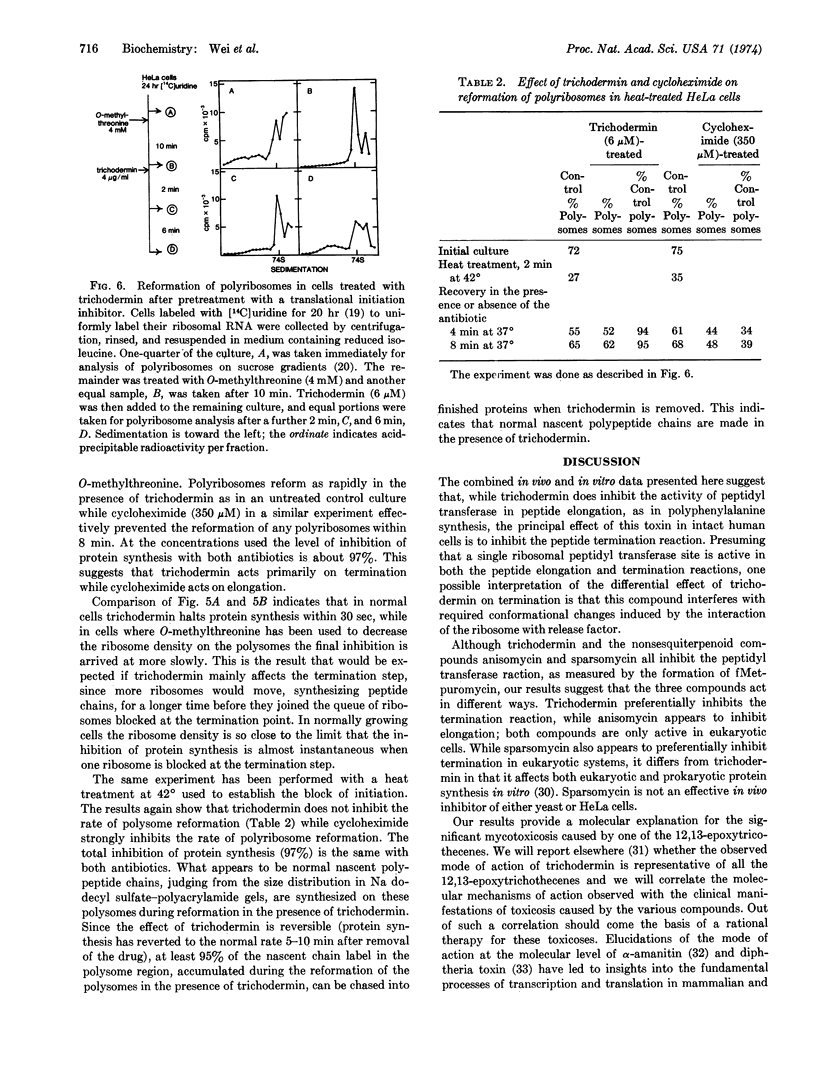
Abstract
Trichodermin is a member of a group of closely related compounds—the 12,13-epoxytrichothecenes—that form a medically and economically important class of mycotoxins produced by fungi that spoil fruit and grain. Our studies show that trichodermin is a very potent inhibitor of protein synthesis in mammalian cells. Since ribosomes remain in polyribosomes in inhibited cells, trichodermin inhibits the elongation and/or termination processes of protein synthesis. In vitro, trichodermin is a potent inhibitor of the peptidyl transferase activity required for elongation and/or termination. An in vitro comparison of the effects of three peptidyl transferase inhibitors on elongation and termination indicates that anisomycin acts primarily on elongation while trichodermin and sparsomycin act primarily on termination. A new in vivo test to distinguish elongation inhibitors from termination inhibitors confirms that trichodermin inhibits primarily the termination process. Thus trichodermin inhibits protein synthesis by blocking the activity of peptidyl transferase required for termination. These studies suggest that the toxicosis caused by one of the 12,13-epoxytrichothecenes is due to its action as a protein synthesis inhibitor involving the peptidyl transferase activity of the eukaryotic ribosomes.
Keywords: human cells, protein synthesis, release factor, peptidyl transferase, antitumor
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A. L., Caskey C. T. Mammalian peptide chain termination. II. Codon specificity and GTPase activity of release factor. Proc Natl Acad Sci U S A. 1971 Mar;68(3):619–624. doi: 10.1073/pnas.68.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L., Barbacid M., Vazquez D. The trichodermin group of antibiotics, inhibitors of peptide bond formation by eukaryotic ribosomes. Biochim Biophys Acta. 1973 Jun 23;312(2):368–376. doi: 10.1016/0005-2787(73)90381-x. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A. L., Scolnick E. M., Rosman M. Hydrolysis of fMet-tRNA by peptidyl transferase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3163–3167. doi: 10.1073/pnas.68.12.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Eppley R. M., Bailey W. J. 12,13-Epoxy-delta 9-trichothecenes as the probable mycotoxins responsible for stachybotryotoxicosis. Science. 1973 Aug 24;181(4101):758–760. doi: 10.1126/science.181.4101.758. [DOI] [PubMed] [Google Scholar]
- Glaz E. T., Csanyi E., Gyimesi J. Supplementary data on crotocin--an antifungal antibiotic. Nature. 1966 Nov 5;212(5062):617–618. doi: 10.1038/212617b0. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H., Stewart M. L., Ayuso M., Kappen L. S. On the mechanisms of inhibition of polypeptide synthesis by the antibiotics sparsomycin and pactamycin. Fed Proc. 1973 Jun;32(6):1688–1697. [PubMed] [Google Scholar]
- Kosuri N. R., Grove M. D., Yates S. G., Tallent W. H., Ellis J. J., Wolff I. A., Nichols R. E. Response of cattle to mycotoxins of Fusarium tricinctum isolated from corn and fescue. J Am Vet Med Assoc. 1970 Oct 1;157(7):938–940. [PubMed] [Google Scholar]
- Kosuri N. R., Smalley E. B., Nichols R. E. Toxicologic studies of Fusarium tricinctum (Corda) Snyder et Hansen from moldy corn. Am J Vet Res. 1971 Nov;32(11):1843–1850. [PubMed] [Google Scholar]
- LAMFROM H., KNOPF P. M. PROPERTIES OF RETICULOCYTE 80 S RIBOSOMES. J Mol Biol. 1965 Mar;11:589–599. doi: 10.1016/s0022-2836(65)80013-4. [DOI] [PubMed] [Google Scholar]
- Lamfrom H., McLaughlin C. S., Sarabhai A. Direction of reading the genetic message in reticulocytes. J Mol Biol. 1966 Dec 28;22(2):355–358. doi: 10.1016/0022-2836(66)90138-0. [DOI] [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- McCormick W., Penman S. Regulation of protein synthesis in HeLa cells: translation at elevated temperatures. J Mol Biol. 1969 Jan;39(2):315–333. doi: 10.1016/0022-2836(69)90320-9. [DOI] [PubMed] [Google Scholar]
- Menninger J. R. A simple assay for protein chain termination using natural peptidyl-tRNA. Biochim Biophys Acta. 1971 Jun 30;240(2):237–243. doi: 10.1016/0005-2787(71)90663-0. [DOI] [PubMed] [Google Scholar]
- Rüsch M. E., Stähelin H. Uber einige biologische Wirkungen des Cytostaticum Verrucarin A. Arzneimittelforschung. 1965 Aug;15(8):893–897. [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Stafford M. E., McLaughlin C. S. Trichodermin, a possible inhibitor of the termination process of protein synthesis. J Cell Physiol. 1973 Aug;82(1):121–128. doi: 10.1002/jcp.1040820114. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Hosoya M., Morita Y., Ueno I., Tatsuno T. Inhibition of the protein synthesis in rabbit reticulocyte by Nivalenol, a toxic principle isolated from Fusarium nivale-growing rice. J Biochem. 1968 Oct;64(4):479–485. doi: 10.1093/oxfordjournals.jbchem.a128919. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Ishikawa Y., Saito-Amakai K., Tsunoda H. Environmental factors influencing the production of fusarenon-X, a cytotoxic mycotoxin of Fusarium nivale Fn 2B. Chem Pharm Bull (Tokyo) 1970 Feb;18(2):304–312. doi: 10.1248/cpb.18.304. [DOI] [PubMed] [Google Scholar]
- Vaughan M. H., Jr, Pawlowski P. J., Forchhammer J. Regulation of protein synthesis initiation in HeLa cells deprived of single essential amino acids. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2057–2061. doi: 10.1073/pnas.68.9.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]



