Abstract
Gender difference has been suggested as a risk factor for developing cardiovascular and renal diseases in humans and experimental animals. As a major sex hormone, progesterone was reported to compete with cardiotonic steroid binding to Na/K-ATPase. Our previous publication demonstrated that cardiotonic steroids (e.g., marinobufagenin) play an important role in the development of experimental uremic cardiomyopathy. We also observed that the putative mineralocorticoid antagonists, spironolactone and its major metabolite canrenone, antagonize binding of cardiotonic steroids to Na/K-ATPase in a competitive manner and also ameliorate experimental uremic cardiomyopathy induced by partial nephrectomy. In the following studies, we noted that progesterone displayed competitive inhibition of cardiotonic steroid binding to Na/K-ATPase and partially inhibited collagen synthesis induced by marinobufagenin in cultured cardiac fibroblasts. Therefore, we sought to examine whether female rats displayed less uremic cardiomyopathy than male rats when subjected to partial nephrectomy. Although partial nephrectomy caused the induction of smaller increases in blood pressure of female rats, they appeared to be similarly susceptible to cardiac remodeling induced by partial nephrectomy in terms of hypertrophy and fibrosis as age-matched male rats. The possible explanations for our findings are therefore discussed.
Keywords: Uremic cardiomyopathy, Renal failure, Cardiotonic steroids, Collagen, Fibrosis, Progesterone, Gender
Introduction
We have previously shown that the Cardiotonic Steroid (CTS), Marinobufagenin (MBG), plays an important role in the pathogenesis of experimental uremic cardiomyopathy induced by partial (i.e., 5/6th) nephrectomy (PNx) in the rat [1]. Specifically, we have demonstrated that infusion of MBG via an osmotic minipump to achieve similar elevations in plasma MBG concentration as that seen with PNx, produces a very similar cardiac phenotype. Moreover, we have also shown that both active and passive immunization against MBG both prevents and reverses phenotypic changes induced by PNx i.e., cardiac hypertrophy, diastolic dysfunction and cardiac fibrosis [2,3]. Additionally, we have observed that the putative aldosterone antagonists, spironolactone and its major metabolite, canrenone, prevent binding of radiolabeled ouabain to Na/K-ATPase in a competitive manner, additionally these compounds also prevented MBG-induced increases in fibroblast collagen production [4]. In the same report we also demonstrated that administration of spironolactone through osmotic minipump attenuates development of experimental uremic cardiomyopathy.
Indications are that men possess a greater risk for cardiovascular and renal disease than age-matched, premenopausal women [5]. The National Health and Nutrition Evaluation Survey (NHANES III) also showed that, in general, men had higher blood pressure than women through middle age [6]. Progesterone, a major female sex hormone, was found to affect salt-sensitive hypertension in women [7]. Due to structural similarity, progesterone was reported to compete with CTS binding to Na/K-ATPase, an important Na+ and K+ ion transporter found on the plasma membrane of all mammalian cells [8]. The current study evaluates whether the competitive effects of progesterone ameliorate cardiovascular change in an experimental setting in which MBG concentration is increased, viz., PNx operated rats.
Methods
Animals
Male and Female Sprague-Dawley rats were used for surgical studies. All of the experiments presented in this manuscript were conducted in accordance with the National Institutes of Health, Guide for the Care and Use of Laboratory Animals, using protocols approved by the University of Toledo, Health Science Campus, Institutional Animal Care and Use Committee.
Experimental groups
Male rats weighing 250 g to 300 g and female rats weighing 150 g to 200 g were divided into 4 groups with 6-12 rats surviving surgical manipulation in each group. Group 1 (male) and Group 2 (female) were sham-operated controls whereas Group 3 (male) and Group 4 (female) received PNx surgery as previously described [3].
Blood pressure and progesterone measurement
Conscious Blood pressure (BP) was measured by the tail cuff method immediately prior to and once a week for three consecutive weeks following surgery. Blood pressure measurements were taken using equipment from IITC, Inc. (Amplifier model 229, Monitor Model 31, Test chamber model 306, IITC Life Science, Woodland Hills, CA), as previously described [3]. Four weeks following surgery animals were euthanized. At that time their body and heart weights were recorded and blood was collected in heparinized tubes for plasma preparation. Left ventricle tissue of these animals was halved and fixed in 4% formaldehyde overnight before processing for histology. For progesterone measurement, plasma samples were first diluted 10 times and progesterone concentrations were measured using an Enzyme immunoassay kit from Cayman Chemical (Ann Arbor, MI) following the manufacturer’s protocol.
Isolation of cardiac fibroblasts
Isolation of cardiac fibroblasts was carried out as described previously by Brilla and Rupp [9], with modifications [10]. Briefly, hearts of adult male Sprague-Dawley rats weighing between 250-300 g, were used to obtain fibroblasts. The rats were anesthetized with pentobarbital (50mg/kg), and their hearts then removed and perfused under sterile conditions via the ascending aorta with Joklik’s medium (Sigma-Aldrich, St. Louis, MO) on a modified Langendorff Apparatus. After 5 min of perfusion, perfusate was switched to Joklik’s medium containing 0.1% Collagenase type 2 (Worthington Biochemical, Lakewood, NJ) and 0.1% bovine serum albumin (BSA) which was circulated for 15-25 min or until the heart became flaccid. After perfusion the heart was removed from the apparatus and the ventricles were excised, finely cut, and shaken in Joklik’s modified medium with 0.1% Collagenase and 0.1% BSA for 15 min. The resulting cell/tissue suspension was allowed to settle for 15 min and was centrifuged at 500 rpm for 10 min. The supernatant was then centrifuged at 1500 rpm for 15 min. The resulting Pellet was suspended in Dulbecco’s Modified Eagle Medium (DMEM, Sigma-Aldrich) supplemented with antibiotics (penicillin/streptomycin/fungizone) and 15% fetal bovine serum (FBS, Hyclone, Logan, UT). The resulting resuspended pellet, which contained Cardiac fibroblasts, was seeded onto plates and incubated for 1 h. Unattached cells were removed by changing the media and any remaining attached fibroblast cells were allowed to grow to confluence. Subsequently they were trypsinized and passaged once at 1:3 dilution. Cells were then allowed to grow to confluence before being utilized for experiments.
(3H) Ouabain binding studies
Ouabain binding studies were performed using purified Na/K-ATPase from porcine renal proximal tubules and 3H-labeled ouabain as described previously [4]. Purified Na/K-ATPase was incubated with or without progesterone for 30 min in a K+-free Kreb’s solution at 37°C, followed by adding varying amounts of 3H-ouabain. After another 30 min of incubation at the same temperature, the entirety of the solution was transferred onto a 0.2 μm membrane and washed three times with ice-cold K+-free Kreb’s solution. After being washed the membrane was then transferred to a scintillation vial for measurement of radioactivity. The dissociation constant (Kd) value of 3H-ouabain was calculated using one binding site curve fitting.
Western blot analysis
Cardiac fibroblasts were grown in DMEM with 15% FBS to confluence and then starved by changing the medium to DMEM containing 1% FBS for an additional 24 h. The starved cells were then treated with varying concentrations of MBG, Progesterone or both for 24 h. Subsequently, treated cells were washed twice with ice cold Phosphate Buffered Saline (PBS), and exposed to RIPA lysis buffer from Santa Cruz Biotechnology (Santa Cruz, CA). Gel Electrophoresis for western blotting was carried out using precast Ready Gels 4-15% Tris-HCl, purchased from Bio-Rad (Hercules, CA). The proteins from the gel were electro-transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in 20 mM Tris-HCl (pH 7.5, 150 mM CaCl2, and 0.1% Tween-20). Goat anti-type 1 collagen antibody (Southern Biotech, Birmingham, AL) was used to probe for procollagen-1. Secondary anti-goat horse radish peroxidase conjugated antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The structural protein β-actin was used as loading control.
Histology
Sirius Red/Fast green staining for collagen was performed on left ventricular tissues and fibrosis quantified using a protocol modeled after Junquiera et al. [11] and Puchtler et al. [12]. Briefly, 4 μm sections of paraffin embedded left ventricular sections were mounted on slides and subsequently deparaffinized by incubation in Xylene overnight, the tissue sections were then re-hydrated by sequential 3min incubations in: 100% Ethanol (EtOH, 3X), 95% EtOH (1X), and 80% EtOH (1X). Slides were then incubated in Sirius red solution (0.25 g, Direct Red 80, Sigma; 250 mL, saturated aqueous picric Acid, RICCA Chemical, Arlington, TX; and 250 μL, of Picric acid reagent plus, Sigma) for 1 h. The slides were then washed in 3 changes of acidified water (0.005% acetic acid solution), then incubated in Fast Green dying solution (0.25 g, Fast green FCF, Sigma; 250 mL, saturated aqueous solution of picric acid; 250 μL, of picric acid reagent plus) for 1 h. Slides were then washed again in 3 changes of acidified water, dehydrated in 3 changes of 100% EtOH, cleared in Xylene and mounted with a resinous medium. Bright field images of the stained slides were taken at 20X on an Olympus FSX100 system and analyzed for collagen by ImageJ (NIH).
Statistical analysis
Data presented are the mean ± standard error of the mean. Data obtained were first tested for normality. Based on whether the data were normal either an ANOVA followed by Tukey’s post-hoc test comparing means of all groups (if normal), if data were not normal the Mann-Whitney Rank Sum test was used to compare the data (JMP10, SAS).
Results
Progesterone inhibits ouabain binding to purified Na/K-ATPase
Using purified Na/K-ATPase from porcine kidney medulla, we examined the ability of progesterone to compete for binding with 3H-ouabain. Labeled ouabain was used due to the lack of availability of radiolabeled MBG. As shown in figure 1, pre-incubation of progesterone (100 μmol/L or 1 mmol/L) with purified Na/K-ATPase for 30 min before adding 3H-ouabain shifted the ouabain binding curve and increased the Kd of 3H-ouabain binding. These results are consistent with previous reports that progesterone may compete with ouabain binding to Na/K-ATPase [8,13]. Utilization of estradiol over a wide range of concentrations in these studies did not appear to influence ouabain binding (data not shown).
Figure 1. Progesterone competition with 3H-ouabain binding to purified Na/K-ATPase. Na/K-ATPase was purified from medulla of porcine kidney.
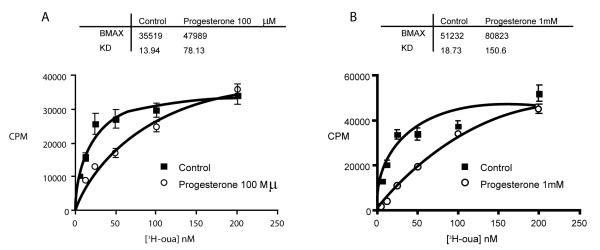
For 3H-ouabain binding assay, 30 μg of aliquots of purified Na/K-ATPase were incubated with different concentrations of 3H labeled ouabain in 500 μl K+-free Krebs solution for 30 min. After washing away the free unbound ouabain on a 0.2 μm filter, bound 3H-ouabain was measured via scintillation counting. To measure effect of progesterone on ouabain binding, 100 μM (A) or 1 mM (B) progesterone was pre- incubated with purified Na/K-ATPase for 30 min before addition of 3H labeled ouabain.
Progesterone ameliorates MBG induced cardiac fibroblast collagen synthesis in vitro
To evaluate whether progesterone ameliorates the generation of fibrosis by competing with cardiotonic steroids for binding to Na/K-ATPase, we examined collagen expression in cardiac fibroblasts treated with MBG alone or in combination with progesterone. Our data demonstrated that collagen protein level was significantly increased by more than two fold when cells were incubated for 24 h with either 1 nM or 100 nM MBG. Conversely, 100 nM progesterone was without effect on collagen synthesis (Figure 2). However, combination of MBG at a concentration of either, 1 nM or 100 nM with 100 nM progesterone shows a partial blocking effect of progesterone on MBG-induced collagen synthesis in adult rat cardiac fibroblasts.
Figure 2. Progesterone partially inhibits MBG-induced collagen-1 synthesis.
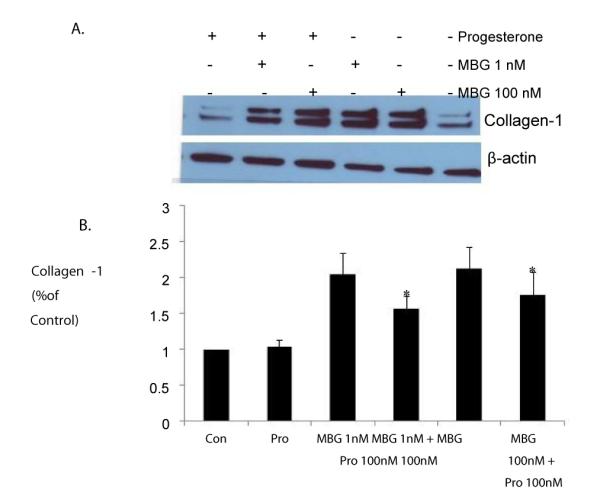
Cardiac fibroblasts isolated from male Sprague-Dawley rats were serum-depleted in DMEM with 1% FBS for 24 h and were then treated with MBG alone or in combination with 100nM progesterone for additional 24 h. Cell lysates were collected in RIPA buffer and probed for collagen-1 using Western Blot. Panel A shows a representative blot; Panel B shows the quantitative data from the mean ± SEM from 6 experiments indicates p<0.05 vs. control.
Physiological differences between male and female CKD
To determine whether progesterone was capable of limiting cardiac hypertrophy and fibrosis induced by PNx, we performed PNx surgeries on male and female rats and monitored physiological measures throughout and at the end of the 3 week course of the experiment. The data shown in figure 3 indicate that PNx induces increased blood pressure in both male and female rats. However, the increase in blood pressure versus controls is significantly lower in female rats (165.0 ± 5.0 vs. 200.0 ± 7.0 mmHg in male rats at the end of 3rd week).
Figure 3. Male and Female blood pressure responses to PNx compared with sham operated controls over the period of study.
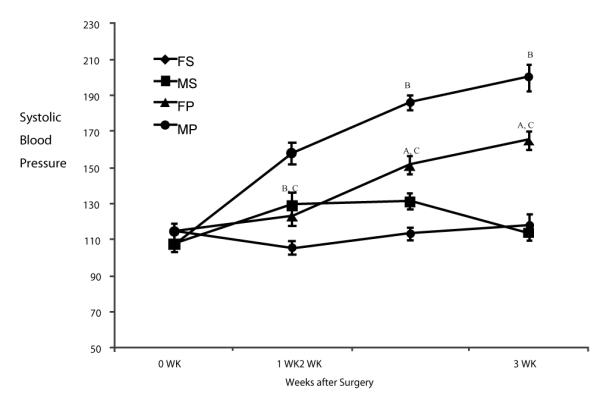
FS: female sham; MS: male sham; FP: female PNx; MP: male PNx. Values are means ± SEM of 6 animals at each point. A, indicates P<0.05 vs. sham; B, indicates p<0.01 vs. sham; C, indicates p<0.05 female vs. male.
An evaluation of cardiac hypertrophy after PNx in male and female rats, as measured by the ratio of heart weight in g (HW) to body weight in kg (BW) at the end of the 4th week following surgery, was carried out. As shown in figure 4, both male and female rats exhibit significant cardiac hypertrophy by 4 weeks after PNx surgery versus sham operated controls. There was no statistical difference in hypertrophy data from male versus female rats. Sirius Red staining as shown in figure 5, revealed significant increases in fibrosis in both male and female rats receiving PNx surgery compared to sham-operated controls. Female rats showed a milder increase compared to male rats, but this mild difference is not statistically significant.
Figure 4. Quantified Organ Data from male and female rats.
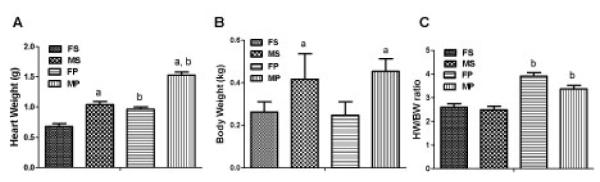
Quantified heart weights (A), body weights (B), and HW/BW ratios (C), 4 weeks after PNx surgery. Data are represented as the means ± SEM of 6 animals in each group. a, indicates P<0.05, female vs male; b, indicates P<0.05, PNx vs Sham.
Figure 5. Fibrosis was induced by PNx surgery in male and female Rats.
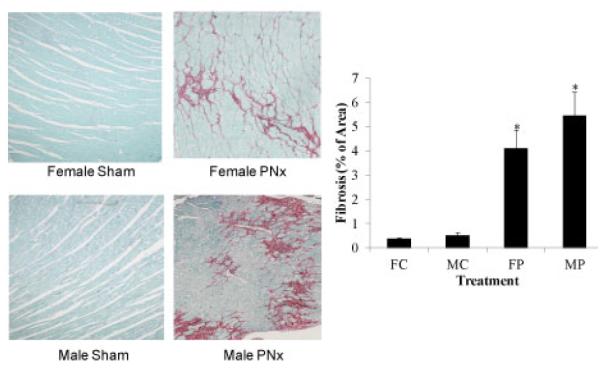
Left Panel, representative Sirius Red/Fast Green stained photomicrographs at 20X magnification obtained from male and female rats subjected to sham and PNx surgeries. Right Panel shows computer aided quantification data of photo micrographs taken from 6 animals in each group. *indicates P<0.05 vs sham.
Since progesterone levels in female rats may fluctuate in a cyclic manner, we also measured the progesterone levels in PNx rats at the end of the 4th week using the plasma collected during the sacrificing process. The result revealed that progesterone levels are 10-fold higher in female rats subjected to PNx than male rats operated in a similar manner (Figure 6). However there was no significant difference in the level of progesterone in Sham or PNx operated female rats (Data not shown).
Figure 6. Effects of PNx on plasma Progesterone concentrations.
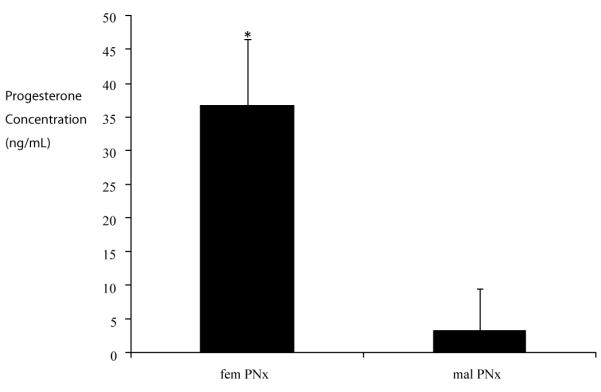
Data shown are the mean ± SEM of the plasma progesterone concentration of female and male rats subjected to PNx surgeries as measured by a commercial ELISA. Values are representative of plasma concentrations of 6 animals in each group.*indicates p<0.01vs male.
Discussion
Uremic cardiomyopathy is characterized by cardiac hypertrophy, diastolic dysfunction and cardiac fibrosis, both in clinical subjects as well as in our experimental rat model, viz., PNx [1,3,10,14]. In this setting diastolic dysfunction can be attributed to both, an active component which is related to downregulation of Sarco (Endo) plasmic reticulum Ca2+ dependent ATPase (SERCA) expression, and cardiac fibrosis [15-17]. Clinically, uremic cardiomyopathy is the leading cause of death in patients with end stage renal disease, and even modest degrees of renal insufficiency are associated with increased risk of cardiovascular disease and death [1,18]. Although morbidity and mortality risk is increased in both male and female patients, it should be noted that mortality is somewhat lower in female subjects [5,8]. These findings as well as our work with isolated purified Na/K-ATPase binding served as the basis for our current study.
In this and other studies progesterone demonstrated competitive inhibition with radiolabeled ouabain binding to purified Na/K-ATPase and it attenuated MBG-induced stimulation of cardiac fibroblast collagen production [8,13]. In our experiments, we did find that PNx induced less degree of blood pressure increase in female rats than in male rats. Fibrosis in female rats tended to be lower in female rats than in male rats, though not significantly. However, based on our data, it is not clear whether these mild effects are sufficient to reduce long-term mortality in the setting of uremic cardiomyopathy in females. While progesterone concentrations appeared to be significantly higher in female PNx rats than in male PNx rats, these concentrations were apparently insufficient to significantly prevent the cardiac fibrosis and hypertrophy observed in PNx operated animals. Several factors may play a role in differences observed between in vitro and in vivo findings noted here. First, progesterone concentrations are either elevated in a manner which is too transient or insufficient to prevent CTS induced cardiac fibrosis observed with experimental renal failure. This is somewhat surprising given that progesterone appeared to prevent ouabain binding to a similar degree as that found with canrenone [4], in addition to the fact that progesterone levels were essentially 10-fold higher in females as compared to that observed in male rats but these elevations may prove not to be enough physiologically to elicit changes on the whole animal level. We emphasize that the binding inhibition observed here did appear to be competitive, and higher levels of circulating MBG might be expected to overwhelm protective effects elicited by progesterone. An additional possibility exists that other sex hormones (e.g. estradiol) which were not studied in this setting in vivo had a profibrotic effect of their own [19-22]. The nature of the current study precluded the examination of these other hormones at this time.
In summation, although we were able to observe substantial interactions between progesterone and CTS, including MBG in our in vitro models, these compounds did not cause substantial gender based differences in physiological response to PNx surgery, a condition known to mimic uremic cardiomyopathy.
Acknowlegements
This work was partially support by NIH grant HL-105649 (JT). Marinobufagenin was kindly supplied by Drs. A.Y. Bagrov and O.V. Fedorova from the National Institutes of Health, National Institute on Aging intramural research program.
Abbreviations
- MBG
Marinobufagenin
- PNx
5/6th Partial Nephrectomy
- NHANES III
National Health and Nutrition Evaluation Survey
- DMEM
Dulbecco’s Modified Eagle Medium
- BSA
Bovine Serum Albumin
- FBS
Fetal Bovine Serum
- PBS
Phosphate Buffered Saline
- EtOH
Ethanol
- Kd
Dissociation Constant
- HW
Heart Weight
- BW
Body weight
- SERCA
Sarco(Endo)plasmic Reticulum Calcium ATPase
- CTS
Cardiotonic Steroids
- BP
Blood Pressure
References
- 1.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haller ST, Kennedy DJ, Shidyak A, Budny GV, Malhotra D, et al. Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure. Am J Hypertens. 2012;25:690–696. doi: 10.1038/ajh.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Shidyak A, Periyasamy SM, Haller S, Taleb M, et al. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension. 2009;54:1313–1320. doi: 10.1161/HYPERTENSIONAHA.109.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 6.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 7.Pechère-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens. 2004;17:994–1001. doi: 10.1016/j.amjhyper.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Morrill GA, Kostellow AB, Askari A. Progesterone binding to the alpha1-subunit of the Na/K-ATPase on the cell surface: insights from computational modeling. Steroids. 2008;73:27–40. doi: 10.1016/j.steroids.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brilla CG, Rupp H. Myocardial collagen matrix remodeling and congestive heart failure. Cardiologia. 1994;39:389–393. [PubMed] [Google Scholar]
- 10.Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49:215–224. doi: 10.1161/01.HYP.0000252409.36927.05. [DOI] [PubMed] [Google Scholar]
- 11.Junquiera LC, Junquiera LC, Brentani RR. A simple and sensitive method for the quantitative estimation of collagen. Anal Biochem. 1979;94:96–99. doi: 10.1016/0003-2697(79)90795-4. [DOI] [PubMed] [Google Scholar]
- 12.Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr Pathol. 1973;150:174–187. doi: 10.1016/s0005-8165(73)80016-2. [DOI] [PubMed] [Google Scholar]
- 13.Morrill GA, Kostellow AB, Askari A. Progesterone modulation of transmembrane helix-helix interactions between the alpha-subunit of Na/K-ATPase and phospholipid N-methyltransferase in the oocyte plasma membrane. BMC struct biol. 2010;10:12. doi: 10.1186/1472-6807-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohmand B, Malhotra DK, Shapiro JI. Uremic cardiomyopathy: role of circulating digitalis like substances. Front Biosci. 2005;10:2036–2044. doi: 10.2741/1679. [DOI] [PubMed] [Google Scholar]
- 15.Inesi G, Prasad AM, Pilankatta R. The Ca2+ ATPase of cardiac sarcoplasmic reticulum: Physiological role and relevance to diseases. Biochem Biophys Res Commun. 2008;369:182–187. doi: 10.1016/j.bbrc.2007.11.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy DJ, Vetteth S, Xie M, Periyasamy SM, Xie Z, et al. Ouabain decreases sarco(endo)plasmic reticulum calcium ATPase activity in rat hearts by a process involving protein oxidation. Am J Physiol Heart Circ Physiol. 2006;291:H3003–3011. doi: 10.1152/ajpheart.00603.2006. [DOI] [PubMed] [Google Scholar]
- 17.Periasamy SM, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 18.Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12:1079–1084. doi: 10.1681/ASN.V1251079. [DOI] [PubMed] [Google Scholar]
- 19.Chotirmall SH, Smith SG, Gunaratnam C, Cosgrove S, Dimitrov BD, et al. Effect of estrogen on pseudomonas mucoidy and exacerbations in cystic fibrosis. N Engl J Med. 2012;366:1978–1986. doi: 10.1056/NEJMoa1106126. [DOI] [PubMed] [Google Scholar]
- 20.Manigrasso MB, Sawyer RT, Marbury DC, Flynn ER, Maric C. Inhibition of estradiol synthesis attenuates renal injury in male streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2011;301:F634–640. doi: 10.1152/ajprenal.00718.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murase T, Hattori T, Ohtake M, Nakashima C, Takatsu M, et al. Effects of estrogen on cardiovascular injury in ovariectomized female DahlS.Z-Lepr(fa)/Lepr(fa) rats as a new animal model of metabolic syndrome. Hypertension. 2012;59:694–704. doi: 10.1161/HYPERTENSIONAHA.111.180976. [DOI] [PubMed] [Google Scholar]
- 22.Villa E, Vukotic R, Camma C, Petta S, Di Leo A, et al. Reproductive status is associated with the severity of fibrosis in women with hepatitis C. PLoS One. 2012;7:e44624. doi: 10.1371/journal.pone.0044624. [DOI] [PMC free article] [PubMed] [Google Scholar]


