Abstract
Uncoupling protein 3 (UPC3) is a candidate protein transporter that uncouples oxidative phosphorylation of mitochondrial respiration in skeletal muscle. A number of studies on UCP3 functions under various physiological conditions have suggested that the function of UCP3 is not limited only to regulation of whole-body energy metabolism but is also involved in regulation of substrate (lipids and glucose) metabolism. The purpose of the present study was to clarify the time course of UCP3 mRNA expression in rat skeletal muscle during a 1 h bout of treadmill exercise and to examine whether changes in fat/glucose metabolism modulates UCP3 mRNA expression. The pattern of UCP3 mRNA expression during the exercise was biphasic in both the soleus and gastrocnemius muscles. UCP3 expression increased at 5 min of exercise (soleus: 232%, p < 0.05, gastrocnemius: 185%, p < 0.05, respectively), and at the end of the exercise (196%, p < 0.05 and 193%, p < 0.05, respectively). UCP3 mRNA expression was still increased at 3 h post-exercise in both muscles, 200% (p < 0.05) and 237% (p < 0.05), respectively. However, at 20 min of the exercise, UCP3 mRNA expression was similar to control levels in both muscles (104% and 97%, respectively). The time course of plasma free fatty acid (FFA) did not follow the same time course as UCP3 mRNA expression. Plasma FFA peaked at the end of the exercise, suggesting that FFA did not play a role in inducing UCP3 mRNA expression. Glucose transporter 4 (GLUT4) mRNA expression did not change during or after exercise. These data indicated a rapid acceleration in UCP3’s transcription activity in response to exercise, and suggest that potential factor(s) other than changes in fat/glucose metabolism regulate UCP3 gene expression during moderate exercise.
Key Points.
A single bout of 1 h moderate exercise induced rapid and bi-phasic alteration of UCP3 gene transcription activity in rat skeletal muscle.
The exercise-induced up-regulation in UCP3 mRNA expression was sustained during 3 h of recovery period.
Key words: UCP3, FFA, GLUT4, exercise
Introduction
Uncoupling proteins (UCPs) are inner mitochondrial membrane transporters that dissipate the proton gradient and uncouple adenosine triphosphate (ATP) production from mitochondrial respiration. Uncoupling protein 3 (UCP3) is expressed predominantly in skeletal muscle in both rodents and humans (Boss et al., 1997; Gong et al., 1997). Metabolic challenges such as starvation (Gonzalez-Barroso et al., 1996; Hildebrandt et al., 2000; Hwang and Lane, 1999; Pilegaard et al., 2003; Samec et al., 1999) or hormone (triiodothyronine) infusion (Barbe et al., 2000; Gong et al., 1997; Lanni et al., 1999), which stimulate fatty acid oxidation, alter UCP3 transcription rate and mRNA expression in rodents and humans. These metabolic perturbations influence free fatty acid (FFA) concentration in the blood (Samec et al., 1998b; Weigle et al., 1998) and a clear up-regulation in UCP3 gene expression induced by intralipid infusion (Khalfallah et al. , 2000; Weigle et al., 1998) has been demonstrated. In fact, UCP3 is believed to be involved in regulation of fat metabolism (Himms-Hagen et al., 2001; Samec et al., 1998a). A single bout of exercise has been demonstrated to induce transient up-regulation of UCP3 transcription and mRNA expression in human skeletal muscle (Pilegaard et al., 2000, 2003; Schrauwen et al., 2002). Also, this exercise-induced up-regulation of UCP3 gene expression has often been observed during the initial hours of recovery. FFA concentration increases as rapidly as ~20min of acute aerobic exercise (Bergman et al., 1999; Klein et al., 1995), however an association between UCP3 gene expression and plasma FFA concentration during exercise has not yet been demonstrated.
The involvement of UCP3 in regulating glucose metabolism has also been demonstrated in previous studies. In L6 myotubes, the presence of UCP3 increased glucose uptake and stimulated glucose transporter 4 (GLUT4) cell surface translocation (Hupperts et al., 2001). In addition, exercise-induced increases in GLUT4 mRNA have been demonstrated in rodents during recovery periods following one bout of exercise with a concomitant increase in UCP3 mRNA expression (Tsuboyama-Kasaoka et al., 1998). However, there are limited data describing the potential signals that induce UCP3 gene transcriptional activity during exercise and there are no data demonstrating whether change in fat or glucose metabolism (FFA concentration) in response to exercise is an initial stimulus inducing UCP3 mRNA expression. We therefore hypothesized that the time course of UCP3 mRNA expression during one bout of exercise may be modulated by changes in lipid and/or glucose metabolism. To examine this hypothesis, the time course of UCP3 mRNA expression, plasma FFA concentration and GLUT4 mRNA expression levels during and after exercise were measured.
Methods
Animals
Eight-week-old male Wistar rats (Sankyo Labo Service, Tokyo, Japan) were provided food and water ad libitum and were maintained at constant room temperature (20-22°C) under controlled lighting conditions (12:12 h light-dark cycle). Rats were assigned to either control (CON; n = 17) or exercise groups (n = 17). Each of the exercise groups had 17 rats for each different time point (0 min, 5 min, 20 min, 60 min and 3 h recovery). All experiments were carried out according to the Guiding Principles for the Care and Use of Animals in the Field of Physiological Science of the Physiological Society of Japan, and all experiments conducted in this study were approved by the Animal Care Committee of Tokyo Metropolitan University Graduate School of Sciences.
Treadmill exercise protocol
Rats were allowed to eat ad libitum overnight. All rats practiced treadmill exercise three times by walking and very light running one week prior to the experimental day. Rats unsuitable for exercise were excluded. Both CON and exercised rats were randomly chosen from the included animals. Exercised rats ran on a treadmill at 5% grade, 17m·min-1 for 5 min, 20 min or 60 min of exercise. The intensity corresponded to ~60-65% of VO2max (Abe et al., 1986).
Isolation of skeletal muscle
Rats were stunned and killed by cervical dislocation after 5 min, 20 min or 60 min of exercise, or 3 h following the end of 1 h of exercise. The soleus and gastrocnemius muscles were harvested and the tissue was quickly frozen in liquid nitrogen and stored at -80°C until RNA isolation.
Determination of UCP3 mRNA and GLUT4 mRNA expression
Total RNA (tRNA) was isolated from skeletal muscle using a quick prep tRNA extraction kit (Amarsham Pharmacia). Complimentary DNA (cDNA) was obtained by reverse transcription using random hexamer primers as described by the manufacture (GIBCO BRL, 95C for 5 min, 50C for 60 min). The PCR Mastermix containing the specific primers and Ampli Taq DNA polymerase was added. The PCR protocol was 95°C for 2 min, then 35 cycles of 94°C for 45 sec, 50°C for 45 sec, and 72°C for 45 sec. Finally, the PCR products were extended for 6 min at 72°C. The PCR-products were loaded on 2% agarose gel, stained with ethidium bromide and analyzed using ATTO Densitograph software library Lane & Spot Analyzer (Tokyo Japan). The ratio between target PCR product and glyceraldehyde-3- phosphate dehydrogenase (GAPDH) PCR product were calculated. PCR primer pairs (Table 1) were designed from rat specific sequence data (Entrez; National Institutes of Health).
Table 1.
Primers used for PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| UCP3 | 5’-CCTCTACGACTCTGTCAAGC-3’ | 5’-GACTCTACCACTGGATACT-3’ |
| GLUT4 | 5’-CTGTATTCTCAGCTGTGCTTG-3’ | 5’-GTAACGAAGACCGATAGTGTC-3’ |
| GAPDH | 5’-ACCAGGTTGTCTCCTGTGAC-3’ | 5’-GCTCTCAGTATCCTTGCTGG-3’ |
Abbreviations: UCP3= uncoupling protein, GLUT4= glucose transporter 4, GAPDH= glyceraldehyde-3-phosphate dehydrogenase.
Plasma free fatty acid content
The blood samples were taken from the alveolar vessels of the heart, approximately 2 min after isolation of the muscle samples and placed into heparinaized tubes. The plasma was immediately separated by centrifugation and the supernatant was frozen until further processing. Plasma free fatty acid (FFA) concentration was determined by a commercial kit (Wako CO, Tokyo, Japan). FFA content was measured after five minutes (5 min), 20 minutes (20 min) or 60 min of exercise (60 min), or 3 h following 1 h of exercise.
Statistical analysis
Statistical comparisons of the groups were calculated by ANOVA. Results are expressed as mean ± SE and the level for statistical significance was set at p < 0.05.
Results
UCP3 mRNA and GLUT4 mRNA expression level
One bout of moderate intensity exercise induced biphasic up-regulation of UCP3 mRNA expression in both the soleus and gastrocnemius muscles (Figure 1). UCP3 mRNA expression was increased (p < 0. 05) in the soleus (232%) and gastrocnemius (185%) muscles at 5 min of exercise compared to the CON animals. However, UCP3 mRNA expression level in both muscles at 20 min of exercise decreased to the control level, respectively (104% and 97% of control, respectively). The UCP3 mRNA expression then increased (p < 0.05) again in both muscles at the end of the exercise session (60 min) (soleus: 196% and gastrocnemius: 193%) and remained elevated in soleus (200%; p < 0.05) and gastrocnemius (237%; p < 0.05) at 3 h post-exercise.
Figure 1.
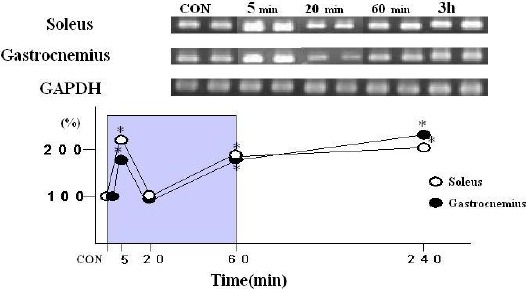
Effects of a single bout of moderate exercise on expression of UCP3 mRNA and GAPDH mRNA in soleus and gastrocnemius muscle. Muscle samples were collected at 5 and 20 minutes after the onset of exercise, at the end of exercise (60min), and 3 h post-exercise. UCP3 mRNA band intensity was determined using ATTO Densitograph software library. * p < 0.05 compared with control.
There were no significant alterations in GLUT4 mRNA expression at the end of any of the exercise bouts or 3 h after the 60 min of exercise in the soleus muscle. GLUT4 mRNA expression in the gastrocnemius muscle reached the highest levels of changes at the end of the 60 min exercise session, but this was not a significant increase (Figure 2).
Figure 2.
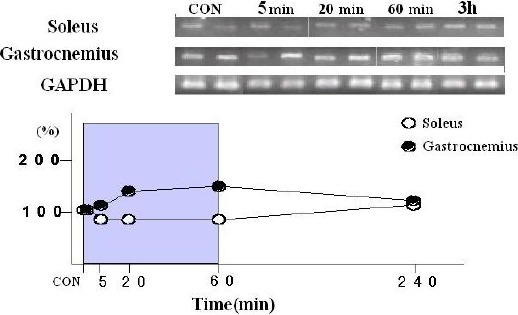
Effects of a single bout of moderate exercise on expression of GLUT4 mRNA and GAPDH mRNA in soleus and gastrocnemius muscle. Muscle samples were collected at 5 and 20 minutes after the onset of exercise, at the end of exercise (60min), and 3 h post-exercise. GLUT4 mRNA band intensity was determined using ATTO Densitograph software library.
Plasma FFA concentration
The plasma FFA concentration increased at the end of the 20 min (0.33±0.09 mmol·l-1, p < 0.05) and 60 min of exercise (0.56±0.21 mmol·l-1, p < 0.05) compare with the control level (0.21±0.09 mmol·l-1). The plasma FFA content returned to the resting levels within 3 h after the exercise (Figure 3).
Figure 3.
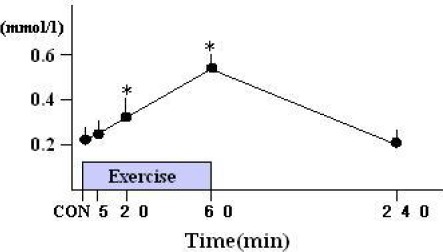
Time course of changes in plasma free ratty acid (FFA) content during and after a single bout of moderate intensity exercise (mmol·l-1). Values are means of n = 9-15 rats per group and SE. * p < 0.05 compared with control.
Discussion
It has been suggested that the function of UCP3 is not limited only to regulation of whole-body energy metabolism but is also involved in regulation of substrate (lipids and glucose) metabolism. This is because UCP3 gene transcriptional activity has been implicated to be affected by FFA/FA oxidation (Khalfallah et al., 2000; Samec et al., 1999; Weigle et al., 1998) and to relate to GLUT4 gene expression (Bashan et al., 1993; Hupperts et al., 2001; Tsuboyama-Kasaoka et al., 1998). There have been a number of studies that demonstrated a strong association between UCP3 gene expression and fat metabolism. Fasting, characterized by an increase in plasma FFA, was shown to up- regulate UCP3 in rodents (Millet et al., 1997; Weigle et al., 1998) and this effect was also confirmed in humans (Khalfallah et al., 2000). The fasting-induced up-regulation of UCP3 and subsequent down-regulation of UCP3 during refeeding provide further evidence that UCP3 is indeed involved in fatty acid metabolism (Samec et al., 1999). UCP3 has also been suggested to be involved in the handling of lipids as fuel, because increases in FFA levels are also accompanied by increased oxidation of FA (Samec et al., 1999), and a strong association between UCP3 and fat oxidation has been reported (Argyropoulos et al., 1998). Previous studies have indicated that acute exercise induces up-regulaion of UCP3 transcription (Pilegaard et al., 2000; 2003), mRNA (Cortright et al., 1999; Pilegaard et al., 2000, 2003; Tsuboyama-Kasaoka et al., 1998), and protein (Zhou et al., 2000) expression. The marked up-regulation of UCP3 gene activation observed during the initial hours of the recovery period was suggested to be due to increased FA/FA metabolism (Schrauwen et al., 2002). The involvement of UCP3 in regulating glucose metabolism has also been demonstrated in previous studies. In L6 myotubes, the presence of UCP3 increased glucose uptake and stimulated GLUT4 cell surface translocation (Hupperts et al., 2001). Further, it was proved that 2,4-dinitrophenol (DNP), which uncouples mitochondrial electron transport from ATP, increased glucose uptake immediately in L6 cells (Bashan et al., 1993), suggesting that UCP3 may influence GLUT4 transcriptional activity. Exercise-induced increases in GLUT4 mRNA have been demonstrated in rodents during recovery periods following one bout of exercise with a concomitant increase in UCP3 mRNA expression (Tsuboyama-Kasaoka et al., 1998). All these studies are suggesting probability that UCP3 mRNA expression during one bout of exercise may also be modulated by lipid and/or glucose metabolism in response to exercise. Accordingly, we hypothesized that exercise-induced changes in lipid and/or glucose metabolism may be involved in regulating of UCP3 mRNA expression during one bout of exercise. However, the increase in FFA concentration in response to exercise did not always correspond to the alteration in UCP3 mRNA expression in the present study. In addition, GLUT4 mRNA expression did not show a direct influence on UCP3 mRNA expression during and after the exercise. These results suggest putative factor(s) other than FFA and GLUT4 may be involved in the regulation of UCP3 mRNA expression during exercise.
It is not clear which factors induced the rapid up-regulation in UCP3 gene transcription activity at 5 min of the exercise, however, previous studies have suggested possible mechanisms. First, other fat sources in addition to FFA, such as muscle triyglycerides (TG), may be involved in regulating UCP3 gene expression. Previous research has demonstrated a relationship between FA oxidation in the mitochondria and UCP3 gene expression (Samec et al., 1999). There is also a rapid acceleration (~5~10min) of fat metabolism (acetyl-CoA carboxylase and malonyl- CoA content) in skeletal muscle in response to exercise (Duan et al., 1992; Hutber et al., 1997; Vavvas et al., 1997; Winder et al., 1990). Accordingly, this rapid acceleration in skeletal muscle fat metabolism may induce the rapid induction in UCP3 mRNA expression. Second, reactive oxygen species (ROS) formation in skeletal muscle in response to exercise/muscle contraction may relate to the rapid increase in UCP3 gene expression. Intense physical exercise is known to induce a marked increase in oxygen consumption in skeletal muscle and induce a higher level of oxygen flux and electron leakage from the mitochondria. In previous papers, muscle contraction-induced increases in free radical signals and ROS formation have been demonstrated in rodent skeletal muscle (Davies et al., 1982; Jackson et al., 1985; Reid et al., 1992). Brand et al. (2002) have suggested that UCP3 prevents the formation of ROS catalyzing a superoxide- inducible proton conductance. They also reported that oxidative damage (ROS formation) was significantly higher in mitochondria from UCP3 under-expressing mice as compared with wild-type controls but was unaltered in those over-expressing UCP3 (Brand et al., 2002). Additionally, it was found that uncoupling induced by superoxide in isolated skeletal muscle mitochondria was mediated by UCP3 (Echtay et al., 2002). These observations support the idea that UCP3 may function as a regulator of ROS formation. Up- regulation of UCP3 gene is thought to be an important mechanism in protecting these organelles from deleterious damage on cells because it can lower the proton gradient (Cline et al., 2001; Gong et al., 2000). There is little evidence of ROS-induced up-regulation of UCP3 mRNA expression during exercise. However, based on previous studies demonstrating an involvement of UCP3 in regulating ROS formation, it is thought that a rapid and continuous up-regulation in UCP3 gene transcription activity throughout exercise may protect the mitochondria from ROS damage, especially damage from ATP synthesis consumed during the exercise.
At present, it is not known what factors suppressed UCP3 mRNA expression activity to the basal level at 20 min of exercise. Although a potent link between UCP3 and FA metabolism has been implicated, the transient down-regulation in UCP3 mRNA expression may suggest a minor role of FFA in regulating UCP3 mRNA expression during exercise. Because it is unlikely that exercise-induced accelerated fat metabolism is attenuated transiently during exercise, thereby inducing down- regulation in UCP3 gene transcription activity. In a study previously demonstrated, 2 h-exercise- induced ~4-fold induction in FFA content did not accompany with significant changes in UCP3 mRNA expression at the end of the exercise, however UCP3 levels increased 4 h after the exercise with sustained FFA content level (Schrauwen et al., 2002). These results suggest that changes in FFA content may be not always a crucial factor which regulates UCP3 gene expression during exercise. The down-regulation of UCP3 mRNA expression during the exercise is not consistent with the inhibitory effect of UCP3 against ROS formation. The UCP3 has been shown to have extremely low concentration in skeletal muscle (200-700-fold lower than the expression of UCP1 in brown adipose tissue) (Harper et al., 2002). In addition, other natural potent uncouplers in the mitochondrial inner membrane, such as ATP/ADP antiporter (ANT), have been identified (Esposito et al., 1999). Therefore these studies suggest a possibility that several uncouplers may be involved in coordinative regulation of antioxidant activity and UCP3 gene expression may have changed transiently during the exercise. Otherwise an inhibitory effect of nucleotides on UCP3 activity (Jaburek et al., 1999), especially by diphosphates (ADP or GDP) (Echtay et al., 1999) might have affected the activity of the UCP3 gene transiently in response to change in ADP content in mitochondrial during the exercise.
At the end of 60 min of exercise, UCP3 mRNA expression was significantly increased. This is in line with the previous reports in humans (Pilegaard et al., 2000; Schrauwen et al., 2002) and in rodents (Jones et al., 2003; Tsuboyama-Kasaoka et al., 1998; Zhou et al., 2000), providing evidence that a single bout of sustained exercise (0.5 h~4 h) induces transient up- regulation in UCP3 mRNA expression. Exercise-induced sustained up-regulation in UCP3 mRNA expression however was not accompanied by FFA induction. Considering that muscle TG is important substrate for muscle metabolism in the post-exercise period (Kiens and Richter, 1998), it is plausible that muscle TG may be involved in regulating the transcription activity. Otherwise, a sustained increase in oxygen consumption (Bahr et al., 1990; 1992) may provide function of UCP3 in preventing ROS formation.
In the present study, GLUT4 mRNA did not show remarkable changes despite of increase in UCP3 mRNA expression suggesting minor effects of glucose metabolism on UCP3 mRNA expression in response to exercise. There are conflicting results about the relationship between UCP3 and GLUT4 mRNA expression in response to acute exercise in rodents (Tsuboyama-Kasaoka et al., 1998; Zhou et al., 2000). The lack of induction of GLUT4 mRNA expression either the soleus or gastrocnemius muscle during and after exercise in the present study may extend previous work indicating dissociation between UCP3 (4~5-fold) and GLUT4 (no change) gene transcriptional activities in rat hind limb skeletal muscle in response to acute running exercise (Zhou et al., 2000). On the contrary, marked up-regulation of both UCP3 (14~18-fold) and GLUT4 mRNA expression (~2-fold) expression were found 3 h after acute exercise in mouse gastrocnemius muscle (Tsuboyama-Kasaoka et al., 1998). There are differences in mode (swimming vs, running), duration of exercise (2 h vs. 1 h) and a time point for measurement (3 h after the exercise vs. at the end of the exercise) between this previous study (Tsuboyama-Kasaoka et al., 1998) and the present study, in addition to differences in species (mice vs. rat) and training status (2 wk training vs. untrained). On the other hand, the differences in the results may have been partly due to insufficient UCP3 mRNA expression to induce GLUT4 mRNA expression in the present study.
Conclusions
In summary, rapid and bi-phasic alteration of UCP3 gene transcription activity in response to moderate exercise was demonstrated. However, physiological significance of these alterations is unclear. Further investigations directed at elucidating which factor(s) are involved in UCP3 gene transcription activity during exercise and how these factors are coordinated in response to exercise are warranted.
Biographies
Keiko KUSUHARA

Employment
Ph.D student, Tokyo Metropolitan University Depart. of Exercise and Sport Science, Japan.
Degree
MD
Research interests
Exercise and uncoupling protein 3 (UCP3) expression.
E-mail: keidragon1964@hotmail.com
Takashi TOBE

Employment
Prof., Showa University, Depart. of Medicine Information, Japan.
Degree
PhD
E-mail: ttobe@pharm.showa-u.ac.jp
Takaharu NEGORO

Employment
Research associate, Showa University, Department of Medicine Information, Japan.
E-mail: tanego@pharm.showa-u.ac.jp
Takashi ABE

Employment
Professor,, Tokyo Metropolitan University, Department of Exercise and Sport Science, Japan.
Degree
PhD
Research interests
Muscle physiology and sports performance.
E-mail: abebe@comp.metro-u.ac.jp
References
- Abe T., Sakamoto T., Asami T., Higashi T., Hirota K. (1986) The effect of vigorous exercise training on cholesterol metabolism in rat. The Journal of The Physiological Society of Japan 48, 775-782 (In Japanese: English abstract). [PubMed] [Google Scholar]
- Argyropoulos G., Brown A.M., Willi S.M., Zhu J., He Y., Reitman M., Gevao S.M., Spruill I., Garvey W.T. (1998) Effects of mutations in the human uncoupling protein 3 gene on the respiratory quotient and fat oxidation in severe obesity and type 2 diabetes. The Journal of Clinical Investigation 102, 1345-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr R. (1992) Excess postexercise oxygen consumption - magnitude, mechanisms and practical implications. Acta Physiol Scandinavica 144, 1-70 [PubMed] [Google Scholar]
- Bahr R., Hansson P., Sejersted O.M. (1990) Triglyceride/fatty acid cycling is increased after exercise. Metabolism 39, 993-999 [DOI] [PubMed] [Google Scholar]
- Barbe P., Larrouy D., Boulanger C., Chevillotte E., Viguerie N., Thalamas C., Trasroy M.O., Roques M., Vidal H., Langin D. (2000) Triiodothyronine-mediated up-regulation of UCP2 and UCP3 mRNA expression in human skeletal muscle without coordinated induction of mitochondrial respiratory chain genes. The FASEB Journal 15, 13-15 [DOI] [PubMed] [Google Scholar]
- Bashan N, Burdett E., Guma A., Sargent R., Tumaiti L., Liu Z., Klip A. (1993) Mechanism of adaptation of glucose transporters to changes in the oxidative chain of muscle and fat cell. American Journal of Physiology Cell 264, 430-440 [DOI] [PubMed] [Google Scholar]
- Bergman B.C., Butterfield G.E., Wolfel E.E., Casazza G.A., Lopaschuk G.D., Brooks G.A. (1999) Evaluation of exercise and training on muscle lipid metabolism. American Journal of Physiology Endocrinology and Metabolism 276, E106-E117 [DOI] [PubMed] [Google Scholar]
- Boss O., Samec S., Paoloni-Giacobino A., Rossier C., Dulloo A., Seydoux J., Muzzin P., Giacobino J.P. (1997) Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Letters 408, 39-42 [DOI] [PubMed] [Google Scholar]
- Brand M.D., Pamplona R, Portero-Otin M, Requena JR, Roebuck SJ, Buckingham JA, Clapham JA, Cadenas S. (2002) Oxidative damage and phospholipid fatty acyl composition in skeletal muscle mitochondria from mice underexpressing or overexpressing uncoupling protein 3. Biochemical Journal 368, 597-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline G.W., Vidal-Puig A.J., Dufour S., Cadman K.S., Lowell B.B., Shulman G.I. (2001) In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. The Journal of Biological Chemistry 276, 20240-20244 [DOI] [PubMed] [Google Scholar]
- Cortright R.N., Zheng D., Jones J.P., Fluckey J.D., DoCarlo S.E., Grujic D., Lowell B.B., Dohm L. (1999) Regulation of skeletal muscle UCP2- and UCP-3 gene expression by exercise and denervation. American Journal of Physiology Endocrinology and Metabolism 276, E217-E221 [DOI] [PubMed] [Google Scholar]
- Davies K.J.A., Quintanilha A.T., Brooks G.A., Packer L. (1982) Free radicals and tissue damage produced by exercise. Biochemcal and Biophysical Research Communication 107, 1198-1125 [DOI] [PubMed] [Google Scholar]
- Duan C, Winder W.W. (1992) Nerve stimulation decreases malonyle-CoA in skeletal muscle. Journal of Applied Physiology 72, 901-904 [DOI] [PubMed] [Google Scholar]
- Echtay K.S., Roussel D., St-Pierre J., Jekabsons M.B., Cadenas S., Stuart J.A., Harper J.A., Roebuck S.J., Morrison A., Pickering S., Clapham J.C., Brand M.D. (2002) Superoxide activates mitochondrial uncoupling proteins. Nature (London) 415, 96-99 [DOI] [PubMed] [Google Scholar]
- Echtay K.S., Liu Q., Caskey T., Winkler E., Frischmuth K., Bienengräber M., Klingenberg M. (1999) Regulation of UCP3 by nucleotides is different from regulation of UCP1. FEBS Letters 450, 8-12 [DOI] [PubMed] [Google Scholar]
- Esposito L.A., Melov S., Panov A., Cottrell B., Wallace D.C. (1999) Mitochondrial disease in mouse results in increased oxidative stress. Proceedings of the National Academy of Sciences of the United States of America 96, 4820-4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D.W., Monemdjow S., Gavrilova O., Leon L.R., Marcus-Samuels B., Chow C.J., Evertt C., Kozak L.P., Li C., Deng C., Harper M.E., Reitman M.L. (2000) Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. The Journal of Biological Chemistry 275, 16251-16257 [DOI] [PubMed] [Google Scholar]
- Gong DW, He Y, Karas M, Reitman M. (1997) Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, β3-adrenergic agonist, and leptin. The Journal of Biological Chemistry 272, 24129-24132 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barroso M.M., Fleury C., Arechaga I., Zaregoza P., Levi-Meyruies C., Raimbault S., Ricquier D., Bouillaud F., Rial E. (1996) Activation of the uncoupling protein by fatty acid is modulated by mutation in the C-terminal region of the protein. European Journal of Biochemistry 239, 445-450 [DOI] [PubMed] [Google Scholar]
- Harper J.A., Stuart J.A., Jekabsons M.B., Roussel D., Brindle K.M., Dickinson K., Jones R.B., Brad M.D. (2002) Artifactual uncoupling by uncoupling protein 3 in yeast mitochondria at the concentraions found in mouse and rat skeletal-muscle mitochondria. Biochemical Journal 361, 46-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt A., Neufer P.D. (2000) Exercise attenuates the fasting-induced transcriptional activation of metaboli genes in skeletal muscle. American Journal of Physiology Endocrinolgy and Metabolism 278, E1078-E1086 [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J., Harper M.E. (2001) Physiological role of UCP3 may be export of fatty acids from mitochondria when fatty acid oxidation prodomintaes: an hypothesis. Experimental Biology and Medicine 226, 78-84 [DOI] [PubMed] [Google Scholar]
- Hupperts C., Ficher B.M., Kim Y.B., Kotani K., Vidal-Puig A., Slieker L.J., Sloop K.W., Lowell B.B., Kahn B.B. (2001) Uncoupling protein 3 (UCP3) stimulate glucose uptake in muscle cell through a phosphoinositide 3-kinase-dependent mechanism. The Journal of Biological Chemistry 276, 12520-12529 [DOI] [PubMed] [Google Scholar]
- Hutber C.A., Hardie D.G., Winder W.W. (1997) Electrical stimulation inactivates muscle acetyl-CoA carbosylase and increases AMP-activated protein kinase. American Journal of Physiology Endocrinology and Metabolism 272, E262-E266 [DOI] [PubMed] [Google Scholar]
- Hwang C.S., Lane M.D. (1999) Up-regulation of uncoupling protein-3 by fatty acid in C2C12 myotubes. Biochemical and Biophysical Research Communications 258, 464-469 [DOI] [PubMed] [Google Scholar]
- Jabůrek M., Vařecha M., Gimeno R.E., Dembski M., Je?ek P., Zhang M., Burn P., Tartaglia L.A., Garlid K.D. (1999) Transport function and regulation of mitochondrial uncoupling protein 2 and 3. The Journal of Biological Chemistry 274, 26003-26007 [DOI] [PubMed] [Google Scholar]
- Jackson M.J., Edwards R.H.T., Sumons M.C.R. (1985) Electro spin resonance studies of intact mammalian skeletal muscle. Biochimica et Biophysica Acta 847, 185-190 [DOI] [PubMed] [Google Scholar]
- Jones T.E., Baar K., Ojuka E., Chen M., Holloszy J.O. (2003) Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. American Journal of Physiology Endocrinology and Metabolism 284, E96-E101 [DOI] [PubMed] [Google Scholar]
- Khalfallah Y., Fages S., Laville M., Langin D., Cidal H. (2000) Regulation of uncoupling protein-2 and uncoupling protein-3 mRNA expression during lipid infusion in human skeletal muscle and subcutaneous adipose tissue. Diabetes 49, 25-31 [DOI] [PubMed] [Google Scholar]
- Kiens B., Richter E,A. (1998) Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. American Journal of Physiology Endocrinology and Metabolism 275, E332-E337 [DOI] [PubMed] [Google Scholar]
- Klein S., Coyle E.F., Wolfe R. (1995) Effect of exercise on lipolytic sensitivity in Endurance-trained athelets. Journal of Applied Physiology 78, 2201-2206 [DOI] [PubMed] [Google Scholar]
- Lanni A., Beneduce L., Lombardi A., Moreno M., Boss O., Muzzin P., Giacobino J.P., Golgia F. (1999) Expression of uncoupling protein-3 and mitochondrial activity in the transition from hypothyroid to hyperthyroid state in rat skeletal muscle. FEBS Letters 444, 250-254 [DOI] [PubMed] [Google Scholar]
- Millet L., Vidal H., Andreelli F., Larrouy D., Riou J.P., RIcquier D., Laville M., Langin D. (1997) Increased uncoupling protein-2 and -3 mRNA expression during fasting in obese and lean humans. The Journal of Clinical Investigation 100, 2665-2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil C.A., Stebbins C.l., Bonigust S., Halliwell B., Longhurst J.C. (1996) Production of hydroxyl radicals in contracting skeletal muscle of cats. Journal Applied of Physiology 83, 1108-1206 [DOI] [PubMed] [Google Scholar]
- Pilegaard H., Saltin B., Neufer P.D. (2003) Effect of short-term fasting and refeeding on transcriptional regulation of metabolic genes in human skeletal muscle. Diabetes 52, 657-662 [DOI] [PubMed] [Google Scholar]
- Pilegaard H., Ordway G.A., Saltin B., Neufer P.D. (2000) Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. American Journal of Physiology Endocrinology and Metabolism 279, E806-E814 [DOI] [PubMed] [Google Scholar]
- Reid M.B., Haack K.E., Franchek K.M., Valverg P.A., Kobzik L., West M.S. (1992) Reactive oxygen in skeletal muscle I. Intracellular oxidant kinetics and fatigue in vitro. Journal of Applied Physiology 73, 1797-1804 [DOI] [PubMed] [Google Scholar]
- Samec S., Seydoux J., Dulloo A.G. (1999) Skeletal muscle UCP3 and UCP2 gene expression in response to inhibition of free fatty acid flux through mitochondrial beta-oxidation. Pflügers Archiv European Journal of Physiology 438, 452-457 [DOI] [PubMed] [Google Scholar]
- Samec S., Seydoux J., Dulloo A.G. (1998a) Role of UCP homologues in skeletal muscle and brown adipose tissue: mediators of thermogenesis of regulators of lipids as fuel substrate?. The FASEB Journal 12, 715-724 [DOI] [PubMed] [Google Scholar]
- Samec S., Seydoux J., Dulloo A.G. (1998b) Interorgan signaling between adipose tissue metabolism and skeletal muscle uncoupling protein homologues. Diabetes 47, 1693-1698 [DOI] [PubMed] [Google Scholar]
- Schrauwen P., Hesselink M.K.C., Vaartjes I., Kornips E., Saris W.H.M., Giacobino J.P., Russell A. (2002) Effect of acute exercise on uncoupling protein 3 is a fat metabolism mediated effect. American Journal of Physiology Endocrinology and Metabolism 282, E11-E17 [DOI] [PubMed] [Google Scholar]
- Tsuboyama-Kasaoka N., Tsunoda N., Murayama K., Takahashi M., Kim H., Ikemoto S., Ezaki O. (1998) Up-regulation of uncoupling protein 3 (UCP3) mRNA by exercise training and down-regulation of UCP3 by denervation in skeletal muscles. Biochemical and Biophysical Research Communications 247, 498-503 [DOI] [PubMed] [Google Scholar]
- Vavvas D., Apazidis A., Saha A.K., Gamble J., Patel A., Kemp B.E., Witters L.A., Ruderman N.B. (1997) Contraction-induced change in acetyl-CoA carboxylase and 5’-AMP-activated kinase in skeletal muscle. The Journal of Biological Chemistry 272, 13255-13261 [DOI] [PubMed] [Google Scholar]
- Weigle D.S., Selfridge L.E., Schwars M.W., Seeley R.J., Cummings D.E., Havel P.J., Knuiper J.L., BeltrandeRio H. (1998) Elevated free fatty acid induce uncoupling protein 3 expression in muscle. Diabetes 47, 1453-1460 [DOI] [PubMed] [Google Scholar]
- Winder W.W., Arogyasami J., Elayan I.M., Cartmill D. (1990) Time course of exercise-induced decline in malonyl-CoA in different muscle types. American Journal of Physiology Endocrinology and Metabolism 259, E266-E271 [DOI] [PubMed] [Google Scholar]
- Zhou M., Lin B.Z., Coughlin S., Vallega G., Pilch P.F. (2000) UCP-3 expression in skeletal muscle: effects of exercise, hypoxia and AMP-activated protein kinase. American Journal of Physiology Endocrinology and Metabolism 279, E622-E629 [DOI] [PubMed] [Google Scholar]


