Abstract
Biological invasion is increasingly recognized as one of the greatest threats to biodiversity. Using ensemble forecasts from species distribution models to project future suitable areas of the “100 of the world’s worst invasive species” defined by the IUCN, we show that both climate and land use changes will likely cause drastic species range shifts. Looking at potential spatial aggregation of invasive species, we identify three future hotspots of invasion in Europe, northeastern North America, and Oceania. We also emphasize that some regions could lose a significant number of invasive alien species, creating opportunities for ecosystem restoration. From the list of 100, scenarios of potential range distributions show a consistent shrinking for invasive amphibians and birds, while for aquatic and terrestrial invertebrates distributions are projected to substantially increase in most cases. Given the harmful impacts these invasive species currently have on ecosystems, these species will likely dramatically influence the future of biodiversity.
Keywords: invasive species, climate change, land use change, species distribution models
INTRODUCTION
Climate changes, including extreme climatic events (i.e., flood, fires), can enhance invasion processes, from initial introduction through establishment and spread (Walther et al., 2009; Diez et al., 2012), and consequently have a profound influence on the environment. In addition, human activities such as transcontinental transportation, land degradation and agricultural systems, lead to the spread of many non-indigenous species (Foley et al., 2005). Thus, the concurrent effects of climate and land use changes can further increase the already dramatic rates of biological invasions. For example, in Europe the number of invasive alien species increased by 76% in the last 30 years (Millennium Ecosystem Assessment et al., 2005). Invasive alien species are likely causing an array of ecological, economic and health impacts for invaded countries (Simberloff et al., 2012), that may become visible only long after introduction (Essl et al., 2011). However, our limited knowledge of the impacts of climate and land use changes on biological invasions hinders our ability to measure, predict, and mitigate the growing effects of these two factors on invasive alien species. Therefore, the question of how the interplay between climate and land use change will influence the global process of invasions is thus becoming of prime relevance for natural resource management. Moreover, anticipating future distributions of invasive alien species is essential to facilitate pre-emptive and effective management actions such as prevention of introductions and opportunities for eradication. Risk maps summarizing land suitability for invaders can be useful tools for anticipating species’ invasions and controlling their spread (Jiménez-Valverde et al., 2011). Identification of areas where policies could benefit from synergies between climate, land use change and invasive species management is also of prime relevance.
Attempts to predict future ecologically suitable areas for the establishment of invasive alien species have been made on single species (Ficetola et al., 2007; O’Donnell et al., 2012; Larson & Olden 2012). While many authors have warned about the potential synergistic feedbacks between climate and land use changes on species distributions (Brook et al., 2008; Hellmann et al., 2008; Walther et al., 2009), few studies have examined these interactions explicitly (Jetz et al., 2007; Butchart et al., 2012; Murray et al., 2012; Leroy et al., 2013) and none have done so specifically for any invasive alien species. To address this gap, we report here a comprehensive evaluation of the dual effects of climate and land use changes on the “100 of the world’s worst invasive species” from the International Union for the Conservation of Nature list of the Invasive Species Specialist Group. This list regroups species with some of the largest impacts on biodiversity and/or human activity and represents a range of ecological strategies over 10 different taxonomic groups.
In this study, we used ensemble forecast projections extracted from multiple Species Distribution Models (SDMs), several global climate models, and land cover change scenarios to predict future suitability for each of the 100 invasive alien species. Second, using these ensemble projections, we mapped the potential level of invasion at different time-slices (current, 2050 and 2100). Third, using projected species range changes for the different taxonomic groups of the 100 invasive alien species, we assessed the future vulnerability of various biome types to these invasive alien species.
Materials and Methods
Data
Climate data
To characterize present-day climate, we used climatic data (averaged from 1950-2000) from the Worldclim database (Hijmans et al., 2005) at a 0.5 degree resolution. We selected six climatic variables: (1) mean diurnal range, (2) maximum temperature of warmest month, (3) minimum temperature of coldest month, (4) annual precipitation, (5) precipitation of wettest month, and (6) precipitation of driest month (Table S1 for references details)). These variables provide a combination of means, extremes and seasonality that are known to influence species distribution (Root et al., 2003) and we selected only variables that were not collinear (pair-wise rPearson<0.75). In the case of freshwater species, many studies have revealed strong correlations between spatial patterns and climatic variables (Jocque et al., 2010), mostly temperature and the availability of water, and have used species distributions models to successfully predict the distribution of fishes (McNyset 2005) and mussels (Drake & Bossenbroek 2004). Future climate data were extracted from the Global Climate Model data portal (http://www.ccafs-climate.org/spatial_downscaling/). These models were statistically downscaled from the original Global Change Model outputs with the Delta method (Ramirez-Villegas & Jarvis 2010). Due to large effects of different Atmosphere Ocean Global Circulation Models (AOGCMs) on species range projections, simulations of future climate were based on three different AOGCMs (HADCM3, CSIRO2 and CGCM2) averaged from 2040 to 2069 (“2050”) and 2070 to 2099 (“2080”). We used two different scenarios (A1B, B2A) that reflect different assumptions about demographic, socio-economic and technological development on greenhouse gas emission (Solomon et al., 2007). A1B scenario represents a maximum energy requirement, emissions balanced across fossil and non-fossil sources and B2A represents a lower energy requirement and thus lower emissions scenario than A1B (Solomon et al., 2007).
Land use data
Current and future global land use and land cover variables were simulated by the Globio3 land model at a 0.5 degree resolution for two different emission scenarios A1B and B2A (Millennium Ecosystem Assessment et al., 2005; Alkemade et al., 2009). These data together with climate were used to model the potential distribution of the species in the “100 world’s worst invasive species” list (see below). For the two selected emission scenarios, we re-classified the 30 different land cover types from the Globio3 data (Bartholomé & Belward 2005) into 12 land cover type variables by grouping some of them together. These land use variables consisted of the proportion of the grid cell covered by (1) tree cover, (2) tree cover regularly flooded, (3) mosaic habitat, (4) tree cover burnt, (5) shrub cover, (6) herbaceous cover, (7) cultivated and managed areas, (8) bare areas, (9) water bodies, (10) snow and ice, (11) artificial surfaces and associated areas and (12) pasture. We calculated for each pixel the proportion of each land cover type in 1970-2000 (“current”), “2050” and “2080”.
Biome data
We used 14 different type of biomes: (1) boreal forest, (2) cool coniferous forest, (3) grassland and steppe, (4) hot desert, (5) ice, (6) savanna, (7) scrubland, (8) temperate deciduous forest, (9) temperate mixed forest, (10) tropical forest, (11) tropical woodland, (12) tundra, (13) warm mixed forest, and (14) wooded tundra, that we extracted from the IMAGE 2.4 model for each 0.5 degree cell (Prentice et al., 1992; Leemans & Born 1994). The biome data were used to compare the future potential number of invasive alien species per biome.
Invasive alien species data
We collected current distribution data for the species on the list “100 of the world’s worst invasive alien species”, compiled by the International Union for the Conservation of Nature (IUCN, Lowe et al., 2000). Developed in 2000 by the ISSG global network of over 1,000 invasion biology experts, this synthesis included input from the wider community of practitioners and scientists with expertise on all taxonomic groups and environments. The list provides the most geographically and taxonomically representative set of the most dangerous invasive alien species around the world, causing significant impacts on biodiversity and/or human activity in all ecosystems.
We chose these 100 species in an attempt to objectively explore patterns of change (i.e., range expansion versus retractions) and geographic patterns (i.e., into some regions and biomes but not others) for different taxonomic groups. They share the following specifications: (1) large impact on biodiversity and/or human activity; (2) threaten a variety of taxonomic groups, ecosystems, types of impacts; (3) illustration of important issues surrounding biological invasions. This list includes three micro-organisms, five macro-fungi, four aquatic plants, 30 terrestrial plants, nine aquatic invertebrates, 17 terrestrial invertebrates, three amphibians, eight fishes, three birds, two reptiles and 14 mammals (Lowe et al., 2000)). Rinderpest virus was removed from the list of the 100 because it is now eradicated. We made an extensive search for records from both the native and invaded ranges as recommended by Gallien et al., (2010) for all the 99 species. Data were collected from a variety of online databases, references and personal communications (Table S2 for details).
We collected on average 3,850 records per species with a minimum of 46 records for the least documented species. We found records of the 99 species from all over the world (except in Sahara region, northeastern Russia, northern Canada and Greenland).
Species Distribution Model projections
Modeling process
We modeled the potential distribution of the 99 invasive species by combining available occurrences with a set of six climatic variables and twelve land use variables that we assumed to be important for invasive species. Analysis of the climate and land use preferences of a species can therefore be used to predict areas where the species could occur at global scales. Although other factors such as soil properties or micro-climate also determine the presence or absence of a species at the local scale, we assumed that climate and land use were the most important explanatory variables of species distribution at the global scale. We used six different SDMs, within the biomod v.2.0 platform (Thuiller et al., 2009) carried out on the R platform. These models were: Generalized Linear Model, Generalized Boosting Trees, Multivariate Adaptive Regression Splines, Random Forest, Flexible Discriminant Analysis and Maximum Entropy. More details about the first five modeling techniques can be found in Thuiller et al., (2009) and in Elith et al., (2011) for Maximum Entropy. All models required presence and pseudo-absences (PAs or background). Five sets of PAs were generated by selecting from 1000 to 10,000 random points across the globe, according to the number of presences N (if N≤1000 then 1000 PAs were selected, else 10,000 PAs were selected) (as recommended by Barbet-Massin et al., (2012)). Equal weightings were given to presences and PAs.
Evaluating model performance
We evaluated the predictive performance of each model using a repeated split sampling approach in which models were calibrated over 70% of the data and evaluated over the remaining 30%. This procedure was repeated four times. We used two different statistical metrics: the True Skill Statistics (TSS) (Allouche et al., 2006) and Area Under the ROC Curve (AUC) (Fielding & Bell 1997). The TSS accounts for both omission and commission errors, and ranges from −1 to +1, where +1 indicates perfect agreement and 0 represents a random fit (Allouche et al., 2006). AUC values range from 0 to 1, and according to Swets (1988), an AUC above 0.8 is considered to have “good” discrimination abilities. All calibrated models performed very well with an average AUC value of 0.983 ± 0.002 and an average TSS value of 0.911 ± 0.007 (Fig. S1). We also used the Boyce index to assess model performance (Boyce et al., 2002; Petitpierre et al., 2012). The Boyce index only requires presence data, where AUC and TSS require both presence and absence data. Boyce index measures how much model predictions differ from random distribution of the observed presences across the prediction gradients. Values of Boyce index vary between −1 and +1. Positive values indicate a model with present predictions that are consistent with the distribution of presences in the evaluation dataset; values close to zero mean that the model is not different from a random model. We calculated the Boyce index for each of the 60 models (GLM, MARS and MaXent) per species. On average, MARS (0.819 +/− 0.322) and MaXent (0.817 +/− 0.225) gave a very good evaluation measure, whereas GLM (0.367 +/− 0.286) was less robust, but still gave fair predictions based on the Boyce index. This makes the interpretation of our results consistent over all species.
Ensemble modeling approach
The final calibration of every model for generating invasion scenarios used 100% of available data. We used an ensemble forecast approach to account for the variability among the six species distribution models and the three general circulation models to get the central tendency (Araújo & New 2007). To make sure no spurious models were used in the ensemble projections, we only kept the projections for which the model’s evaluation estimated by AUC and TSS were higher than 0.8 and 0.6, respectively (e.g. Gallien et al., 2012), and a weight proportional to their TSS evaluation was associated with each model. Because of the potential problems raised by Lobo et al., (2008) on the use of AUC as a measure of model performance, we decided to use TSS for the final consensus distributions. The final current and future consensus distributions were obtained by calculating the weighted mean of the distribution for each scenario (Marmion et al., 2009). This resulted in one current probability distribution map and three future probability distribution maps (because we used three global circulation models) for each emission scenario (A1B and B2A) and each species. Future probability maps were therefore averaged for each scenario. Then, we transformed the probability maps obtained from the ensemble projections into binary suitable/non-suitable maps using the threshold maximising the True Skill Statistics, as proposed by Allouche et al., (2006). This was done to ensure the most accurate predictions since it is based on both sensitivity and specificity (Jiménez-Valverde & Lobo 2007). We obtained one current binary distribution map and three future binary distribution maps per emission scenario and per species. Consensus binary maps were obtained attributing presence when the majority of GCMs (i.e., two of three) predicted presence, otherwise predicting absence. Since projections were not based on an equal-area projection system, cell sizes decrease pole-ward. Therefore, we calculated the area of each cell and weighted the cells of the world map by their area.
Results
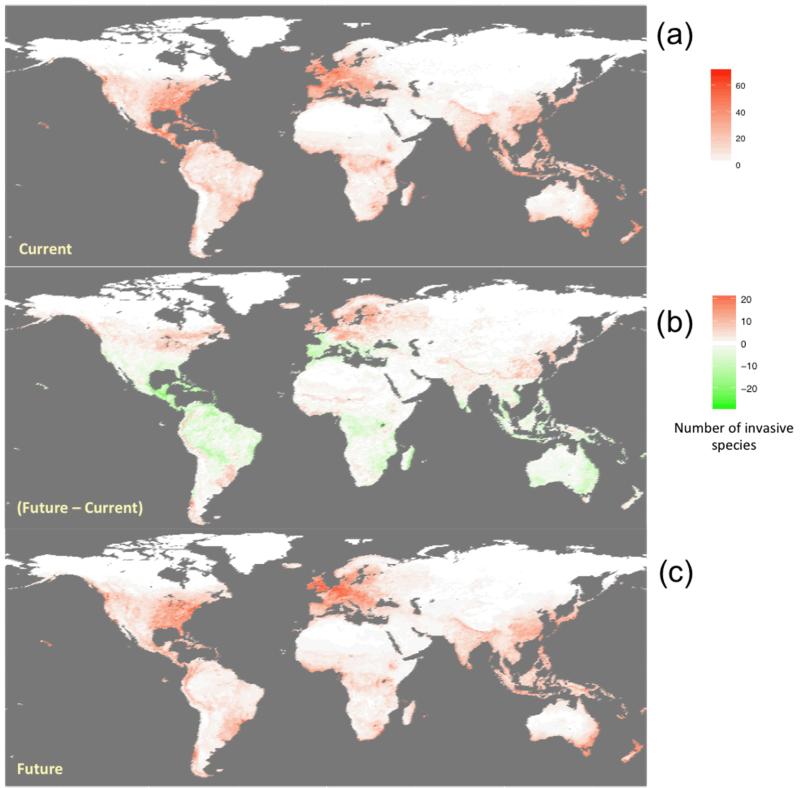
Future hotspots of invasion (i.e., >60 invasive alien species) were projected to mostly occur in eastern United States, northeastern Europe, southwest Australia and New Zealand. Indonesian and Pacific islands region, central Africa, and southern Brazil could be affected at a lower rate (i.e., 20 to 40 invasive alien species), regardless of the time-slice or climate scenario used (Fig.1A). Changes of future number of invasive alien species showed important geographic variation, allowing for the detection of areas more vulnerable to invasions and those that might actually lose invasive alien species. Under all scenarios, an increase in the number of invasive alien species was projected for northwestern Europe and northeastern United States, India and eastern China (Fig.1B). On the contrary, a decrease in the number of invasive alien species was projected for Central and South America, southwestern Europe, central Africa, and Indonesian and Pacific islands regions, eastern Australia. Globally, most areas projected to increase in the number of invasive alien species are located in continental and temperate climatic zones, especially in the northern hemisphere, while the potential number of invasive alien species largely decreased in the tropical regions at low latitudes. Climate and land use changes could create opportunities for many temperate species to spread at higher latitudes but could also lead to a significant lost of tropical invasive alien species. For example, several invasive alien species were projected to expand their ranges into more temperate regions in northern Europe (Fig.1B). Globally, the number of invasive alien species susceptible to invade new regions in the future tended both to be higher in the northern hemisphere compared to the southern, and to decrease at lower latitudes (Fig.1A).
Fig.1. Global distribution of invasive species under current and future scenarios.
(a) Projected richness in invasive species by 2000; (b) Relative change in invasive species richness between 2000 and 2100 and (c) Projected richness in invasive species by 2100.
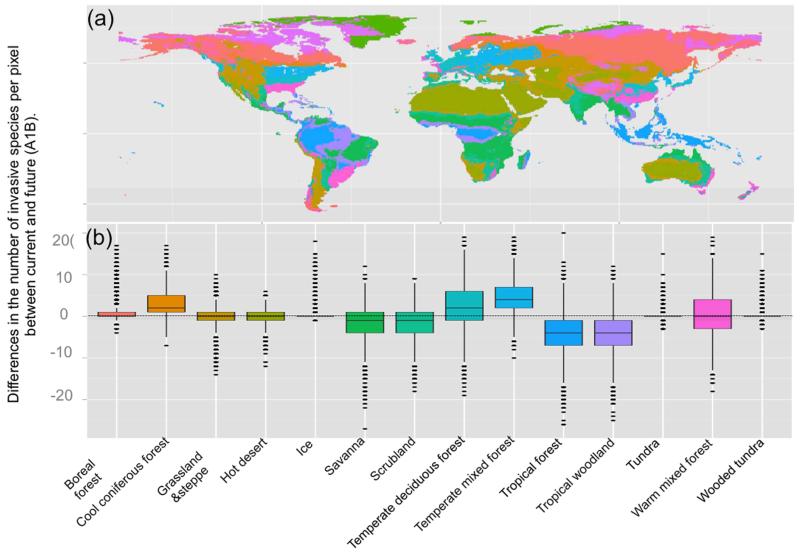
More precisely, our analyses revealed that biomes harboring extreme climatic conditions such as ice, hot desert, tundra and wooded tundra were not predicted to be suitable for invasive alien species by 2100 (Fig.2 and Table S3). Other biomes tended to have suitable environmental conditions for a high number of invasive alien species in the future, especially temperate deciduous forests (27, average number of invasive alien species per pixel), warm mixed forest (22), temperate mixed forest (16) and tropical forest and woodland (12) (Table S3). The highest increase of invasive alien species, among the 14 types of terrestrial biomes, was projected to occur in temperate mixed forests (+4.5%, i.e., average invasive alien species per pixel), followed by cool coniferous forests (+3.4%), whereas tropical forests (−4%) and tropical woodlands (−4.4%) might become suitable for a lower number of invasive alien species in the future. In other words, biomes expected to shift into future extreme climatic zones (e.g. tropical forest) were predicted to lose invasive alien species, whereas biomes (e.g. temperate mixed forest) that occurred at higher latitudes, where the climate will be less extreme, were predicted to gain some invasive alien species.
Fig.2. Effect of climate and land use changes on the number of invasive species per pixel in each biome.
Map representing the biomes (a) and the associated boxplots representing the net potential changes of invasive species number between 2000 and 2100, under the A1B emission scenarios (b).
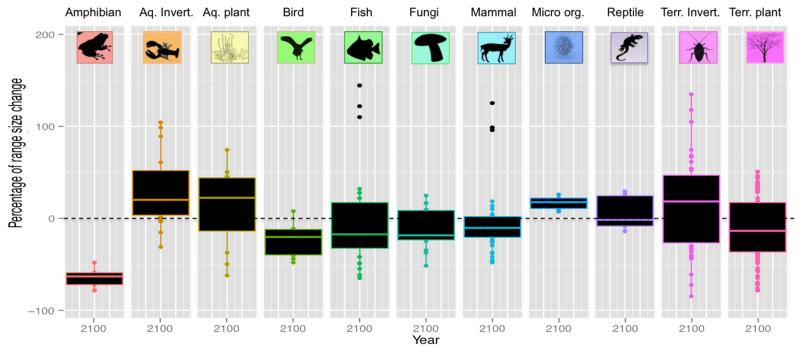
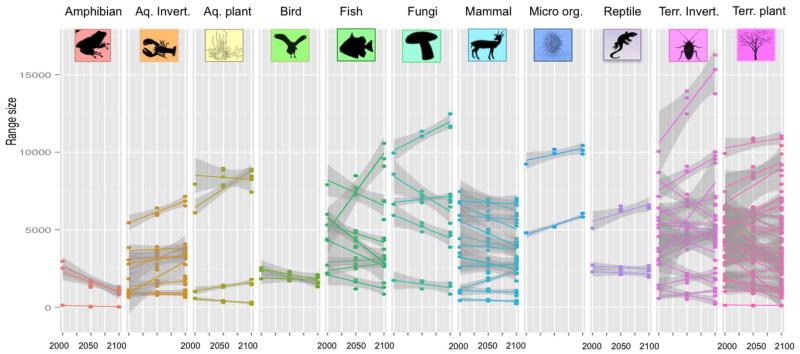
Among the 100 invasive alien species, range size expanded on average from 2 to 6% for both CO2 emission scenarios (Table S4). However, this slight increase masked high variation across taxonomic groups: amphibians (−65%), birds (−24%), and fungi (−11%) could experience strong range size shrinkage under future projections (Fig.3). Range size distribution of fishes (−1%), mammals (−4%) and reptiles (−4%) were predicted to remain stable, while aquatic invertebrates (+59%), aquatic plants (+12%), micro-organisms (+17%), and terrestrial invertebrates (+18%) were predicted to largely expand their potential range distributions. While our results predicted convergent patterns of range size change within amphibians and micro-organisms (i.e., either shrink or expand), divergent patterns were observed within the majority of the other groups, particularly within terrestrial invertebrates, plants, and fungi (Fig.4).
Fig.3. Range size change for the different taxonomic groups of the 100 invasive species.
Boxplot of the effect of climate and land use changes on invasive alien species range size (estimated by counting the number of suitable pixels) under A1B scenario for each species sorted by taxonomic group.
Fig.4. Temporal range size change for the 100 invasive alien species among the different taxonomic groups.
Effect of climate and land use changes on range size (estimated by counting the number of suitable pixels) under A1B scenario for each species along the different time-slices. Smoothing was performed for each species using linear regression.
Discussion
Using state-of-the-art of species distribution models, our findings identify three future hotspots of invasion: Europe, northwestern North America, and south Australia and New Zealand, whatever the climate scenario used. Globally, the number of invasive alien species in the future tended to be higher in the northern hemisphere compared to the southern and to decrease at lower latitudes. Many studies suggest that global change will, on average, increase the risk of invasion (Walther et al., 2009; Bradley et al., 2012). Surprisingly, we did not find a global increase in invasive species distributions following climate and land use changes. We found contrasting projected changes in species distributions among regions and taxa. For example, Central America, northern South America, Western Europe (e.g. Portugal, Spain and France), central Africa, eastern Australia, and Indonesia all showed a decrease in the expected number of future invasive alien species. We also showed consistent expected distribution shrinkage for invasive amphibians and birds, and a substantial distribution increases for most aquatic and terrestrial invertebrates. The range sizes for fishes, mammals, and reptiles are expected to remain stable on average, although we observed some local shifts in species area distributions.
Although our approach has merits, we also faced limitations that call for further refinements. First, our results should be interpreted with respect to these invasive alien species only, as they are not a representative sample of all global invasive alien species (i.e., they are mostly invasive in temperate areas). Despite this sample bias, these 100 invasive alien species include some of the most harmful and widespread invasive alien species in the world, and they have already demonstrated their ability to establish and spread into new ecosystems. Therefore, the expansion of these invasive alien species will result in new ecological interactions with consequences that are often hard to predict, but are likely to be negative for native ecosystems, economies and human health (Simberloff et al., 2012). In addition, new invasive alien species are also expected to emerge as a consequence of the ongoing climate and land use changes (Hellmann et al., 2008). However, the range contractions we have predicted for some of these dangerous invasive alien species should provide some good news for those concerned with their management and eradication. In fact, reduced climatic suitability on currently invaded areas may make invasive species less competitive, therefore potentially leading to retreat (Pyke et al., 2008; Bradley et al., 2009).
We are aware that the methodology of projecting species distributions into the future is known to be limited by the amount of information able to be incorporated in the models (Schwartz 2012). Likewise, in our study, climate and land use are assumed to be the only drivers of change for these invasive alien species distributions. We only considered abiotic variables, our analyses disregarded dispersal capacities, biotic interactions, and opportunity of invasive alien species introduction (Gaston & Fuller 2009). Recent studies provided strong evidence that biotic and abiotic factors are determinants in the pattern of invasive alien species (Roura-Pascual et al., 2011; Gallardo & Aldridge 2013). The potential presence of suitable environmental conditions for a “new” species does not automatically lead to successful establishment, meaning SDMs may often overestimate the full extent of predicted invasions. Species must travel across major geographic barriers to their new location, and they must survive and tolerate environmental and biotic conditions at the arrival site. However, these cannot presently be incorporated into our analyses. Results of SDMs are also potentially limited in their ability to predict species occurrences in novel environmental space, because we could not forecast if species will or will not occupy the new conditions that were not available to them before. Finally, we assume that invasive alien species are in equilibrium with their environment, which is not necessary the case as many invasive alien species have recently been introduced into new ecosystems.
A careful investigation of some methodological uncertainties in our studies confirms the consistency of our findings (see Methods). The results from the three different global circulation models at the two different periods (i.e., 2050 and 2100) are consistent, supporting our main conclusions.
Here, we show for the first time that the magnitude and the pattern of invasion around the world is projected to change in the 21st century for a large sample of invasive alien species, following expected changes in climate and land use. Our projections show that the number of invasive alien species should increase in northeastern Europe and northeastern United States more than elsewhere. These resulting maps indicating future hotspots of invasion help point to global zones in which emphasis should be placed on prevention and early detection, for the protection of future biodiversity in these areas. We also clearly showed that some regions (i.e., at lower latitudes) will lose invasive alien species. The potential absence of suitable conditions for an invasive alien species is one of the stronger findings since it is based on real presence in the current distribution. This result could be explained both because these regions will suffer from extreme climate conditions for invasive alien species (Neelin et al., 2006; Beaumont et al., 2010) and future important local climate changes have been shown to occur at low latitudes, including the Caribbean/Central America region and equatorial South America (Williams et al., 2007). Habitat degradation through deforestation was also projected to increase in tropical regions (Laurance et al., 2012). Additionally, it has been shown that tropical regions might become extremely sensitive to climate change because the increase in absolute temperatures relative to the past variability is relatively large compared to temperate regions (Beaumont et al., 2010). These results are of primary importance as it suggests that some invasive alien species could suffer from climate and land use changes. These potential local extinctions could create restoration opportunities for some regions such as Central America or southeast of Australia because management of invasive alien species could be easier for species that are currently at the limit of their abiotic tolerance. Globally, our results suggest strongly that biological communities will see important reorganizations in the future owing to shifting area distribution of many invasive alien species. SDMs are cost effective, giving the opportunity to prioritize and focalize actions including financial investments for certain regions. Our results provide valuable insights of plausible and possible future hotspots of invasions. They also highlight the need to account for both climate and land use change when focusing on biotic exchanges. Overall, the general picture painted here is that the question of whether climate and land use changes will favor invasive alien species is not likely to have a simple universal answer.
To conclude, we provide a new picture of future invasions, highlighting that responses of invasive alien species to global change will depend both on the region considered and on the taxa considered. These results are of major importance for Europe, northeast United States, Central America and Africa, Indonesian and Pacific islands regions because suitability of these regions to invasive alien species could be strongly modify. The impacts of these invasive alien species on these regions might become even more important in the future. We suggest that prompt responses to the introduction of invasive alien species and control of invasions should be a key component of the global efforts to mitigate the effects of climate change. Governments should also regulate the importation of species through black-listing, and activate early warning and rapid response frameworks, since such actions are decisive in preventing invasions and ecologically less risky than postponed interventions (Simberloff et al., 2012). Finally, our models predict that restoration opportunities resulting from climate change will exist in the future. Eradication and control programs should be continued for invasive alien species that will suffer from global changes, but also encouraged by our findings.
Supplementary Material
Acknowledgments
The article was written while the authors were supported by various grants, CB from the CNRS (PhD contract), FC and WT from the French “Agence Nationale de la Recherche” (ANR) with the RARE (2009 PEXT01001) and SCION (ANR-08-PEXT-03) projects. It has also been supported by an European program BIODIVERSA : FFII. WT received also funding from the European Research Council under the European Community’s Seven Framework Programme FP7/2007-2013 Grant Agreement no. 281422 (TEEMBIO). We thank Gloria Luque and Elsa Bonnaud for constructive criticism and discussions on an earlier version of the manuscript. We also thank Josh Donlan and Jessica Albate for English corrections on the manuscript. All co-authors have no conflict of interest to declare.
References
- Alkemade R, Oorschot M, Miles L, Nellemann C, Bakkenes M, ten Brink B. GLOBIO3: A Framework to Investigate Options for Reducing Global Terrestrial Biodiversity Loss. Ecosystems. 2009;12:374–390. [Google Scholar]
- Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models : prevalence, kappa and the true skill statistic (TSS) Journal of Applied Ecology. 2006;46:1223–1232. [Google Scholar]
- Araújo MB, New M. Ensemble forecasting of species distributions. Trends in ecology & evolution. 2007;22:42–7. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Barbet-Massin M, Jiguet F, Albert CH, Thuiller W. Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution. 2012;3:327–338. [Google Scholar]
- Bartholomé E, Belward AS. GLC2000: a new approach to global land cover mapping from Earth observation data. International Journal of Remote Sensing. 2005;26:1959–1977. [Google Scholar]
- Beaumont LJ, Pitman A, Perkins S, Zimmermann NE, Yoccoz NG, Thuiller W. Impacts of climate change on the world’s most exceptional ecoregions. Proceedings of the National Academy of Science. 2010;108:2306–2311. doi: 10.1073/pnas.1007217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FKA. Evaluating resource selection functions. Ecological Modelling. 2002;157:281–300. [Google Scholar]
- Bradley B a, Blumenthal DM, Early R, et al. Global change, global trade, and the next wave of plant invasions. Frontiers in Ecology and the Environment. 2012;10:20–28. [Google Scholar]
- Bradley B, Oppenheimer M, Wilcove DS. Climate change and plant invasions: restoration opportunities ahead? Global Change Biology. 2009;15:1511–1521. [Google Scholar]
- Brook BW, Sodhi NS, Bradshaw CJ. Synergies among extinction drivers under global change. Trends in ecology & evolution. 2008;23:453–60. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Butchart SHM, Scharlemann JPW, Evans MI, et al. Bennett PM, editor. Protecting Important Sites for Biodiversity Contributes to Meeting Global Conservation Targets . PLoS ONE. 2012;7:e32529. doi: 10.1371/journal.pone.0032529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez JM, D’Antonio CM, Dukes JS, et al. Will extreme climatic events facilitate biological invasions? Frontiers in Ecology and the Environment. 2012;10:249–257. [Google Scholar]
- Drake JM, Bossenbroek JM. The Potential Distribution of Zebra Mussels in the United States. BioScience. 2004;54:931. [Google Scholar]
- Elith J, Phillips SJ, Hastie T, Dudi M, Dudík M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions. 2011;17:43–57. [Google Scholar]
- Essl F, Dullinger S, Rabitsch W, et al. Socioeconomic legacy yields an invasion debt. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:203–7. doi: 10.1073/pnas.1011728108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficetola GF, Thuiller W, Miaud C. Prediction and validation of the potential global distribution of a problematic alien invasive species - the American bullfrog. Diversity and Distributions. 2007;13:476–485. [Google Scholar]
- Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation. 1997;24:38–49. [Google Scholar]
- Foley JA, Defries R, Asner GP, et al. Global consequences of land use. Science (New York, N.Y.) 2005;309:570–4. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- Gallardo B, Aldridge DC. Frid C, editor. The “dirty dozen”: socio-economic factors amplify the invasion potential of 12 high-risk aquatic invasive species in Great Britain and Ireland. Journal of Applied Ecology. 2013;50:757–766. [Google Scholar]
- Gallien L, Douzet R, Pratte S, Zimmermann NE, Thuiller W. Invasive species distribution models - how violating the equilibrium assumption can create new insights. Global Ecology and Biogeography. 2012;21:1126–1136. [Google Scholar]
- Gallien L, Münkemüller T, Albert CH, Boulangeat I, Thuiller W. Predicting potential distributions of invasive species: where to go from here? Diversity and Distributions. 2010;16:331–342. [Google Scholar]
- Gaston KJ, Fuller R a. The sizes of species’ geographic ranges. Journal of Applied Ecology. 2009;46:1–9. [Google Scholar]
- Hellmann JJ, Byers JE, Biderwagen BG, Dukes JS. Five potential consequences of climate change for invasive species. Conservation Biology. 2008;22:534–543. doi: 10.1111/j.1523-1739.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Jetz W, Wilcove DS, Dobson AP. Projected impacts of climate and land-use change on the global diversity of birds. PLoS biology. 2007;5:e157. doi: 10.1371/journal.pbio.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Valverde A, Lobo JM. Threshold criteria for conversion of probability of species presence to either-or presence-absence. Acta Oecologica. 2007;31:361–369. [Google Scholar]
- Jiménez-Valverde A, Peterson AT, Soberón J, Overton JM, Aragón P, Lobo JM. Use of niche models in invasive species risk assessments. Biological Invasions. 2011;13:2785–2797. [Google Scholar]
- Jocque M, Field R, Brendonck L, De Meester L. Climatic control of dispersal-ecological specialization trade-offs: a metacommunity process at the heart of the latitudinal diversity gradient? Global Ecology and Biogeography. 2010;19:244–252. [Google Scholar]
- Larson ER, Olden JD. Using avatar species to model the potential distribution of emerging invaders. Global Ecology and Biogeography. 2012;21:1114–1125. [Google Scholar]
- Laurance WF, Carolina Useche D, Rendeiro J, et al. Averting biodiversity collapse in tropical forest protected areas. Nature. 2012;489:290–294. doi: 10.1038/nature11318. [DOI] [PubMed] [Google Scholar]
- Leemans R, Born GJ. Determining the potential distribution of vegetation, crops and agricultural productivity. Water, Air, & Soil Pollution. 1994;76:133–161. [Google Scholar]
- Leroy B, Paschetta M, Canard A, Bakkenes M, Isaia M, Ysnel F. First assessment of effects of global change on threatened spiders : Potential impacts on Dolomedes plantarius (Clerck) and its conservation plans. Biological Conservation. 2013;161:155–163. [Google Scholar]
- Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography. 2008;17:145–151. [Google Scholar]
- Lowe S, Browne M, Boudjelas S, De Poorter M. In: 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database. Speciali N, editor. Invasive Species Specialist Group; 2000. [Google Scholar]
- Marmion M, Parviainen M, Luoto M, Heikkinen RK, Thuiller W. Evaluation of consensus methods in predictive species distribution modelling. Diversity and Distributions. 2009;15:59–69. [Google Scholar]
- McNyset KM. Use of ecological niche modelling to predict distributions of freshwater fish species in Kansas. Ecology of Freshwater Fish. 2005;14:243–255. [Google Scholar]
- Millennium Ecosystem Assessment. Arico S, Bridgewater P, et al. Ecosystems and Human Well-being: Synthesis. Washington, DC: 2005. [Google Scholar]
- Murray JV, Stokes KE, Klinken RD. Predicting the potential distribution of a riparian invasive plant: the effects of changing climate, flood regimes and land-use patterns. Global Change Biology. 2012;18:1738–1753. [Google Scholar]
- Neelin JD, Münnich M, Su H, Meyerson JE, Holloway CE. Tropical drying trends in global warming models and observations. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6110–5. doi: 10.1073/pnas.0601798103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell J, Gallagher RV, Wilson PD, Downey PO, Hughes L, Leishman MR. Invasion hotspots for non-native plants in Australia under current and future climates. Global Change Biology. 2012;18:617–629. [Google Scholar]
- Petitpierre B, Kueffer C, Broennimann O, Randin C, Daehler C, Guisan A. Climatic niche shifts are rare among terrestrial plant invaders. Science (New York, N.Y.) 2012;335:1344–8. doi: 10.1126/science.1215933. [DOI] [PubMed] [Google Scholar]
- Prentice I, Cramer W, Harrison S, Leemans R, Monserud R, Solomon A. A global biome model based on plant physiology and dominance, soil properties and climate. Journal of Biogeography. 1992;19:117–134. [Google Scholar]
- Pyke CR, Thomas R, Porter RD, Hellmann JJ, Dukes JS, Lodge DM, Chavarria G. Current practices and future opportunities for policy on climate change and invasive species. Conservation biology : the journal of the Society for Conservation Biology. 2008;22:585–92. doi: 10.1111/j.1523-1739.2008.00956.x. [DOI] [PubMed] [Google Scholar]
- Ramirez-Villegas J, Jarvis A. Downscaling Global Circulation Model Outputs: The Delta Method Decision and Policy Analysis Working Paper No.1. Policy Analysis. 2010 [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Roura-Pascual N, Hui C, Ikeda T, et al. Relative roles of climatic suitability and anthropogenic influence in determining the pattern of spread in a global invader. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:220–5. doi: 10.1073/pnas.1011723108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW. Using niche models with climate projections to inform conservation management decisions. Biological Conservation. 2012;155:149–156. [Google Scholar]
- Simberloff D, Martin J-L, Genovesi P, et al. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology & Evolution. 2012;28:58–66. doi: 10.1016/j.tree.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Solomon S, Qin D, Manning M, et al. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. United Kingdom and New York, NY, USA: 2007. [Google Scholar]
- Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Thuiller W, Lafourcade B, Engler R, Araújo MB. BIOMOD - a platform for ensemble forecasting of species distributions. Ecography. 2009;32:369–373. [Google Scholar]
- Walther G, Roques A, Hulme PE, Sykes MT, Pys P, Robinet C, Semenchenko V. Alien species in a warmer world : risks and opportunities. Evolution. 2009;12:686–693. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Walther G-R, Roques A, Hulme PE, Sykes MT, Pysek P, et al. Alien species in a warmer world: risks and opportunities. Trends in ecology & evolution. 2009;24:686–93. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Williams JW, Jackson ST, Kutzbach JE. Projected distributions of novel and disappearing climates by 2100 AD. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5738–42. doi: 10.1073/pnas.0606292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.