Abstract
A new spectrofluorometric assay is described for quantitating uroporphyrinogen I synthase (EC 4.3.1.8) activity in volumes of human blood as small as 2 μl. By this sensitive assay the inheritance of the enzyme's activity has been studied and the genetic defect for acute intermittent porphyria has been confirmed to be autosomal dominant in nature. There is a 3-fold range of uroporphyrinogen I synthase activity in erythrocytes in the normal population, with a mean Vmax ± SD of 35.7 ± 8.4 nmol of uroporphyrinogen I formed per ml of erythrocytes per hr, at 37°. One-half this level of enzyme activity (18.0 ± 5.0) is found in erythrocytes from patients with clinically manifest acute intermittent porphyria; and in erythrocytes from those of their relatives, including prepubertal children, who have the latent gene defect for the disease. The Km of erythrocyte enzyme of normal people is 12.3 ± 3.9 μM, whereas the Km of the erythrocyte enzyme of patients with acute intermittent porphyria is 6.2 ± 3.9 μM, as determined on whole blood lysates. Three enzymic changes have now been identified in patients with acute intermittent porphyria; a high level of δ-aminolevulinate synthase activity; a low level of uroporphyrinogen I synthase activity; and a deficiency of steroid Δ4-5α reductase activity.
Keywords: spectrofluorimetry, erythrocytes
Full text
PDF
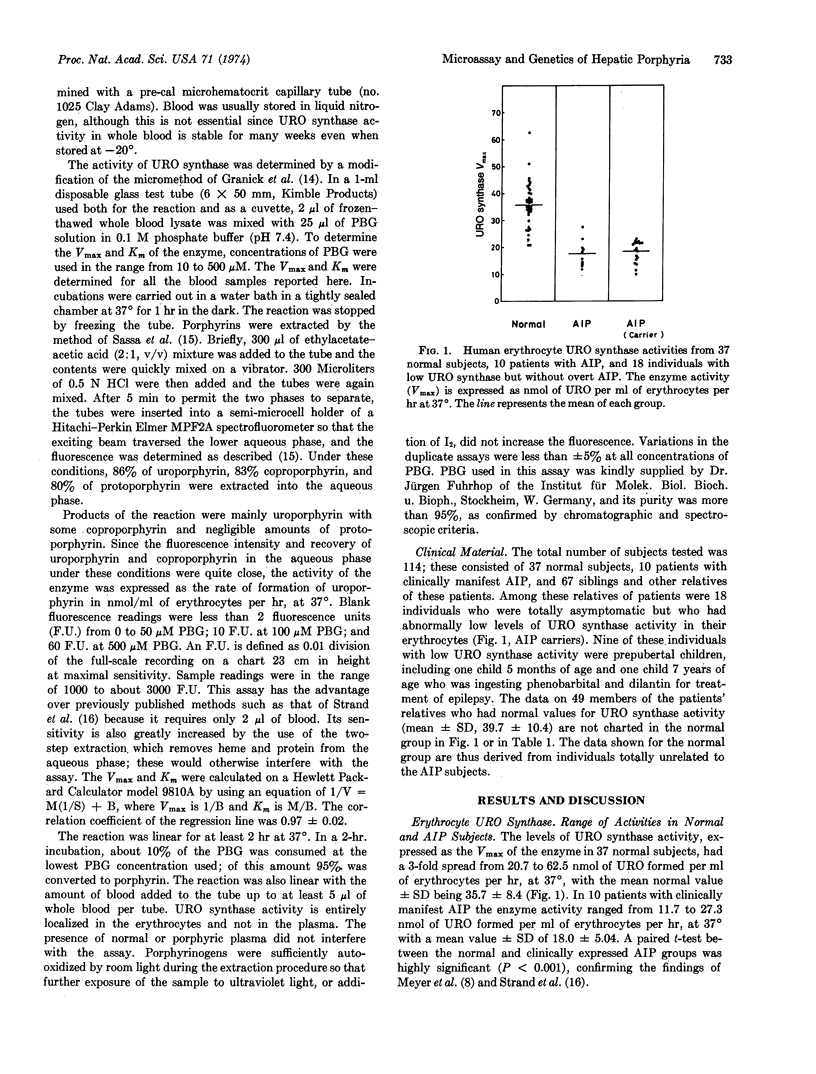
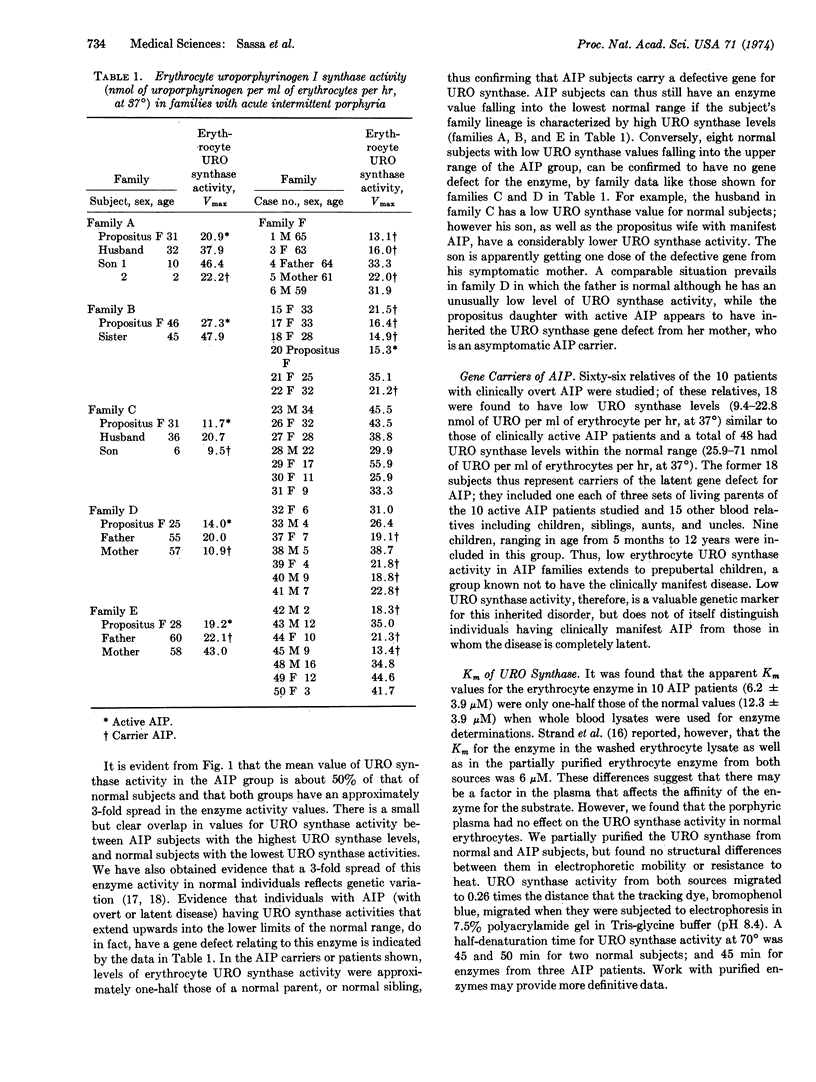


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradlow H. L., Gillette P. N., Gallagher T. F., Kappas A. Studies in porphyria. II. Evidence for a deficiency of steroid delta-4-5-alpha-reductase activity in acute intermittent porphyria. J Exp Med. 1973 Oct 1;138(4):754–763. doi: 10.1084/jem.138.4.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Granick S., Kappas A. Steroid induction of porphyrin synthesis in liver cell culture. I. Structural basis and possible physiological role in the control of heme formation. J Biol Chem. 1967 Oct 25;242(20):4587–4593. [PubMed] [Google Scholar]
- Granick S., Sassa S., Granick J. L., Levere R. D., Kappas A. Assays for porphyrins, delta-aminolevulinic-acid dehydratase, and porphyrinogen synthetase in microliter samples of whole blood: applications to metabolic defects involving the heme pathway. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2381–2385. doi: 10.1073/pnas.69.9.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmeyer L., Clotten R. Zur biochemischen Pathogenese der Pophyria acuta intermittens. Klin Wochenschr. 1969 Jan 15;47(2):71–74. doi: 10.1007/BF01745768. [DOI] [PubMed] [Google Scholar]
- Kappas A., Bradlow H. L., Gillette P. N., Gallagher T. F. Studies in porphyria. I. A defect in the reductive transformation of natural steroid hormones in the hereditary liver disease, acute intermittent porphyria. J Exp Med. 1972 Nov 1;136(5):1043–1053. doi: 10.1084/jem.136.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Granick S. Steroid induction of porphyrin synthesis in liver cell culture. II. The effects of heme, uridine diphosphate glucuronic acid, and inhibitors of nucleic acid and protein synthesis on the induction process. J Biol Chem. 1968 Jan 25;243(2):346–351. [PubMed] [Google Scholar]
- Kappas A., Song C. S., Levere R. D., Sachson R. A., Granick S. THE INDUCTION OF delta-AMINOLEVULINIC ACID SYNTHETASE in vivo IN CHICK EMBRYO LIVER BY NATURAL STEROIDS. Proc Natl Acad Sci U S A. 1968 Oct;61(2):509–513. doi: 10.1073/pnas.61.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., Marver H. S. Biochemical defects in two types of human hepatic porphyria. N Engl J Med. 1970 Oct 29;283(18):954–958. doi: 10.1056/NEJM197010292831803. [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Strand L. J., Doss M., Rees A. C., Marver H. S. Intermittent acute porphyria--demonstration of a genetic defect in porphobilinogen metabolism. N Engl J Med. 1972 Jun 15;286(24):1277–1282. doi: 10.1056/NEJM197206152862401. [DOI] [PubMed] [Google Scholar]
- Nakao K., Wada O., Kitamura T., Uono K., Urata G. Activity of amino-laevulinic acid synthetase in normal and porphyric human livers. Nature. 1966 May 21;210(5038):838–839. doi: 10.1038/210838b0. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick J. L., Granick S., Kappas A., Levere R. D. Studies in lead poisoning. I. Microanalysis of erythrocyte protoporphyrin levels by spectrophotometry in the detection of chronic lead intoxication in the subclinical range. Biochem Med. 1973 Aug;8(1):135–148. doi: 10.1016/0006-2944(73)90017-3. [DOI] [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Meyer U. A., Felsher B. F., Redeker A. G., Marver H. S. Decreased red cell uroporphyrinogen I synthetase activity in intermittent acute porphyria. J Clin Invest. 1972 Oct;51(10):2530–2536. doi: 10.1172/JCI107068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSCHUDY D. P., PERLROTH M. G., MARVER H. S., COLLINS A., HUNTER G., Jr, RECHCIGL M., Jr ACUTE INTERMITTENT PORPHYRIA: THE FIRST "OVERPRODUCTION DISEASE" LOCALIZED TO A SPECIFIC ENZYME. Proc Natl Acad Sci U S A. 1965 Apr;53:841–847. doi: 10.1073/pnas.53.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDENSTROM J. The porphyrias as inborn errors of metabolism. Am J Med. 1957 May;22(5):758–773. doi: 10.1016/0002-9343(57)90126-2. [DOI] [PubMed] [Google Scholar]



