Abstract
Background
Opioid substitution treatment (OST) can increase quality of life (WHOQOL-BREF) and reduce addiction severity index (ASI) scores among participants over time. OST program participants have noted that improvement in quality of life is one of the most important variables to their reduction in drug use. However, there is little systematic understanding of WHOQOL-BREF and ASI domain changes among OST participants in low and middle-income countries (LMIC).
Methods
Utilizing PRISMA guidelines we conducted a systematic literature search to identify OST program studies documenting changes in WHOQOL-BREF or ASI domains for participants in buprenorphine or methadone programs in LMIC. Standardized mean differences for baseline and follow-up domain scores were compared along with relationships between domain scores, OST dosage, and length of follow-up.
Results
There were 13 OST program studies with 1801 participants from seven countries eligible for inclusion in the review. Overall, statistically significant changes were noted in all four WHOQOL-BREF domain and four of the seven ASI domain scores (drug, psychological, legal, and family) documented in studies. Dosage of pharmacologic medication and length of follow-up did not affect changes in domain scores.
Conclusion
WHOQOL-BREF and ASI domain scoring is a useful tool in measuring overall quality of life and levels of addiction among OST participants. Coupled with measurements of blood-borne infection, drug use, relapse, and overdose, WHOQOL-BREF and ASI represent equally important tools for evaluating the effects of OST over time and should be further developed as integrated tools in the evaluation of participants in LMIC.
Keywords: Addiction Severity Index, Quality of Life (WHOQOL-BREF), opioid dependence, People who inject drugs, methadone, buprenorphine, opioid substitution treatment (OST), low and middle income countries
1. BACKGROUND
Opioid substitution treatment (OST) programs have been in place for decades, and recently, the number of OST programs has increased in low and middle-income countries (LMIC), especially in LMIC with large populations of opiate users. The majority of studies that have evaluated OST programs have focused on relapse, overdose, and drug use consumption as variables associated with positive changes among OST participants (McLellan, 2002). However, in recent years, there has been an increased interest in looking at changes in overall quality of life (WHOQOL-BREF, which include aspects of physical, social, psychological and environmental health; World Health Organization, 1993) and addiction severity (ASI, which includes aspects of substance use, physical and psychological health, legal status and employment status; McLellan et al., 1980) domain scores among drug users that are retained in OST programs over time. As opioid dependence is a complex chronic condition involving physical, psychological and social dimensions (McLellan et al., 2000), it is important to understand how these different dimensions are improved among individuals in treatment over time, and how they contribute to abstinence and lowered rates of drug use (World Health Organization, 2003).
Quality of life is defined as an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns. The concept is very broad, and includes variables related to one's physical health, psychological health, level of independence, social relationships and their relationships to salient features of their environment (World Health Organization, 1993). A questionnaire measuring these domains (physical, social, psychological and environmental) that is utilized in analysis of drug users in many countries is the World Health Organization Quality of Life assessment (WHOQOL-BREF) (World Health Organization, 1993).
The WHOQOL-BREF is useful in measuring participant quality of life beyond traditional indicators including morbidity and mortality (World Health Organization, 1996). The assessment covers measurements of the impact of disease on daily activities, perceived health measures, and disability/functional status measures (Bergner et al., 1981; Turner-Bowker et al., 2008; Wiklund, 1990). Several studies have reviewed WHOQOL-BREF domain scores among drug users participating in OST programs in high-income countries, documenting significant changes in all four domains over time among participants who remain in treatment (Ghodse et al., 2003; Giacomuzzi et al., 2005; Habrat et al., 2002). A randomized control trial utilizing OST treatment in Australia noted significant improvements from baseline to 3 months across physical, psychological and environmental WHOQOL-BREF domains (Bell et al., 2007). Similarly, a study from the US found significant improvements after 6 months across all quality of life domains (Tracy et al., 2012).
The Addiction Severity Index (ASI) is designed as a semi structured interview, and addresses seven potential problem drug areas including employment, drug use, alcohol use, legal status, family/social status and psychiatric status (Bultler et al., 2001; Leonhard et al., 2000; Moos et al., 2000; Rosen et al., 2000). Along with the WHOQOL-BREF, the ASI is a second, but equally useful tool used in evaluating opioid users retained in OST programs (Brown et al., 1993; Strain et al., 1996). Several studies focused in high-income countries have reported changes in ASI scores among OST participants, documenting reductions in several of the ASI domains over time (Brown et al., 1993; Kakko et al., 2003).
There are many variables that affect addiction severity and quality of life among opioid users. Studies have found that opioid users who are living with human immunodeficiency virus (HIV), hepatitis C (HCV), have psychiatric disorders, or have committed drug-related crimes, are more likely to have low scores on the WHOQOL-BREF questionnaire (Korthuis et al., 2011). Studies have also found that drug users have lower WHOQOL-BREF across all domains when compared to the general population, and the scores are comparable to WHOQOL-BREF among psychiatric patients (De Maeyer et al., 2010). Although there have been many studies that have used WHOQOL-BREF and ASI domain scores as outcomes among individuals in specific harm reduction programs throughout the world there is a lack of a systematic review that examines these outcomes among OST participants. This is especially true in LMIC, where the majority of OST programs have only been in operation for five to 10 years.
As the ASI and WHOQOL-BREF questionnaires have gained wider acceptance as chosen methods for evaluating OST participants, it is important to have a systematic understanding of how successful OST programs have been in increasing participant quality of life or reducing addiction severity. Diverse studies in high-income countries have noted significant improvements in quality of life domains among OST participants.
In this review, we will examine studies in LMIC that have used one of these two domain measurements (WHOQOL-BREF or ASI) among OST participants who are retained in OST programs over time. The goal is to establish if LMIC, many who have only recently implemented OST programs, have been able to improve ASI and WHOQOL-BREF scores in different domains among drug users in the same way established programs have in high income countries (De Maeyer et al., 2010).
2. METHODS
2.1 Search Strategies
The literature search conducted for this review utilized strict PRISMA guidelines (Liberati et al., 2009; Yin et al., 2011). Studies were selected from several sources including PubMed, EMBASE, NLM Gateway, and abstracts from International AIDS Society (IAS) 2000-2012, International Harm Reduction Association (IHRA) 2000-2012 conferences, and other harm reduction and public health conferences [including the American Public Health Association (APHA), the College on Problem Drug Dependence (CPDD) and the Harm Reduction Coalition (HRC)]. Systematic literature searches were conducted to identify potentially eligible articles from journals and conference presentations, utilizing search terms related to quality of life, pharmacologic substitution treatment (including methadone, buprenorphine), and opiate or other substance use/abuse. We also searched references from review articles regarding drug-using populations for any country designated as a LMIC. Figure 1 presents the search terminology used to locate potentially eligible studies.
Figure 1. Search String for Eligible Studies.
List of search terms used for conducting systematic literature reviews of relevant databases and conference abstracts
2.2 Study Selection and Eligibility Criteria
In order for a study to be included in the review, there had to be implementation or ongoing OST treatment in a sample of drug users, with longitudinal measurements (defined by at least 6 months of follow-up) documenting changes in either ASI or WHOQOL-BREF domain scores. We excluded any study that did not utilize buprenorphine or methadone, or studies that only included participants or individuals from prison or institutionalized locations due to the structured nature of these settings, and unique factors not present in outpatient settings could affect quality of life or the domains considered in the ASI. Locations were restricted to countries that fit the LMIC designation defined by the World Bank (World Bank, 2011); all high-income location studies were excluded (unless they included a separate analysis of a LMIC as well).
We included all samples of opioid drug users, not just samples of people who inject drugs. While there are many opioid users who choose to inject drugs, there are several geographic areas where other routes of administration, such as smoking or snorting, are prevalent (Gerstein et al., 1994; Goldman et al., 1973). Including all opioid using populations in OST programs was especially important among OST studies, as participants in the majority of studies were not asked directly about their preferred drug route of administration.
2.3 Data extraction and analysis
A standardized coding form was developed to document pertinent information for each study. Information collected included demographics of the drug using population, study design characteristics of the OST program including location and services offered, type of pharmacologic substance used in the program (methadone vs. buprenorphine), and information related to WHOQOL-BREF or ASI domain changes over time, including multivariate analysis, if conducted. Each study was assigned a reference ID number after completion of coding. Data was extracted from each coding form and entered into a database in order to assess changes in domain scores over time for each study. Attempts were made to contact all authors for studies in which dosage values for methadone or buprenorphine were not documented.
Standardized mean differences (SMD) were calculated for baseline and follow-up WHOQOL-BREF and ASI scoring domains using pooled domain values from studies to determine if statistically significant differences were documented among studies.
As domain scores may change more significantly for participants who spend more time in treatment, we performed a separate analysis to determine if there was any relationship between the number of months in treatment, and changes in overall WHOQOL-BREF or ASI domain scores. This analysis was based on comparisons between standard mean differences among studies collected with follow-up at 6 months vs. follow-up periods 12 months, 18 months, or 24 months (the longest follow-up period for any of the studies included).
As pharmacologic treatment (methadone vs. buprenorphine) differed in studies, we also compared SMD values for each type of treatment to determine if changes in overall WHOQOL-BREF or ASI domain scores were affected by the type of pharmacologic treatment offered in each OST program. We also compared dosage level of pharmacologic treatment, where available, to determine if dosage level had a direct effect on improvements in quality of life domain scores.
Standardized mean differences between baseline and follow-up domain scores were compared to establish if any of the changes documented were statistically significant. I2, a test that is traditionally used in meta-analysis and systematic reviews, was used to assess heterogeneity among OST program studies.
2.4 Assessment of Risk of Bias in Studies
A quality check was performed to document the strengths and weaknesses of each of the studies; items in the quality checklist included recruitment method, comparability in loss to follow-up and retained participants, assessment of follow-up period (including drop-out rate and appropriate time period between baseline and follow-up measurements), and controlling for potential confounders that could affect the association between OST treatments and the WHOQOL-BREF or ASI scores among OST participants (including use of other harm reduction services such as needle exchange programs, or antiretroviral therapy for people living with HIV). This checklist was modified from quality checklists for primary studies compiled by the Cochrane Collaboration of systematic reviews (Higgins and Green, 2011).
3. RESULTS
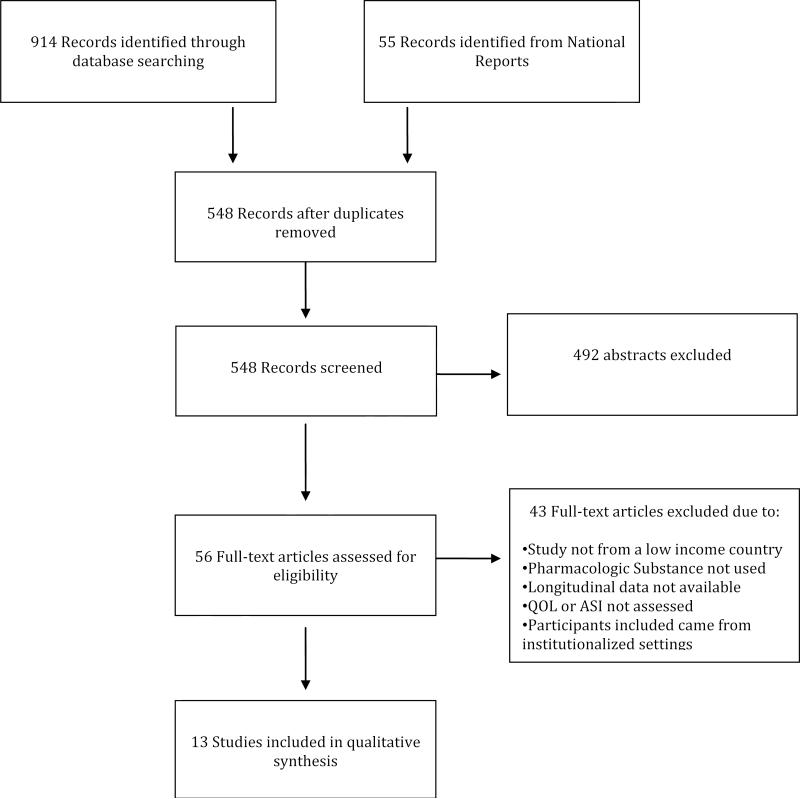
Figure 2 shows the PRISMA diagram for the search that led to the final number of studies included in this review. The search included all published studies from January 1 2000 through November 15 2012. Searching identified 1059 article titles. After removal of 511 duplicate papers, we hand screened 548 abstracts against the inclusion criteria (specified in the methods) and retrieved 56 full text articles for further screening. Abstracts were excluded for multiple reasons, including location (high income setting vs. low income settings, with high income settings excluded), lack of longitudinal ASI or WHOQOL-BREF domain scores, or lack of medicated assisted treatment with methadone or buprenorophine. After removal of obvious ineligible studies from review of abstracts, we reviewed the remaining 56 abstracts and obtained full-text articles for each of the abstract citations. Of the 56 full text articles and reports retrieved, 13 met all criteria for inclusion and were coded for our review, while 43 full text articles were ineligible. These 13 studies described 14 different OST samples from 5 different countries. The included studies contained a total of 1849 participants from China (OST implemented in 2004), Malaysia (OST implemented in 2005), Taiwan (OST implemented in 2005), Ukraine (OST implemented in 2005) and Vietnam (OST implemented in 2009).
Figure 2. PRISMA Diagram.
Flow chart of studies identified through systematic literature search reviewed for inclusion in the systematic review
The studies included in this review used a prospective cohort design, and were conducted at OST clinics located at stand-alone locations or within hospitals. Seven of the 13 studies included reported OST dosage. For methadone, dosages levels ranged from 52mg to 64.7mg, while for buprenorphine, dosage levels ranged from 5mg to 13mg. While 6 studies did not report methadone or buprenorphine dosages, the remaining 7 studies that that did report dosage fell within the guidelines of WHO dosage recommendations for OST participants (Uchtenhagen et al., 2007).
There were eight studies with nine samples that examined WHOQOL-BREF domains among OST participants, with follow-up periods ranging from 6 months to 24 months. Changes in domain scores occurred regardless of type of OST treatment used (buprenorphine vs. methadone) or dose of OST use in the program. All studies reported increases in psychological, social, and environmental quality of life, while seven of the eight studies reported increased quality of life for the physical domain (Table 1). One study from China reported a slight decrease in WHOQOL-BREF domain scoring for physical health; however, the change was not statistically significant, and the change in the domain score from baseline to follow-up was only 0.3.
Table 1.
Prospective cohort studies examining changes in WHOQOL-BREF among opioid users in OST programs in LMIC
| Study | Location | Study Years | Followup Months | OST Type (Dosage) | Study (n) | WHOQOL-BREF Psychological | WHOQOL-BREF Physical | WHOQOL-BREF Social | WHOQOL-BREF Environmental | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Followup | Baseline | Followup | Baseline | Followup | Baseline | Followup | ||||||
| Lua 2012 (Lua and Talib, 2012) | Terengganu, Malaysia | 2009-2010 | 12 | Methadone (64.7mg) | 75 | 11.97 | 14.30 | 12.69 | 14.58 | 12.74 | 13.79 | 11.96 | 13.93 |
| Musa 2011 (Musa et al., 2012) | Pahang, Malaysia | 2007-2009 | 24 | Methadone (N/A)** | 107 | 15.42 | 17.73 | 17.58 | 19.86 | 9.68 | 10.64 | 19.47 | 22.02 |
| Baharom 2012 (Baharom et al., 2012) | Negeri Sembilan, Malaysia | 2009-2010 | 6 | Methadone (N/A)** | 122 | 12.03 | 14.52 | 12.25 | 14.40 | 12.49 | 13.67 | 12.93 | 14.46 |
| Dvoriak 2008 (Dvoryak and Grishayeva) | Kherson and Kiev, Ukraine | 2004 | 6 | Buprenorphine (13.0mg) | 76 | 11.05 | 15.15 | 11.17 | 15.68 | 11.26 | 12.38 | 12.05 | 13.60 |
| Tran 2012 (Tran et al., 2012) | Hai Phong and Ho Chi Minh, Vietnam | 2009 | 9 | Methadone (N/A)** | 370 | 11.44 | 14.11 | 12.70 | 14.58 | 12.24 | 13.65 | 14.51 | 15.00 |
| Wang 2010 (Wang et al., 2010) | Hunan, China | 2007-2008 | 9 | Methadone (N/A)** | 50 | 11.55 | 13.67 | 13.22 | 15.24 | 11.55 | 13.64 | 11.13 | 13.98 |
| Wang 2010b (Wang et al., 2010) | Hunan, China | 2007-2008 | 9 | Methadone (N/A)** | 50 | 10.99 | 10.95 | 13.30 | 12.27 | 11.36 | 11.54 | 11.40 | 11.58 |
| Wang 2012 (Wang et al., 2012) | Southern Vietnam | 2007-2008 | 18 | Methadone (61.9mg) | 368 | 17.33 | 20.08 | 21.91 | 24.79 | 12.28 | 13.73 | 27.96 | 31.49 |
| Chen 2012 (Chen et al., 2012) | Multicenter, Taiwan | 2009 | 3 | Methadone (N/A)** | 127 | 11.21 | 12.52 | 12.37 | 13.63 | 12.01 | 12.63 | 12.41 | 13.19 |
N/A: not available (OST dosage not given in study)
There were four studies with five samples of OST participants that measured changes in ASI scores over time, with follow-up periods ranging from 6 months to 12 months. The ASI is scored with lower scores representing better functioning, so that decreases in ASI scores represent clinical improvement, and increases in ASI scores represent worse functioning among participants. With the exception of one study that reported a slight increase in ASI employment scores among OST participants in China (which was not statistically significant), all other domains among OST study samples remained low or decreased over the study periods (see Table 2).
Table 2.
Prospective cohort studies examining changes in ASI scores among opioid users in OST programs in LMIC
| Study | Location | Study Years | Followup Months | OST Type | Followup (n) | ASI Drug | ASI Alcohol | ASI Medical | ASI Psych | ASI Legal | ASI Employment | ASI Family | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Followup | Baseline | Followup | Baseline | Followup | Baseline | Follow-up | Baseline | Followup | Baseline | Followup | Baseline | Followup | ||||||
| Schaub 2009 (Schaub et al., 2009) | Kiev Ukraine | 2008-2009 | 12 | Buprenorphine (5mg) | 120 | 0.3 | 0.01 | 0.01 | 0 | 0.3 | 0.2 | 0.3 | 0.1 | 0.2 | 0.1 | 0.7 | 0.07 | 0.2 | 0.1 |
| Schaub 2010 (Schaub et al., 2010) | Kiev Ukraine | 2008 | 6 | Buprenorphine (5mg) | 162 | 0.2 | 0.1 | 0.1 | 0 | 0.3 | 0.3 | 0.3 | 0.1 | 0.1 | 0.01 | 0.8 | 0.7 | 0.2 | 0.2 |
| Schaub 2010 (Schaub et al., 2010) | Kiev Ukraine | 2008 | 6 | Methadone (60mg) | 119 | 0.2 | 0.1 | 0 | 0 | 0.2 | 0.01 | 0.1 | 0.1 | 0.2 | 0 | 0.8 | 0.7 | 0.2 | 0.1 |
| Hser 2011 (Hser et al., 2011) | Shanghai China | 2009-2010 | 6 | Methadone (N/A)** | 50 | 0.12 | 0.02 | 0.07 | 0.05 | 0.11 | 0.09 | 0.08 | 0.05 | 0.11 | 0.02 | 0.61 | 0.63 | 0.17 | 0.11 |
| Donoghoe 2009 (Donoghoe M., 2009) | Donetsk Ukraine | 2008 | 12 | Buprenorphine (10mg) | 53 | 0.37 | 0.08 | 0.09 | 0.02 | 0.28 | 0.15 | 0.21 | 0.08 | 0.13 | 0.02 | NS* | NS* | 0.19 | 0.1 |
NS: not scored
N/A: not available (OST dosage not given in study)
Among the studies included, seven reported dosage values for pharmacologic treatment; these studies were compared to determine if methadone or buprenorphine regimens, or dosages, affected changes in WHOQOL-BREF or ASI domain score over time. After examining the changes in domain scores for these seven studies there was no statistically significant difference among WHOQOL-BREF domains or ASI scores when examining type of OST treatment (buprenorphine vs. methadone) or the dose of OST use in the program. Additionally, studies with longer follow-up periods (six months vs. twelve months or longer) did not document statistically significant differences in ASI scores.
Standardized mean differences were calculated for each ASI and WHOQOL-BREF domain score (Table 3). There were statistically significant changes in WHOQOL-BREF scores for all domains (psychological, physical, social and environmental) (p<0.001). For ASI scoring domains, there were statistically significant differences in standardized mean scores for drug, psychological, legal and family domains (p<0.001). However, there was no statistically significant change in standardized mean differences for ASI alcohol, medical or employment domains over time.
Table 3.
Pooled Standardized Mean Differences in QOL and ASI Scoring
| Standardized Mean Difference (SMD) | 95% CI | p value | |
|---|---|---|---|
| WHOQOL-BREF Domain Scores | |||
| Psychological | 0.491 | 0.351, 0.631 | <0.0001 |
| Physical | 0.454 | 0.276. 0.631 | <0.0001 |
| Social | 0.292 | 0.164, 0.421 | <0.0001 |
| Environmental | 0.417 | 0.207, 0.626 | <0.0001 |
| ASI Domain Scores | |||
| Drug | −1.513 | −2.195, −0.831 | <0.0001 |
| Alcohol | −0.324 | −0.881, 0.233 | 0.254 |
| Medical | −0.212 | −0.531, 0.108 | 0.194 |
| Psychological | −0.513 | −0.968, 0.058 | 0.027 |
| Legal | −0.649 | −1.130, −0.167 | 0.008 |
| Employment | −0.177 | −0.368, 0.014 | 0.070 |
| Family | −0.434 | −0.729,−0.139 | 0.004 |
There was moderate heterogeneity among the studies included. For WHOQOL-BREF, I2 values for psychological, physical, social, and environmental domains were 63.7%, 77.9%, 57.8% and 84.5% respectively. For ASI, I2 values for drug, alcohol, medical, psychological, legal, employment and family domains were 95.3%, 94.3%, 83.2%, 91.5%, 92.3%, 80%, and 53.2% respectively. Overall, heterogeneity was greater among studies that measured ASI domains among OST participants.
3.1 Assessment of Risk of Bias
There was one study that did not use systematic sampling to recruit participants into the study (Donoghoe, 2009). There were six studies that did not assess differences in variables among participants who remained and those who dropped out of the respective OST program locations (Baharom et al., 2012; Chen et al., 2012; Donoghoe, 2009; Lua and Talib, 2012; Musa et al., 2012; Tran et al., 2012). There were also six studies that did not control for confounders in their analysis of follow up ASI or WHOQOL-BREF domain scores among participants (Donoghoe, 2009; Dvoryak and Grishayeva; Hser et al., 2011; Lua and Talib, 2012; Musa et al., 2012; Schaub et al., 2010). While there was clearly bias introduced into the studies that were included in this review, we did not note any systematic bias in the studies that would lead to removal from the analysis. As a result, all of these studies were included in the final measurements of ASI or WHOQOL-BREF domain scores.
4. DISCUSSION
Traditionally, OST program success has been measured by the number of patients retained in programs over time or by changes in drug use or risky drug use behaviors (Ferri et al., 2010; Hedrich et al., 2012; Mattick et al., 2008). While these measures are clearly important indicators of success, more recently there has been increased attention given to changes in quality of life and addiction severity domain scores among participants who remain in OST programs over time (De Maeyer et al., 2010; Deng et al., 2009). This increased attention may in part be attributed to the recognition of opioid dependence as a chronic, relapsing condition that may have negative outcomes across multiple life domains (De Maeyer et al., 2010). Emerging literature on quality of life and opioid dependence highlights the importance of these instruments to inform drug policy and to tailor drug treatment to individual needs (De Maeyer et al., 2010).
This study found statistically significant changes WHOQOL-BREF and several ASI scoring domains among OST participants in LMIC. In quality of life studies, statistically significant standardized mean differences were documented in all domains including physical, psychological, employment, and social interaction.
Among OST studies utilizing the addiction severity index, there were low levels scores maintained across all ASI domains including drug use, alcohol use, medical status, psychological status, legal status, family status, and employment. Additionally, there were statistically significant standardized mean differences noted for drug, psychological, legal, and family domains. We did not find statistically significant differences across ASI domains for alcohol, medical, and employment, although ASI scores for employment were close to reaching statistical significance (p=0.074). The authors did not give reasons as to why there were not statistically significant changes in these domains, although the authors did acknowledge that the short follow-up period may have contributed to the lack of significant change seen for the alcohol, medical, and employment domains. Additionally, as the scores at baseline for these domains were already low, it was unlikely that there was going to be a significant decrease seen from baseline to follow-up among participants in the OST programs; the low scores for these domains were maintained during the OST treatment period. And as the levels for these domains did not increase, the OST program was successful in maintaining low level domain scores over time for those with low baseline scores.
Previous studies among opioid users in OST treatment have found that positive changes in WHOQOL-BREF domains is associated with longer periods of drug abstinence (Apodaca and Longabaugh, 2009). As heroin use is associated with high relapse rates, it is important to ensure both high levels of retention of participants in programs, coupled with noticeable increases in quality of life, or decreases in addiction severity over time. Studies conducted on OST participants in the past have found that improved satisfaction with life in all domains was one of the most important reasons for initiating and continuing treatment in OST and other programs aimed at reducing drug use (Stark and Campbell, 1991).
The changes in domain scores seen here over time among OST participants are similar to those seen in studies evaluating WHOQOL-BREF and ASI domain scores in high income countries including Austria (Giacomuzzi et al., 2005), Germany (Karow et al., 2011), and Italy (Maremmani et al., 2007). The similarity in changes in domain scores is especially noteworthy as many LMIC with recently introduced OST programs have comparable WHOQOL-BREF and ASI outcomes to high-income countries, of which some have had methadone programs in place since the 1960s (Dole and Nyswander, 1965; Paulus and Halliday, 1967). Among the countries included, all but one implemented OST programs within the last ten years. Improvements in WHOQOL-BREF and ASI scores in LMIC highlight the effectiveness of OST to deliver comparable improvements in resource-poor settings as those in high income settings, despite wide economic disparities.
Although many of the OST programs reviewed here did not specify dosage levels for methadone or buprenorphine, in all studies, researchers noted that they adhered to recommended dosage of methadone or buprenorphine from the WHO (Uchtenhagen et al., 2007). Examination of studies reporting dosage did not show that higher doses of methadone were associated with higher levels of quality of life changes, though as noted earlier, the range of methadone dosages was quite limited. However, for addiction severity index scores, there was a slightly greater decrease in domain scores among participants in the high dose studies (10mg buprenorphine dosage) vs. studies with lower doses of buprenorphine (5mg). However, standardized mean differences in domain scores did not reach statistical significance.
Those studies comparing 6 months of follow-up to studies with greater than 6 months of follow-up showed greater increases in WHOQOL-BREF scores across all domains, but the standardized mean differences in domain scores did not reach statistical significance. However, even without a statistically significant change in scores, the positive changes seen are consistent with previous studies have noted the importance of continuous treatment with OST to ensure increases in life quality as participants spend longer periods of time in OST programs (Maremmani et al., 2007; Torrens et al., 1999). Studies have found that OST participation can strengthen motivation to remain drug free, resulting in increased positive treatment outcomes (Apodaca and Longabaugh, 2009). As many entrants into OST programs have severe psychological and physical impairments as a result of their heroin use and associated drug use behaviors (Ryan and White, 1996) the WHOQOL-BREF and ASI domain tools are particularly helpful, as they contain specific domains addressing these impairments.
Measurement of WHOQOL-BREF domains among OST participants is especially useful as many opioid users have a chronic dependence with drugs (Ferri et al., 2010; Saxon et al., 1996), and as a result, it is important to measure long term changes in well-being over time. This is particularly important for social and environmental domains, as opiate users may suffer from stigmatization or discrimination due to their drug dependence; this stigma is particularly prevalent among persons who inject drugs. For example, persons who inject drugs are often are confined to specific racial and low socioeconomic groups (Raymond and McFarland, 2009). These groups are often made up of large numbers of drug users, and as a result, the social ties can influence drug use and sexual risk behavior, increasing the likelihood of blood-borne virus transmission and risky behaviors (Crawford et al., 2013). As there has been a long established stigma associated with healthcare workers and drug users (McLaughlin and Long, 1996), many will not seek care for their co-morbidities at healthcare facilities (Solomon et al., 2008). As a result of these different factors, it is important to measure how particular domains, particular environmental and social domains, may be improved through participation in OST programs. The WHOQOL-BREF instrument is very helpful in tracking domain changes over long periods of time for those in OST, while offering support in reference to domain areas that do not see noticeable improvement over the course of their treatment.
4.1 Limitations
There are several limitations for this review that should be noted. In several of the studies, the dosage of methadone or buprenorphine was not given. While we attempted to contact authors to get dosage information, we were not able to acquire this information for several of the quality of life studies, especially those in China, Malaysia, and Vietnam. However, it was noted in all studies that the researchers adhered to the WHO guidelines for OST treatment, which specify a minimum dose of 40-60mg of methadone or 2mg of buprenorphine for treatment of opioid addiction (Uchtenhagen et al., 2007). The measurements that were used in this study were based on domain scores taken from self-report responses from participants; this could lead to social desirability bias. Finally, it is conceivable that other harm reduction services (such as needle syringe programs and antiretroviral therapy), ancillary services, treatment goals, age of participants and staff attitudes (Caplehorn et al., 1998; Saxon et al., 1996; Ward et al., 1994) could also have influenced quality of life or addiction severity domain scores, but these were not reported as part of services in the OST programs included here.
Other variables in the samples of drug users recruited in each study could have also influenced the results obtained here, including injecting behaviors of the participants, types of drug used, and other social determinants that would influence the domains reported here. However, as the authors of the primary studies did not include information on these variables, and did not integrate this information into the results reported, we were not able to speculate on how these variables may have influenced the results obtained in the review.
There was great heterogeneity in the pooled domain scores for ASI and WHOQOL-BREF domains among studies included in this review, and caution must be used in interpreting the standard mean differences for each domain. Mean medication dosage did not explain any of the heterogeneity. There are many additional variables, such as eligibility criteria, staff training, services offered in addition to medication, frequency of required clinic attendance, and program discharge policies that might explain some of the heterogeneity, but we were not able to examine these variables.
4.2 Conclusion
This review has documented changes in standardized mean differences in quality of life and addiction severity index domain scores among opioid users who remain in OST programs over time, with statistically significant changes seen in all of the WHOQOL-BREF domains and four domains in the ASI questionnaire. A thorough understanding of life quality is important in understanding the complexity of opioid dependence across multiple domains and tailoring treatment to address individual needs. Together with measurements of risk behavior, these tools allow researchers to acquire a better overall picture of the success of their programs and positive changes among their OST participants. The findings of this study also demonstrate that WHOQOL-BREF improvements in LMIC are comparable to high income countries, and resource-poor settings should not be viewed as a barrier or impasse for improving the quality of life of people who use opioids. The information from this review will be valuable for future OST program clinicians, especially in LMIC; with knowledge of these past programs and the documented successes among participants, future programs can be tailored to the particular drug using population, recognizing the importance of long term treatment and follow-up, in order to maximize changes in life quality over time for this chronic condition.
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIH Grant R01 AI 083035; NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
J. Feelemyer designed the study and wrote the protocol. J. Feelemyer managed the literature searches and summaries of previous related work and extracted pertinent information with quality checks performed by H. Hagan. K. Arasteh undertook the statistical analysis, and author J. Feelemyer wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors have no conflicts of interest with respect to the submitted manuscript
REFERENCES
- Apodaca TR, Longabaugh R. Mechanisms of change in motivational interviewing: a review and preliminary evaluation of the evidence. Addiction. 2009;104:705–715. doi: 10.1111/j.1360-0443.2009.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharom N, Hassan MR, Ali N, Shah SA. Improvement of quality of life following 6 months of methadone maintenance therapy in Malaysia. Subst. Abuse Treat. Policy. 2012;7:32. doi: 10.1186/1747-597X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, Shanahan M, Mutch C, Rea F, Ryan A, Batey R, Dunlop A, Winstock A. A randomized trial of effectiveness and cost-effectiveness of observed versus unobserved administration of buprenorphine-naloxone for heroin dependence. Addiction. 2007;102:1899–1907. doi: 10.1111/j.1360-0443.2007.01979.x. [DOI] [PubMed] [Google Scholar]
- Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med. Care. 1981:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- Brown LS, Jr, Alterman AI, Rutherford MJ, Cacciola JS, Zaballero AR. Addiction Severity Index scores of four racial/ethnic and gender groups of methadone maintenance patients. J. Subst. Abuse. 1993;5:269–279. doi: 10.1016/0899-3289(93)90068-m. [DOI] [PubMed] [Google Scholar]
- Bultler SF, Budman SH, Goldman RJ, Newman FJ, Beckley KE, Trottier D, Cacciola JS. Initial validation of a computer-administered Addiction Severity Index: the ASI-MV. Psychol. Addict. Behav. 2001;15:4. doi: 10.1037/0893-164x.15.1.4. [DOI] [PubMed] [Google Scholar]
- Caplehorn JR, Lumley TS, Irwig L. Staff attitudes and retention of patients in methadone maintenance programs. Drug Alcohol Depend. 1998;52:57–61. doi: 10.1016/s0376-8716(98)00047-7. [DOI] [PubMed] [Google Scholar]
- Chen CY, Ting SY, Tan HK, Yang MC. A multilevel analysis of regional and individual effects on methadone maintenance treatment in Taiwan. Value Health. 2012;15:S60–64. doi: 10.1016/j.jval.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Crawford ND, Ford C, Galea S, Latkin C, Jones KC, Fuller CM. The relationship between perceived discrimination and high-risk social ties among illicit drug users in New York City, 2006–2009. AIDS Behav. 2013;17:419–426. doi: 10.1007/s10461-012-0201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer J, Vanderplasschen W, Broekaert E. Quality of life among opiate-dependent individuals: a review of the literature. Intl. J. Drug Policy. 2010;21:364–380. doi: 10.1016/j.drugpo.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Deng CF, Ma X, Zhou H, Liu QL, Yang Y, Song Z, Wu F. Quality of life of heroin dependent patients with methadone maintenance therapy. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:539–543. [PubMed] [Google Scholar]
- Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction. A clinical trial with methadone hydrochloride. JAMA. 1965;193:646–650. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- Donoghoe M. Prove It: A Standardised Approach to Evaluating Opioid Substitution Treatment in Ukraine.. Proceedings of the 20th International Harm Reduction Conference.2009. [Google Scholar]
- Dvoryak S, Grishayeva I. First experience of opioid therapy with buprenorphine in Ukraine. Heroin Addiction and Related Clinical Problems. 13 [Google Scholar]
- Ferri M, Davoli M, Perucci CA. Heroin maintenance for chronic heroin-dependent individuals. Cochrane Database Syst. Rev. 2010:CD003410. doi: 10.1002/14651858.CD003410.pub3. [DOI] [PubMed] [Google Scholar]
- Gerstein D, Johnson R, Harwood H, Fountain D, Suter N, Malloy K, Center NOR, America U.S.o., Lewin-VHI I. Evaluating recovery services: the California Drug and Alcohol Treatment Assessment (CALDATA); General Report. National Opinion Research Center (NORC); 1994. [Google Scholar]
- Ghodse H, Clancy C, Oyefeso A, Rosinger C, Finkbeiner T, Schifano F, Copez C. The impact of methadone substitution therapy (MST) on illicit drug use and drug abuse-related quality of life: a european study. Heroin Addiction and Related Clinical Problems. 2003;5:5–16. [Google Scholar]
- Giacomuzzi SM, Ertl M, Kemmler G, Riemer Y, Vigl A. Sublingual buprenorphine and methadone maintenance treatment: a three-year follow-up of quality of life assessment. The Scientific World Journal. 2005;5:452–468. doi: 10.1100/tsw.2005.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman F, Berman J, Imboden J. A methadone maintenance program in a community general hospital: a first year's experience. MD State Med. J. 1973;22:48–51. [PubMed] [Google Scholar]
- Habrat B, Chmielewska K, Baran-Furga H, Keszycka B, Taracha E. Subjective quality of life in opiate-dependent patients before admission after six months and one-year participation in methadone program. Przegl Lek. 2002;59:351–354. [PubMed] [Google Scholar]
- Hedrich D, Alves P, Farrell M, Stover H, Moller L, Mayet S. The effectiveness of opioid maintenance treatment in prison settings: a systematic review. Addiction. 2012;107:501–517. doi: 10.1111/j.1360-0443.2011.03676.x. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0. John Wiley & Sons; 2011. [Google Scholar]
- Hser YI, Li J, Jiang H, Zhang R, Du J, Zhang C, Zhang B, Evans E, Wu F, Chang YJ, Peng C, Huang D, Stitzer ML, Roll J, Zhao M. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction. 2011;106:1801–1809. doi: 10.1111/j.1360-0443.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361:662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- Karow A, Verthein U, Pukrop R, Reimer J, Haasen C, Krausz M, Schafer I. Quality of life profiles and changes in the course of maintenance treatment among 1,015 patients with severe opioid dependence. Subst. Use Misuse. 2011;46:705–715. doi: 10.3109/10826084.2010.509854. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, Tozzi MJ, Nandi V, Fiellin DA, Weiss L, Egan JE, Botsko M, Acosta A, Gourevitch MN, Hersh D, Hsu J, Boverman J, Altice FL. Improved quality of life for opioid-dependent patients receiving buprenorphine treatment in HIV clinics. J. Acquir. Immune Defic. Syndr. 2011;56:S39–S45. doi: 10.1097/QAI.0b013e318209754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard C, Mulvey K, Gastfriend DR, Shwartz M. The Addiction Severity Index a field study of internal consistency and validity. J. Subst. Abuse Treat. 2000;18:129–135. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, GÃ,tzsche PC, Ioannidis JP, Clarke M, Devereaux P, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Int. Med. 2009;151:W–65-W-94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- Lua PL, Talib NS. A 12-month evaluation of health-related quality of life outcomes of methadone maintenance program in a rural Malaysian sample. Subst. Use Misuse. 2012;47:1100–1105. doi: 10.3109/10826084.2012.679840. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Pani PP, Pacini M, Perugi G. Substance use and quality of life over 12 months among buprenorphine maintenance-treated and methadone maintenance-treated heroin-addicted patients. J. Subst. Abuse Treat. 2007;33:91–98. doi: 10.1016/j.jsat.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McLaughlin D, Long A. An extended literature review of health professionals’ perceptions of illicit drugs and their clients who use them. J. Psychiatr. Ment. Health Nurs. 1996;3:283–288. doi: 10.1111/j.1365-2850.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction. 2002;97:249–252. doi: 10.1046/j.1360-0443.2002.00127.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J. Nerv. Ment. Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Moos RH, Finney JW, Federman EB, Suchinsky R. Specialty mental health care improves patients’ outcomes: findings from a nationwide program to monitor the quality of care for patients with substance use disorders. J. Stud. Alcohol Drugs. 2000;61:704. doi: 10.15288/jsa.2000.61.704. [DOI] [PubMed] [Google Scholar]
- Musa R, Abu Bakar AZ, Ali Khan U. Two-year outcomes of methadone maintenance therapy at a clinic in Malaysia. Asia Pac. J. Public Health. 2012;24:826–832. doi: 10.1177/1010539511404396. [DOI] [PubMed] [Google Scholar]
- Paulus I, Halliday R. Rehabilitation and the narcotic addict: results of a comparative methadone withdrawal program. CMAJ. 1967;96:655–659. [PMC free article] [PubMed] [Google Scholar]
- Raymond HF, McFarland W. Racial mixing and HIV risk among men who have sex with men. AIDS Behav. 2009;13:630–637. doi: 10.1007/s10461-009-9574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CS, Henson BR, Finney JW, Moos RH. Consistency of self-dministered and interview-based Addiction Severity Index composite scores. Addiction. 2000;95:419–425. doi: 10.1046/j.1360-0443.2000.95341912.x. [DOI] [PubMed] [Google Scholar]
- Ryan CF, White JM. Health status at entry to methadone maintenance treatment using the SF-36 health survey questionnaire. Addiction. 1996;91:39–45. doi: 10.1046/j.1360-0443.1996.911397.x. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Wells EA, Fleming C, Jackson TR, Calsyn DA. Pre-treatment characteristics, program philosophy and level of ancillary services as predictors of methadone maintenance treatment outcome. Addiction. 1996;91:1197–1209. doi: 10.1046/j.1360-0443.1996.918119711.x. [DOI] [PubMed] [Google Scholar]
- Schaub M, Chtenguelov V, Subata E, Weiler G, Uchtenhagen A. Feasibility of buprenorphine and methadone maintenance programmes among users of home made opioids in Ukraine. Intl. J. Drug Policy. 2010;21:229–233. doi: 10.1016/j.drugpo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Schaub M, Subata E, Chtenguelov V, Weiler G, Uchtenhagen A. Feasibility of buprenorphine maintenance therapy programs in the Ukraine: first promising treatment outcomes. Eur. Addict. Resource. 2009;15:157–162. doi: 10.1159/000217586. [DOI] [PubMed] [Google Scholar]
- Solomon S, Hawcroft C, Narasimhan P, Subbaraman R, Srikrishnan A, Cecelia A, Kumar MS, Solomon S, Gallant J, Celentano D. Comorbidities among HIV-infected injection drug users in Chennai, India. Ind. J. Med. Res. 2008;127:447. [PMC free article] [PubMed] [Google Scholar]
- Stark MJ, Campbell BK. A psychoeducational approach to methadone maintenance treatment: a survey of client reactions. J. Subst. Abuse Treat. 1991;8:125–131. doi: 10.1016/0740-5472(91)90003-s. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: self-reports, urinalysis, and Addiction Severity Index. J. Clin. Psychopharmacol. 1996;16:58–67. doi: 10.1097/00004714-199602000-00010. [DOI] [PubMed] [Google Scholar]
- Torrens M, Domingo-Salvany A, Alonso J, Castillo C, San L. Methadone and quality of life. Lancet. 1999;353:1101. doi: 10.1016/S0140-6736(05)76462-X. [DOI] [PubMed] [Google Scholar]
- Tracy EM, Laudet AB, Min MO, Kim H, Brown S, Jun MK, Singer L. Prospective patterns and correlates of quality of life among women in substance abuse treatment. Drug Alcohol Depend. 2012;124:242–249. doi: 10.1016/j.drugalcdep.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran BX, Ohinmaa A, Duong AT, Do NT, Nguyen LT, Nguyen QC, Mills S, Jacobs P, Houston S. Changes in drug use are associated with health-related quality of life improvements among methadone maintenance patients with HIV/AIDS. Qual. Life Res. 2012;21:613–623. doi: 10.1007/s11136-011-9963-y. [DOI] [PubMed] [Google Scholar]
- Turner-Bowker D, DeRosa M, Ware Jr JE. SF-36 health survey. 2008 [Google Scholar]
- Uchtenhagen A, Ladjevic T, Rehm J. WHO Guidelines for Pyschosocially Assisted Pharmacotherapy of Opioid Dependence. Geneva, Switzerland: 2007. WHO Guidelines for Psychosocially Assisted Pharmacological Teatment of Persons Dependent on Opioids. [Google Scholar]
- Wang H, Zhou J, Huang L, Li X, Fennie KP, Williams AB. Effects of nurse-delivered home visits combined with telephone calls on medication adherence and quality of life in HIV-infected heroin users in Hunan of China. J. Clin. Nurs. 2010;19:380–388. doi: 10.1111/j.1365-2702.2009.03048.x. [DOI] [PubMed] [Google Scholar]
- Wang PW, Wu HC, Yen CN, Yeh YC, Chung KS, Chang HC, Yen CF. Change in quality of life and its predictors in heroin users receiving methadone maintenance treatment in Taiwan: an 18-month follow-up dtudy. Am. J. Drug Alcohol Abuse. 2012;38:213–219. doi: 10.3109/00952990.2011.649222. [DOI] [PubMed] [Google Scholar]
- Ward J, Mattick RP, Hall W. The effectiveness of methadone maintenance treatment: an overview. Drug Alcohol Rev. 1994;13:327–336. doi: 10.1080/09595239400185431. [DOI] [PubMed] [Google Scholar]
- Wiklund I. The Nottingham Health Profile--a measure of health-related quality of life. Scand. J. Prim. Health Care Suppl. 1990;1:15. [PubMed] [Google Scholar]
- World Bank . World Bank Country and Lending Groups. World Bank; Washington, DC: 2011. Country and Lending Groups by Income. [Google Scholar]
- World Health Organization Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Qual. Life Res. 1993;2:153–159. [PubMed] [Google Scholar]
- World Health Organization . WHOQOL-BREF: Introduction, administration, scoring and generic version of the assessment. WHO; Geneva: 1996. [Google Scholar]
- World Health Organization . The WHO Collaborative Study on Substitution Therapy of Opioid Dependence and HIV/AIDS. World Health Organization; Geneva: 2003. [Google Scholar]
- Yin W, Hao Y, Sun X, Gong X, Li F, Li J, Rou K, Sullivan SG, Wang C, Cao X. Scaling up the national methadone maintenance treatment program in China: achievements and challenges. Intl. J. Epidemiol. 2011;39:ii29–ii37. doi: 10.1093/ije/dyq210. [DOI] [PMC free article] [PubMed] [Google Scholar]