Abstract
Carotid-femoral pulse wave velocity (PWV), a marker of arterial stiffness, is an established independent cardiovascular (CV) risk factor. Little information is available on the pattern and determinants of the longitudinal change in PWV with aging. Such information is crucial to elucidating mechanisms underlying arterial stiffness and the design of interventions to retard it. Between 1988 and 2013, we collected 2 to 9 serial measures of PWV in 354 men and 423 women of the Baltimore Longitudinal Study of Aging, who were 21 to 94 years of age and free of clinically significant CV disease. Rates of PWV increase accelerated with advancing age in men more than women, leading to gender differences in PWV after the age of 50. In both sexes, not only systolic blood pressure (SBP) ≥140mmHg, but also SBP of 120–139mmHg was associated with steeper rates of PWV increase compared to SBP<120mmHg. Furthermore, there was a dose-dependent effect SBP in men with marked acceleration in PWV rate of increase with age at SBP ≥140mmHg compared to SBP of 120–139mmHg. Except for waist circumference in women, no other traditional CV risk factors predicted longitudinal PWV increase. In conclusion, the steeper longitudinal increase of PWV in men than women led to gender difference that expanded with advancing age. Age and systolic blood pressure are the main longitudinal determinants of pulse wave velocity and the effect of systolic blood pressure on PWV trajectories exists even in the pre-hypertensive range.
Keywords: Arterial stiffness, blood pressure, aging
Introduction
Arterial stiffness is an age-related trait that has been long recognized as an important risk factor for cardiovascular disease. Carotid-femoral pulse wave velocity (PWV) is generally considered the gold standard measure of aortic stiffness in clinical practice as well as in research studies 1–3. PWV is a risk factor for the development of hypertension in normotensive populations4,5, and an independent risk factor for coronary artery disease and stroke in healthy subjects6. In addition, higher PWV is a significant predictor of mortality in the general community-dwelling population7,8, in hospitalized older subjects9, as well as in patients with hypertension10, and end-stage renal disease 11.
Despite its clinical significance12,13, essentially no information is available on (1) the pattern and rate of longitudinal changes in PWV within individuals of a general community-dwelling population of a broad age spectrum, and (2) how longitudinal PWV trajectories are affected by blood pressure and other cardiovascular risk factors. Cross-sectional and short-term longitudinal studies observed that age and blood pressure are major correlates of arterial stiffness14–19, with little difference between genders, although some studies have reported higher PWV in men 18,19.
The Baltimore Longitudinal Study of Aging (BLSA), a large prospective cohort study with repeated measures of PWV and risk factor assessment, provides an excellent opportunity to examine the pattern and rate of longitudinal change in PWV within and among individuals. We analyzed data from the BLSA aiming to (1) examine pattern and rate of longitudinal trajectories in PWV, and to determine whether these trajectories differ in men and women, and (2) examine whether blood pressure and other cardiovascular risk factors affect the longitudinal rates of change in PWV. Such information is not only crucial with respect to elucidating mechanisms that underlie arterial stiffening and predominately systolic hypertension that accompanies advancing age, but also to the design of interventions and clinical trials aimed at reducing or slowing arterial stiffness20.
METHODS
Study Sample
The BLSA (Baltimore Longitudinal Study of Aging) is a prospective study of community-dwelling volunteers who undergo approximately 2.5 to 3 days of medical, physiological, and psychological examinations at regular intervals21. Between 1988 and 2013, repeated PWV measurements and medical, physiological assessments were performed on a subset of 943 BLSA participants. Those selected for the present analysis were chosen on the basis of having repeated PWV and blood pressure measurements over time. Written, informed consent was obtained from all study participants. The BLSA has continuing approval from the Institutional Review Board (IRB) of the MedStar Research Institute, and the protocol for the present study is also approved by the IRB of the Johns Hopkins School of Medicine. The final sample consisted of 775 subjects and a total of 2400 repeated measures; a total of 168 subjects were excluded for evidence of clinical atherosclerosis and or aberrant PWV as described in supplemental methods. Distribution of participants and follow ups visit by age at entry is shown in Supplemental Table 1.
Carotid-femoral Pulse wave velocity
Carotid-femoral PWV was calculated as the distance traveled by the pulse wave divided by the time difference between the feet of carotid and femoral arterial waveforms gated to electrocardiogram. The distance traveled by the pulse wave was measured to the nearest centimeter with an external tape measure over the body surface. Since body surface measurements might result in overestimation of distance, and subsequently PWV, in those with larger body habitus, we performed a supplementary analysis using an alternative estimation of distance derived from body height at initial visit (distance=first height ×0.29)22. Over the long follow up period, the device used to record arterial waveforms changed. In the present analysis different devices had been used: 1. Transcutaneous Doppler probes (model 810A, 9 to 10- Mhz probes, Parks Medical Electronics, Inc., Aloha, Oregon) 2. Complior SP device (Artech Medical, Paris, France)2,23, and 3. SphygmoCor system (AtCor Medical, Sydney, Australia)24. Please refer to supplemental methods for a detailed description of the PWV measurement protocol and process of standardization across the three devices.
Blood Pressure
Oscillometric brachial blood pressure was measured at the time of PWV measurement. Hypertension was defined as blood pressure ≥140/90mm Hg or the use of antihypertensive medications (see supplemental methods). Subjects in each visit were categorized based on their systolic blood pressure into 3 categories: <120, 120–139, and ≥140mmHg based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure25,
Clinical variables
Height and weight were measured on all patients. Body mass index was determined as kg/m2. Waist circumference (WC) was measured as the minimal abdominal circumference between the lower edge of the rib cage and the iliac crests. Subjects were classified to ever vs. never a smoker. Use of medications was determined at each study visit. Diabetes mellitus was defined as meeting the 2011American Diabetes Association criteria26 or the use of diabetes medications. Hypercholesterolemia was defined as total serum cholesterol of 200 mg/dl or the use of lipid-lowering treatment.
Laboratory studies
Fasting plasma glucose, triglycerides (TG), total cholesterol, and high-density lipoprotein (HDL) cholesterol were measured. Low- density lipoprotein (LDL) cholesterol concentrations were estimated by using the Friedewald formula27. Please, refer to the supplemental methods for further details.
Statistical Analysis
Baseline variables for the men and women were reported and compared by Student’s t-test and chi-square test. Linear mixed-effect models (LME) were implemented to assess the rate and pattern of longitudinal change in PWV and the impact of SBP on these trajectories. The LME model easily accommodates unbalanced, unequally spaced observations and, consequently, is an ideal tool for analyzing longitudinal changes in data from an observational study such as the BLSA28; all models included date of first visit to adjust for cohort and period effects. Age was expressed as First-age (age at entry), and Time (follow up time), to differentiate cross-sectional differences from longitudinal changes over time. Gender difference was tested by running the model in the pooled data for men and women with gender, gender× First-age, and gender × Time interaction terms. We fitted models in different age subgroups to identify the age after which gender difference became significant. Models were then run on men and women, separately. Predicted PWV, using gender-age specific mean values for SBP and HR, and its rate of change per decade were plotted against age in men and women. Additional models tested for the cross-sectional and longitudinal association with other cardiovascular health-related covariates by including Time-covariate interaction terms. Significant interaction terms for variables affecting longitudinal rates of changes in PWV were included in the final model. LME regression with categories of SBP was performed to further assess the difference in the longitudinal change in PWV between SBP categories after applying backward elimination of statistically non-significant terms in the full model (Table 3). Quality of models was tested by the correlation between predicted and observed PWV. All analyses were done using SAS for Windows (Version 9·2, Cary, NC).
Table 3.
Linear mixed-effects Models examining cross-sectional and longitudinal association between PWV and traditional cardiovascular risk factors.
| Variables | Men (N=318) | Women (N=373) | ||
|---|---|---|---|---|
|
| ||||
| B | P | β | P | |
| First-age (10 years) | −0.16 | 0.7582* | −0.38 | 0.1892 |
| Time (10 years) | −1.55 | 0.2401* | −3.9 | <.0001 |
| First-age2 | 0.04 | 0.1792* | 0.06 | 0.0101 |
| First-age × Time | −0.41 | 0.3189* | 0.24 | 0.0030 |
| First-age2 × Time | 0.07 | 0.0445 | - | - |
| Heart Rate (10 beat per minute) | - | - | 0.19 | 0.0006 |
| Systolic blood pressure (10 mm Hg) | 0.09 | 0.0905 | 0.10 | 0.0262 |
| Systolic blood pressure × Time | 0.17 | 0.0040 | 0.13 | 0.0167 |
| Waist circumference (SD) | - | - | 0.02 | 0.7974* |
| Waist circumference × Time | - | - | 0.21 | 0.0175 |
| Race (non-white) | 0.32 | 0.0521 | 0.13 | 0.0601 |
| Glucose (SD) | - | - | 0.06 | 0.0278 |
| Triglycerides (SD) | 0.21 | 0.0155 | - | - |
Models were adjusted for variables shown, smoking, LDL, Triglycerides, HDL, glucose, Creatinine, anti-hypertensive medications, anti-hyperlipidemic medications, and initial date of visit; results were reported for terms with P<0.1. First-age and Time interaction with covariates were tested and only significant interaction terms were included in the final model.
P<0.05 for the main term before inclusion of interaction terms, please see explanation in the footnote of Table 2. Cor (observed, predicted): was 0.85 and 0.83 in men and women, respectively.
RESULTS
Men and women had similar age distribution and length of follow up (Table 1). Compared to women, men were taller, heavier, had higher BMI, larger WC, and were more likely to have ever smoked. Men also had lower HR and higher PWV, SBP, DBP, and MAP. There was no gender difference in use of anti-hypertensive medications among hypertensive subjects at baseline or by the end of follow up. Men had lower HDL cholesterol and higher TG and glucose. Anti-HLP and anti-DM medications use was similar in both genders (Table 1).
Table 1.
Baseline Characteristics of the Study Cohort by Gender
| Variables | Total (N=775) Mean ± SD |
Men (N=352) Mean ± SD |
Women (N=423) Mean ± SD |
P |
|---|---|---|---|---|
| Age (year) | 59.0 ± 15.7 | 59.9 ± 16.1 | 58.2 ± 15.3 | 0.1400 |
| Time of follow up (year) | 9.3 ± 6.0 | 9.3 ± 6.1 | 9.2 ± 5.9 | 0.6623 |
| Race (White) | 423 (57.2) | 237 (56.0) | 206 (58.5) | 0.4816 |
| Height, (cm) | 169.2 ± 9.5 | 176.5 ± 7.3 | 163.2 ± 6.3 | < .0001 |
| Weight (kg) | 75.3 ± 15.3 | 83.4 ± 12.8 | 68.5 ± 13.9 | < .0001 |
| BMI (kg/m2) | 26.2 ± 4.4 | 26.7 ± 3.8 | 25.7 ± 4.9 | 0.0008 |
| Waist circumference (WC) (cm) | 87.5 ± 12.2 | 94.4 ± 9.7 | 81.8± 11.0 | < .0001 |
| Smoking (ever) | 360 (46.5) | 195 (55.4) | 165 (39.0) | < .0001 |
| HR (bpm) | 67.0 ± 11.0 | 65.3 ± 11.5 | 68.8 ± 10.2 | <.0001 |
| SBP (mm Hg) | 125.7 ± 17.9 | 128.5 ± 17.3 | 123.4 ± 18.1 | < .0001 |
| DBP (mm Hg) | 72.7 ± 11.6 | 75.5 ± 11.3 | 70.4 ± 11.4 | < .0001 |
| MAP (mm Hg) | 90.4 ± 11.8 | 93.2 ± 11.5 | 88.0 ± 11.6 | < .0001 |
| Pulse pressure (mm Hg) | 53.0 ± 16.0 | 53.0 ± 15.1 | 53.0 ± 16.7 | 0.9650 |
| PWV (m/s) | 8.6 ± 1.9 | 8.8 ± 1.9 | 8.3 ± 1.9 | 0.0002 |
| Traditional cardiovascular risk factors | ||||
| Total Cholesterol (mg/dl) | 190.2 ± 38.3 | 184.5 ± 35.5 | 194.9 ± 40.0 | 0.0002 |
| LDL (mg/dl) | 113.9 ± 34.3 | 112.6 ± 31.9 | 115.0 ± 35.8 | 0.3426 |
| HDL (mg/dl) | 54.9± 16.8 | 48.9 ± 14.9 | 59.9 ± 16.7 | < .0001 |
| Triglycerides (mg/dl) | 104.6 ± 65.6 | 112.2 ± 75.3 | 98.4 ± 55.7 | 0.0036 |
| Glucose (mg/dl) | 98.6 ± 15.7 | 101.5 ± 18.5 | 96.3 ± 12.6 | < .0001 |
| Creatinine (mg/dl) | 0.98 ± 0.24 | 1.09 ± 0.22 | 0.89 ± 0.21 | <.0001 |
| Systolic BP categories | ||||
| SBP Category 1 (< 120 mmHg) | 308 (39.7) | 119 (33.8) | 189 (44.7) | 0.0023 |
| SBP Category 2 (120–139 mmHg) | 291 (37.6) | 132 (37.5) | 159 (37.5) | 0.9858 |
| SBP Category 3 (≥ 140 mmHg) | 176 (22.7) | 101 (28.7) | 75 (17.7)‡ | <.0001 |
| Hypertension (HTN), n (%) | 321 (41.4) | 170 (48.3) | 151 (35.7)† | 0.0004 |
| On HTN meds, baseline, n (%) | 234 (30.2) | 122 (34.7) | 112 (26.5) | 0.0135 |
| On HTN meds, followup, n (%) | 400 (51.6) | 203 (57.7) | 197 (46.6) | 0.0021 |
| Hyperlipidemia, n (%) | 428 (55.2) | 194 (55.1) | 234 (55.3) | 0.9104 |
| On hyperlipidemia meds, n (%) | 185 (23.9) | 88 (25.0) | 97 (22.9) | 0.5018 |
| Diabetes, n (%) | 74 (9.6) | 49 (13.9) | 25 (5.9) | 0.0345 |
| Diabetes on meds, n (%) | 28 (37.8) | 19 (38.8) | 9 (36.0) | 0.1356 |
BMI: Body mass index HR (bpm) Heart Rate (beat per minute). PWV: pulse wave velocity. SBP: systolic blood pressure. DBP: diastolic blood pressure. MAP: mean arterial pressure. LDL: low-density lipoprotein, HDL: high-density lipoprotein.
Adjusting for systolic blood pressure (SBP), heart rate (HR), anti-hypertensive medication use, and waist circumference we found no overall difference in PWV between men and women when the full age spectrum was considered (β=0.12, P=0.298). However, we found significant First-age×Gender (β=0.25, P<.0001) and Time×Gender (β=0.27, P=0.04) interactions (Supplemental Table S2) indicating that gender differences emerged with increasing age with men having steeper increase of PWV with age than women. To follow-up on this finding, we fitted age-stratified models and found that, indeed, men had significantly higher PWV than women only after age of 50 (β=0.305, P=0.045). To investigate whether gender differences are a result of falsely elevated PWV in men due to more prevalent central, android obesity, we repeated the analysis with PWV calculated using distance estimated as 29% of height at first visit (see methods), and found similar results (Supplemental Table S3)
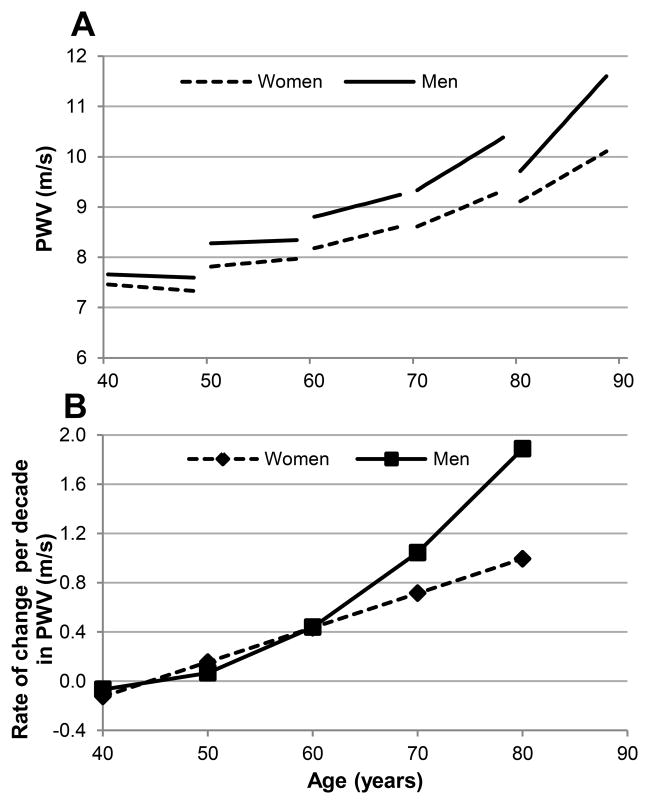
When we fitted separate models for men and women, we found that in both sexes PWV was higher with older age, and increased longitudinally within a given individual over time. The First-age2 and First-age×Time interaction terms were significant in men and women indicating an accelerating increase in PWV with aging. However, men had a more pronounced acceleration with aging evidenced by a significant First-age2×Time interaction (Table 2). As expected, SBP, HR, and waist circumference were significantly and independently associated with higher PWV in both men and women, whereas, anti-hypertensive medications were not. To illustrate gender differences in changes in PWV with aging, model-predicted PWV for age-gender specific mean values for SBP and HR were plotted against age for men and women, separately (Figure 1A). Note the accelerating rates of PWV increase per decade in men beyond the 5th decade (Figure 1B).
Table 2.
Linear mixed-effects Models examining the cross-sectional differences and longitudinal changes in PWV with age.
| Variables | Men (N=352) | Women (N=423) | ||
|---|---|---|---|---|
|
| ||||
| β | P | β | P | |
| First-age (10 years) | 0.55 | 0.1177* | −0.25 | 0.3145* |
| Time (10 years) | 1.88 | 0.0420 | −1.27 | 0.0012 |
| First-age2 | −0.01 | 0.8408* | 0.05 | 0.0214 |
| First-age × Time | −0.99 | 0.0074 | 0.29 | <.0001 |
| First-age2× Time | 0.13 | 0.0004 | - | - |
| Systolic arterial pressure (10 mm Hg) | 0.19 | <.0001 | 0.14 | <.0001 |
| Heart Rate (10 beat per minute) | 0.12 | 0.0352 | 0.21 | <.0001 |
| Waist circumference (SD) | 0.11 | 0.1107 | 0.22 | <.0001 |
| Anti-hypertension drugs | 0.03 | 0.8258 | 0.02 | 0.859 |
β. Unstandardized regression coefficient. Models were adjusted for initial date of visit.
P <0.05 for the main term before inclusion of interaction terms, note that due the significant interaction between First-age and Time, correlation coefficients for the main terms cannot be interpreted separately from the interaction term. Correlation between observed and model-predicted PWV was 0.77 and 0.80 in men and women, respectively.
Figure 1.
A. Predicted longitudinal changes in PWV in men and women, B. Predicted rate of change in PWV per decade in men and women.
We tested for the association of PWV with SBP and other traditional cardiovascular risk factors in additional models (Table 3). Higher SBP was associated with higher rate of PWV increase, as evidenced by a significant SBP×Time interaction in both men (β=0.17, p=0.004) and women (β=0.13, p=0.0167). Note that in men, there was a cross-sectional independent association between PWV and TG levels, while there was a cross-sectional association of HR, waist-circumference, and fasting glucose with PWV in women. Interestingly, in women higher WC was associated with a higher rate of PWV increase over time, evidenced by a significant WC×Time interaction term. There was a trend towards an association between non-white race and higher PWV that did not reach statistical significance. There was no direct association with smoking, or LDL, major traditional CV risk factors.
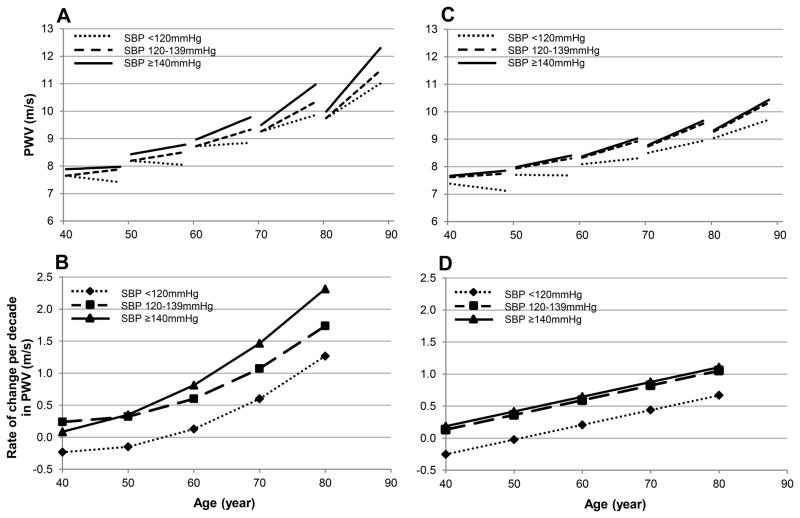
We further dissected into the association between SBP and PWV by stratifying SBP into three categories. Interestingly, in both men and women not only SBP≥140mmHg, but also SBP 120–139mmHg was associated with larger longitudinal increase in PWV compared to SBP<120mmHg in a dose dependent fashion (Table 4). Furthermore, in men with SBP≥140 mmHg, the magnitude of PWV increase over time accelerated with advancing age as evident by a significant First-age×Time×SBP-category-3 interaction term (Table 4). Model-predicted PWV and its rate of change per decade were plotted against age for the SBP categories in men and women (Figure 2A–B). Note in Figure 2B that among men, SBP 120–139mmHg was consistently associated with faster rates of PWV increase overtime (0.3–1.7m/s per decade) compared to that at SBP<120mmHg (−0.2– 1.3 m/s per decade). Note also in Figure 2B, among men SBP≥140mmHg is not only associated with higher rate of change over time than the other SBP categories, but that the rate of increase accelerated over time beyond the age of 50 illustrating the First-age×Time×SBP-category-3 triple interaction shown in Table 4. Similarly, in women there was a separation in the trajectories of PWV between the SBP sub-groups (Figure 2C). It is worth noting that the rates of PWV increase in women with SBP ≥ 140mmHg was similar to that of SBP 120–139mmHg and relatively more constant across the age spectrum compared to men.
Table 4.
Linear mixed-effects models examining the impact of systolic blood pressure categories on the longitudinal change of PWV
| Variables | Men (N=318)
|
Women (N=373)
|
||
|---|---|---|---|---|
| B | P | B | P | |
|
|
|
|||
| Systolic BP categories | ||||
| SBP Category 1 (< 120 mmHg) | 0 | 0 | 0 | 0 |
| SBP Category 2 (120–139 mmHg) | 0.08 | 0.6356* | 0.40 | 0.0082 |
| SBP Category 3 (≥ 140 mmHg) | 0.39 | 0.0454 | 0.53 | 0.0071 |
| Time × SBP Category 2† | 0.48 | 0.0105 | 0.36 | 0.0430 |
| Time × SBP Category 3† | −0.58 | 0.3805 | 0.44 | 0.0493 |
| First-age × Time × SBP Category 3† | 0.23 | 0.0455 | - | - |
Models are adjusted for terms in Table 3 with p <0.05. SBP: Systolic arterial pressure. B. Unstandardized regression coefficients.
P <0.05 for the main term before inclusion of interaction terms, correlation coefficients for the main terms can’t be interpreted separately from those for the interaction terms.
Coefficients for Time and First-age are calculated for 10-year increments. Cor (observed, predicted)was 0.85 and 0.83 in men and women, respectively
Figure 2.
A. Predicted longitudinal changes in PWV in men by SBP groups. B. Predicted rate of change per decade in PWV among men at different first ages by SBP groups. C. Predicted PWV against age in women by SBP groups. D. Predicted rate of change per decade in PWV among women at different first ages by SBP groups.
DISCUSSION
Principle findings
This is the first extended longitudinal study of PWV in the general population of men and women of a broad age spectrum and extensive clinical information available. We report rates of PWV increase per decade for men and women over a broad age range. Knowing the magnitude and patterns of PWV increase over time is crucial to better estimate the duration and sample size of clinical trials aimed at reducing or slowing arterial stiffening. One novel findings of this study is that men had steeper longitudinal increase in PWV with aging leading to higher PWV in men than women beyond the fifth decade. Another novel finding is that elevated SBP, even in the prehypertensive range, was associated with higher rates of PWV increase that persisted after adjusting for anti-hypertensive medications and other traditional CV risk factors.
Differential rate of change in PWV between men and women leads to gender differences
We observed gender differences in PWV at higher but not younger age resulting from accelerating rates of PWV increase with aging in men compared to slower rates of increase in women. This difference was independent of distance measurement errors due to gender differences in body habitus. Previous longitudinal studies did not report age-gender specific rates of change in PWV5,29,30, hence did not reveal such an observation. On the one hand cross-sectional studies, limited by their design, report controversial results regarding gender differences in PWV with only 15 out of 54 studies assessed in a systemic review showed higher PWV in men19. It is not clear why women did not experience acceleration in the rates of PWV increase in the higher decades, however this might be linked to a similar pattern of gender-differences in aortic remodeling31 with women having slower rates of aortic dilatation, another aging phenomenon that accompanies arterial stiffness.
Arterial stiffness and systolic blood pressure: A vicious cycle?
The relationship between arterial stiffness and blood pressure is complex. Based upon findings of this longitudinal analysis that higher SBP was associated with accelerated rate of PWV increase, and the results of previous reports of higher PWV predicting the longitudinal increase in SBP4,5, the association between blood pressure and arterial stiffness may be best described as “feed forward”, i.e. a vicious cycle. Our current findings of a dose-dependent association of SBP with higher rates of PWV increase in SBP≥140mmHg compared to 120–139mmHg and <120mmHg provide a rather strong indication of a potential role of higher SBP in accelerated arterial stiffness. Specifically in men, this dose dependence of SBP on PWV and age gives rise to a triple interaction among age, time, and SBP (Table 4 and Figure 2A, B). In other terms the impact of high systolic blood pressure (≥140 mmHg) on the rate of PWV increase over time in men increases with each successive decade. In contrast, in females, the rate of increase in PWV in SBP≥140mmHg did not accelerate with aging.
The findings of this study are in agreement with a previous 2-wave longitudinal study showing that PWV accelerates at a greater rate in uncontrolled hypertensive patients compared to controlled hypertensive patients and normotensive subjects29. However, the aforementioned study did not address the association between SBP and rate of PWV increase in the pre-hypertensive range. On the other hand, our findings differ from a recent report from Framingham study where antecedent SBP was not independently associated with PWV measurement at a future visit5. However, PWV was measured only 2 times, 7 years apart in the cited study. It is possible that the longer follow-up period and multiple repeated measures of PWV at shorter intervals of 2.5 years on average might have increased the power to detect the effect of SBP on the rate of PWV change with time.
Central obesity in women is associated with arterial stiffness
We observed an association between WC and higher rates of PWV increase in women with higher WC having an additive rather than synergistic effect with SBP. This association between PWV and waist circumference was attenuated, but remained significant when PWV was calculated using body height-derived distance (Supplemental Table S3). This indicates that overestimation of distance, and subsequently PWV, using body surface measurement explained a part, but not all of the association of WC with PWV. We had previously found that metabolic syndrome amplifies the age associated increase in arterial stiffness assessed other measurements such as carotid distensibility32. We have also found the cross-sectional association between adiposity and arterial stiffness is stronger in women than men33. A prior report has shown that weight increase is associated with an increase in PWV in healthy subjects30. These finding suggest a role for obesity and its metabolic derangements in the pathogenesis of arterial stiffness, and encourage close examination of this relationship, while introducing another benefit for weight loss beyond its already well-established and impressive benefits.
Dissociation of longitudinal changes in arterial stiffness from other CVD risk factors
Our longitudinal study results confirm the recently reviewed findings of prior cross-sectional studies 19 that show dissociation or at the maximum minimal association between arterial stiffness and traditional cardiovascular risk factors except for age and systolic blood pressure 19. We observed a trend toward higher PWV in non-white, mainly African-American, subjects that is consistent with previous literature34. This might provide an explanation of the higher incidence of hypertension in African-Americans; further studies with larger representation of minorities are needed to evaluate for racial differences in the longitudinal change in PWV. The association we observed with TG and glucose was cross-sectional only which make them less likely causative agents. These facts seem to negate a major role of atherosclerosis in arterial stiffness in this healthy population free of clinical atherosclerosis throughout the period of the study. The exclusion of any subject who developed clinical atherosclerosis at enrollment or during follow up in the recent study reduced the potential effect of plaque burden on the association between risk factors for atherosclerosis and arterial stiffness.
Our study has some limitation that should be considered while interpreting the results. First, the relatively small sample size specially when categorizing blood pressure subgroups might limit the utility of the absolute values of the rates of PWV change, however, the frequent observations and long follow up insured enough power to detect dose-dependent effect of SBP on PWV increase. Secondly, estimation of PWV using body-surface distance might introduce measurement errors and produce overestimation of PWV due to central obesity. However, adjusting all models to waist circumference should minimize this effect, in addition, using body-height derived distance measurements showed similar findings.
Perspectives
Age and systolic blood pressure are the main longitudinal determinants of pulse wave velocity and the effect of systolic blood pressure on PWV trajectories exists even in the pre-hypertensive range. Arterial stiffness and SBP interact in a vicious cycle; hence the improvement observed in PWV beyond blood pressure control in pharmacological clinical trials 35–37, might be interpreted as winding down of this vicious cycle. Our results emphasize the importance of both blood pressure control and the development of novel interventions at the level of central arteries to limit the progress of this detrimental vicious cycle.
Supplementary Material
Novelty and Significance.
1. What Is New?
This is the first report of rates and determinants of the age-related longitudinal change in PWV
Higher SBP was associated with larger longitudinal PWV increase
Except obesity in women, other cardiovascular risk factors did not alter the rates of PWV change
2. What Is Relevant?
Arterial stiffness is the major player in hypertension and interacts with it in a vicious cycle
SBP control and clinical trials targeting arterial stiffness are essential to halt this vicious cycle
3. Summary
Higher SBP is associated with a faster PWV increase in a dose-dependent fashion, even in the prehypertensive range.
Acknowledgments
FUNDING
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging (USA) and Medstar Research Institute.
Footnotes
DISCLOSURE
None
References
- 1.Lehmann ED, Parker JR, Hopkins KD, Taylor MG, Gosling RG. Validation and reproducibility of pressure-corrected aortic distensibility measurements using pulse-wave-velocity Doppler ultrasound. Journal of biomedical engineering. 1993;15:221–228. doi: 10.1016/0141-5425(93)90118-i. [DOI] [PubMed] [Google Scholar]
- 2.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac A-MM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 3.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network N. Expert consensus document on arterial stiffness: methodological issues and clinical applications. European Heart Journal. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 4.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. Journal of the American College of Cardiology. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA3: the journal of the American Medical Association. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattace-Raso FUS, Van der Cammen TJM, Hofman A, Van Popele NM, Bos ML, Schalekamp M, Asmar R, Reneman RS, Hoeks APG, Breteler MMB, Witteman JCM. Arterial stiffness and risk of coronary heart disease and stroke - The Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 7.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A, Hlth ABCS, Health ABCS. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 8.Hansen TW, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 9.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects > 70 years of age. Arteriosclerosis Thrombosis and Vascular Biology. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 10.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 11.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 12.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 13.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 14.Benetos A, Adamopoulos C, Bean J, Temmar M, Labat C, Bean K, Thomas E, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Gerontologist. 2002;42:129–130. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 15.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T, Sutton-Tyrrell K. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–192. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 17.McEniery CM, Spratt M, Munnery M, Yarnell J, Lowe GD, Rumley A, Gallacher J, Ben-Shlomo Y, Cockcroft JR, Wilkinson IB. An Analysis of Prospective Risk Factors for Aortic Stiffness in Men 20-Year Follow-Up From the Caerphilly Prospective Study. Hypertension. 2010;56:36–43. doi: 10.1161/HYPERTENSIONAHA.110.150896. [DOI] [PubMed] [Google Scholar]
- 18.Mattace-Raso FUS, Hofman A, Verwoert GC, Witteman JCM, Wilkinson I, Cockcroft J, McEniery C, Yasmin, Laurent S, Boutouyrie P, Bozec E, Hansen TW, Torp-Pedersen C, Ibsen H, Jeppesen J, Vermeersch SJ, Rietzschel E, De Buyzere M, Gillebert TC, Van Bortel L, Segers P, Vlachopoulos C, Aznaouridis C, Stefanadis C, Benetos A, Labat C, Lacolley P, Stehouwer CDA, Nijpels G, Dekker JM, Ferreira I, Twisk JWR, Czernichow S, Galan P, Hercberg S, Pannier B, Guerin A, London G, Cruickshank JK, Anderson SG, Paini A, Rosei EA, Muiesan ML, Salvetti M, Filipovsky J, Seidlerova J, Dolejsova M Reference Values Arterial S, Collaboration RV for ASC-P. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson IB, McEniery CM, Cockcroft JR. Arteriosclerosis and atherosclerosis: guilty by association. Hypertension. 2009;54:1213–1215. doi: 10.1161/HYPERTENSIONAHA.109.142612. [DOI] [PubMed] [Google Scholar]
- 21.Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. The Baltimore Longitudinal Study of Aging. Washington, DC: US Government Printing Office; Normal human aging. [Google Scholar]
- 22.Huybrechts SaM, Devos DG, Vermeersch SJ, Mahieu D, Achten E, De Backer TLM, Segers P, Van Bortel LM. Carotid to femoral pulse wave velocity: a comparison of real travelled aortic path lengths determined by MRI and superficial measurements. Journal of hypertension. 2011;29:1577–1582. doi: 10.1097/HJH.0b013e3283487841. [DOI] [PubMed] [Google Scholar]
- 23.Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci LC-P. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. American journal of hypertension. 2009;22:74–79. doi: 10.1038/ajh.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. Journal of hypertension. 1998;16(12 Pt 2):2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA3: the journal of the American Medical Association. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 26.Standards of medical care in diabetes--2011. Diabetes care. 2011;34 (Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 28.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64:215–222. doi: 10.1093/gerona/gln024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benetos a. Determinants of Accelerated Progression of Arterial Stiffness in Normotensive Subjects and in Treated Hypertensive Subjects Over a 6-Year Period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 30.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T, Sutton-Tyrrell K. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–192. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 31.Lam CSP, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, Mitchell GF, Benjamin EJ, Vasan RS C-2956587. Aortic root remodeling over the adult life course: longitudinal data from the Framingham Heart Study. Circulation. 2010;122:884–90. doi: 10.1161/CIRCULATIONAHA.110.937839. ST –Aortic root remodeling over the adult. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. Journal of the American College of Cardiology. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 33.Scuteri A, Orru M, Morrell CH, Tarasov K, Schlessinger D, Uda M, Lakatta EG. Associations of large artery structure and function with adiposity: effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis. 2012;221:189–197. doi: 10.1016/j.atherosclerosis.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heffernan KS, Jae SY, Wilund KR, Woods JA, Fernhall B. Racial differences in central blood pressure and vascular function in young men. American journal of physiology Heart and circulatory physiology. 2008;295:H2380–2387. doi: 10.1152/ajpheart.00902.2008. [DOI] [PubMed] [Google Scholar]
- 35.Kool MJ, Lustermans FA, Breed JG, Struyker Boudier HA, Hoeks AP, Reneman RS, Van Bortel LM. The influence of perindopril and the diuretic combination amiloride+hydrochlorothiazide on the vessel wall properties of large arteries in hypertensive patients. Journal of Hypertension. 1995;13:839–848. doi: 10.1097/00004872-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Ahimastos AA, Natoli AK, Lawler A, Blombery PA, Kingwell BA. Ramipril reduces large-artery stiffness in peripheral arterial disease and promotes elastogenic remodeling in cell culture. Hypertension. 2005;45:1194–1199. doi: 10.1161/01.HYP.0000168945.44069.aa. [DOI] [PubMed] [Google Scholar]
- 37.Lacourcière Y, Béliveau R, Conter HS, Burgess ED, Lepage S, Pesant Y, et al. Effects of perindopril on elastic and structural properties of large arteries in essential hypertension. The Canadian journal of cardiology. 2004;20:795–799. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.