Abstract
Objective: Managing patients with multiple adrenal masses is technically challenging. We present our experience with minimally invasive partial adrenalectomy (PA) performed for synchronous multiple ipsilateral pheochromocytomas in a single setting.
Materials and Methods: We reviewed records of patients undergoing PA for pheochromocytoma at the National Cancer Institute between 1994 and 2010. Patients were included if multiple tumors were excised from the ipsilateral adrenal gland in the same operative setting. Perioperative, functional, and oncologic outcomes of PA for multiple pheochromocytomas are shown.
Results: Of 121 partial adrenalectomies performed, 10 procedures performed in eight patients for synchronous multiple ipsilateral pheochromocytomas were identified. All eight patients were symptomatic at presentation. The mean patient age was 30.6 years, median follow up was 12 months. The average surgical time was 228 minutes, average blood loss of 125 mL, and average number of tumors removed was 2.6 per adrenal. In total, 26 tumors were removed, 24 were pathologically confirmed pheochromocytomas, while two were adrenal cortical hyperplasia. After surgery, all patients had resolution of their symptoms, one patient required steroid replacement postoperatively. On postoperative imaging, one patient had evidence of ipsilateral adrenal nodule at the prior resection site 2 months postoperatively, which was consistent with incomplete resection.
Conclusions: Minimally invasive surgical resection of synchronous multiple pheochromocytomas is feasible with acceptable perioperative, functional, and short-term oncologic outcomes.
Introduction
Partial adrenalectomy (PA) is gaining acceptance as a treatment option for patients with small adrenal tumors, including pheochromocytomas. Pheochromocytomas occur bilaterally in 3%–11% of all patients1 and in up to 60% of patients with hereditary syndromes, including Von Hippel-Lindau (VHL) and multiple endocrine neoplasia two (MEN2).2 In these patients, the importance of preserving normal adrenal tissue is underscored by the risk and decreased quality of life associated with chronic exogenous steroid replacement.3,4 A recent review of 417 patients undergoing PA found that the procedure was associated with minimal morbidity, a 3% recurrence rate, and the freedom from steroid replacement in 90% of patients.5 It has been demonstrated that even the majority of patients with a solitary adrenal gland maintain independence from steroid use following PA.6
To our knowledge, there is no literature on PA performed for synchronous multiple ipsilateral pheochromocytomas in a single setting. In this study, we report the perioperative, functional, and short-term oncologic outcomes of PA for multiple pheochromocytomas.
Methods
We reviewed the records of all patients who underwent PA for pheochromocytoma at the National Cancer Institute (NCI) between January 1994 and November 2010. Patients were included in the current study if multiple tumors were excised from the ipsilateral adrenal gland in the same operative setting. Informed consent was obtained in all patients according to the National Cancer Institute's Institutional Review Board.
Hospital records and operative reports were reviewed for demographic and perioperative data. The preoperative diagnosis of pheochromocytoma was established by a laboratory evaluation establishing biochemical function and imaging studies. Postoperative pathological evaluation confirmed the diagnosis. All operations were performed after a least 2 weeks of biochemical blockade using phenoxybenzamine 10 mg twice daily and metyrosine 250 mg three times daily. During the perioperative period, an adrenocorticotrophin stimulation test was performed on patients who demonstrated clinical or laboratory evidence of adrenocortical deficiency. Steroid supplementation was initiated when clinically indicated.
Minimally invasive PA was performed as described in detail in previous publications from our group.7,8 In brief, patients are positioned in flank and standard laparoscopic partial nephrectomy ports are placed and shifted laterally by 8–10 cm and cranially to facilitate a direct approach to the adrenal gland. Once the gland is identified, laparoscopic ultrasound is used to identify the pheochromocytomas and the margin between the normal adrenal glands. Careful, gentle dissection is then used to identify the pheochromocytoma pseudocapsule within the normal adrenal gland. Dissection of the superior and medial aspects of the adrenal gland is minimized, as these are the sites of arterial blood supply. The mass is carefully enucleated, taking great care to keep the tumor pseudocapsule intact and to respect tissue planes. Locking clips and pinpoint cautery are used for hemostasis. The entire specimen and tumor capsule are inspected to ensure complete resection. The adrenal bed is inspected for residual tumor visually and with laparoscopic ultrasound to ensure adequate hemostasis. A frozen section of the base is not routinely performed.
Patients were followed with biochemical and imaging studies at 3–6 months postoperatively and annually thereafter. For those discharged on steroid supplementation, repeat functional studies were performed at 3–6 months to identify patients with sufficient adrenal function to discontinue steroid supplementation. Functional outcomes were determined by the resolution of symptoms, normalization of serum catecholamines, and the need for steroid replacement. Oncologic outcomes were determined by the presence of a new adrenal lesion in the adrenal remnant on follow-up imaging studies.
Results
Of 121 partial adrenalectomies performed during the study period, we identified 10 procedures performed in eight patients for synchronous multiple ipsilateral pheochromocytomas. Six partial adrenalectomies were performed robotic assisted and 4 by laparoscopy. One patient underwent staged robotic-assisted bilateral PA (see Fig. 1) and another underwent simultaneous laparoscopic bilateral PA for multiple tumors. There were no conversions to open and no attempted cases were converted to total adrenalectomy. Ultrasound was used in every case to localize the enucleation plane and 5/10 patients underwent adrenal vein clamping. Baseline patient characteristics are detailed in Tables 1 and 2. The mean patient age was 30.6 years (range 15–54), of which 3/8 patients (38%) were males. At presentation, all eight patients had symptoms related to pheochromocytoma. Six patients (75%) had known hereditary syndromes predisposing them to pheochromocytoma, including four patients with VHL, one with MEN2, and one with neurofibromatosis type 1 (NF-1)/tuberous sclerosis complex (TSC) and two patients (25%) had a solitary adrenal gland. Six procedures were performed with robotic assistance and four with conventional laparoscopy.
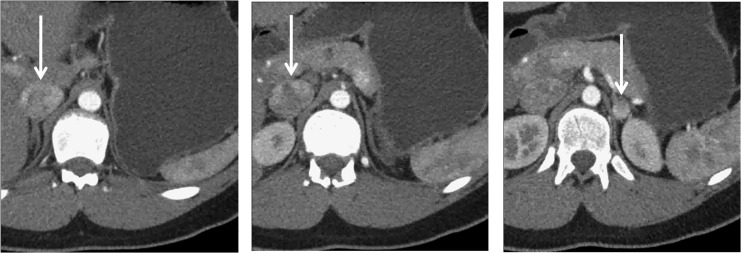
FIG. 1.
Preoperative axial CT scan with IV contrast arterial phase of procedure numbers 7 and 8. This patient had staged bilateral robotic-assisted partial adrenalectomies removing a total of four masses. The left image shows a cephalad 2.5 cm enhancing right-sided adrenal mass (identified by the white arrow). The center image shows a caudad 3.5 cm enhancing right-sided adrenal mass. The right image shows a 1.2 cm enhancing left renal mass (identified by the white arrow).
Table 1.
Summary of Patient Characteristics
| Number patients/procedures |
8/10 |
| Mean age, years (range) |
30.6 (15–54) |
| Male patients (%) |
3 (37%) |
| Right-sided procedure (%) |
5 (50%) |
| Hereditary syndromes (%) | |
| VHL |
4 (50%) |
| MEN2 |
1 (12%) |
| NF-1 |
1 (12%) |
| No known hereditary disorder |
2 (25%) |
| Surgical approach | |
| Robot-assisted laparoscopy |
6 (60%) |
| Conventional laparoscopy |
4 (40%) |
| No. with symptoms | 8 (100%) |
VHL=Von Hippel-Lindau; MEN2=multiple endocrine neoplasia two; NF-1=neurofibromatosis type 1.
Table 2.
Patient Demographics and Preoperative Testing Results
| Procedure | Age | Race | Sex | Familial disorder | Plasma meta preop | Plasma normeta preop | Urine VMA | Urine normeta | Urine meta | Preop MIBG scan |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
30 |
C |
M |
VHL |
− |
+ |
− |
+ |
− |
+ |
| 2 |
16 |
C |
M |
VHL |
− |
+ |
− |
+ |
− |
+ |
| 3 |
42 |
NR |
F |
Pheochromocytoma |
+ |
− |
− |
+ |
+ |
− |
| 4 |
55 |
AA |
F |
NF1-TS |
+ |
+ |
− |
+ |
+ |
+ |
| 5 |
24 |
C |
F |
Adrenal mass NOS |
− |
− |
− |
− |
− |
− |
| 6 |
44 |
C |
F |
MEN2 |
+ |
− |
− |
+ |
+ |
+ |
| 7a |
16 |
C |
M |
VHL |
− |
+ |
− |
+ |
− |
+ |
| 8a |
17 |
C |
M |
VHL |
− |
+ |
− |
+ |
− |
+ |
| 9b |
32 |
C |
F |
Unknown |
+ |
+ |
− |
+ |
+ |
+ |
| 10b | 32 | C | F | Unknown | + | + | − | + | + | + |
7 and 8 were staged bilateral procedures on the same patient.
Procedure 9 and 10 were synchronous bilateral procedures.
VMA=vanilylmandelic acid; MIBG=metaiodobenzyl guanidine; C=Caucasian; NR=data not available; AA=African American; M=male; F=female; VHL=Von Hippel-Lindau; MEN2=multiple endocrine neoplasia two; +=positive; −=negative.
Procedure details and follow-up are shown in Table 3. The average surgical time was 228 minutes (range 110–357) with an average blood loss of 125 mL (range 50–300). The average number of tumors removed was 2.6 per adrenal (range 2–4) with the largest per gland averaging 2.33 cm (range 0.8–5.0), while the smallest averaged 1.19 cm (range 0.7–1.5). In total, 26 tumors were removed, 24 were pathologically confirmed pheochromocytomas, while two were adrenal cortical hyperplasia. There were no perioperative complications.
Table 3.
Procedure Details and Follow-Up Data
| Procedure | Technique | Surgery Time (minutes) | EBL (mL) | # lesions removed | Smallest lesion (cm) | Largest lesion (cm) | Pathology | Periop steroid needed | Postop steroid needed | Follow-up time (months) | Steroid use at follow-up | Recurrence follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
Robotic |
141 |
50 |
3 |
0.8 |
0.8 |
Pheochromocytoma |
N |
N |
1.4 |
N |
Y |
| 2 |
Robotic |
162 |
50 |
2 |
1.5 |
2.5 |
Pheochromocytoma |
N |
N |
2.9 |
N |
N |
| 3 |
Robotic |
285 |
300 |
4 |
1 |
2 |
Pheochromocytoma |
Y |
Y |
20.3 |
Y |
N |
| 4 |
Robot |
NR |
NR |
2 |
0.7 |
1.7 |
Pheochromocytoma |
N |
N |
12.2 |
N |
N |
| 5 |
Laparoscopic |
110 |
50 |
2 |
2 |
5 |
Hyperplasia |
NR |
NR |
4.6 |
N |
N |
| 6 |
Laparoscopic |
220 |
150 |
2 |
1 |
1.6 |
Pheochromocytoma |
NR |
N |
14.8 |
N |
N |
| 7a |
Robot |
357 |
150 |
2 |
0.7 |
0.9 |
Pheochromocytoma |
N |
N |
28.8 |
N |
Y |
| 8a |
Robot |
185 |
50 |
2 |
1.2 |
3.5 |
Pheochromocytoma |
N |
N |
15.7 |
N |
N |
| 9b |
Laparoscopic |
NR |
NR |
4 |
1.5 |
2.5 |
Pheochromocytoma |
N |
N |
1.2 |
N |
N |
| 10b | Laparoscopic | 200 | 200 | 3 | 1.5 | 2.8 | Pheochromocytoma | N | N | 1.2 | N | N |
7 and 8 were staged bilateral procedures on the same patient.
Procedure 9 and 10 were synchronous bilateral procedures on the same patient.
NR=data not available; N=no; Y=yes.
The median follow up was 12 months (range 1.2–28). At the time of last follow up, all patients had resolution of their symptoms. Of the two patients undergoing PA with solitary adrenal glands, one patient required steroid replacement postoperatively following removal of four tumors. No other patient required steroid replacement. On postoperative imaging, one patient had evidence of ipsilateral adrenal nodule at the prior resection site 2 months postoperatively, which was consistent with incomplete resection.
Discussion
Early literature describing the outcomes of partial adrenalectomies focused on patients with hereditary syndromes. Patients with heritable diseases like VHL, MEN2, and NF-1 are at known risk for the development of pheochromocytomas. In addition, nearly 47% of VHL patients will suffer bilateral disease and many with hereditary syndromes are at risk for the development of metachronous tumors.9 Optimal medical and surgical management for these patients dictate adrenal preserving measures. Studies have proven the efficacy of PA both in terms of functional outcomes (i.e., steroid independence) as well as recurrence rates similar to total adrenalectomy.10–12
Prior studies have shown the oncologic successes of bilateral PA.13,14 Few studies, however, have commented on the multiplicity of tumors excised. This factor is important as the current data estimate that ∼15%–30% of adrenal tissue must remain for adequate function.5 Although Walz et al. demonstrated that the preservation of the adrenal vein is not necessary to maintain the function, clearly, multiple tumors within a gland adds a level of difficulty to surgical extirpation. To date, this article is the first to report success in patients with synchronous unilateral pheochromocytomas. From an operative standpoint, mean operative times and estimated blood loss are comparable to prior published work for laparoscopic PA.9,15 At completion of this study, only one patient presented with tumor recurrence thought to be secondary to incomplete resection, and no patients were found to have metastasis. In our study, one patient was found to be steroid dependent, and this patient was one of two patients operated on with a solitary adrenal gland. Within our patient population, the patient who required steroid replacement had a large tumor burden on a solitary adrenal gland. The patient required the removal of four tumors, while the average tumor removal for all other patients was only 2.6 per adrenal. In retrospect, preserving viable adrenal tissue in this patient was attempted, but not possible.
Several limitations to this analysis exist. Our study is retrospective and has a small sample size. Perioperative adrenocorticotrophin stimulation tests were only performed for patients with clinical or laboratory evidence for adrenocortical deficiency; it is possible that the two patients with solitary glands had subclinical adrenal insufficiency before their second adrenal procedure. In addition, we did not test patients with normal perioperative adrenocorticotrophin tests for deficiencies after the PA; thus, the possible deterioration of adrenal function over time was not evaluated. Given our length of follow-up, there was insufficient time to detect recurrences or metastatic disease. Further studies are needed to delineate a possible cutoff for tumor size or volume, after which perioperative steroid supplementation is needed, as well as technical feasibility of complex PA.
Conclusions
Minimally invasive surgical resection of synchronous multiple pheochromocytomas is feasible with acceptable perioperative, functional, and short-term oncologic outcomes.
Abbreviations Used
- MEN2
multiple endocrine neoplasia two
- NF-1
neurofibromatosis type 1
- NIH
National Institute of Health
- PA
partial adrenalectomy
- TSC
tuberous sclerosis complex
- VHL
Von Hippel-Lindau
Acknowledgment
This research was funded by the National Institute of Health (NIH) intramural research program.
Disclosure Statement
No competing financial interests exist.
References
- 1.Walther MM, Keiser HR, Linehan WM. Pheochromocytoma: Evaluation, diagnosis, and treatment. World J Urol 1999;17:35–39 [DOI] [PubMed] [Google Scholar]
- 2.Walther MM, et al. . Clinical and genetic characterization of pheochromocytoma in von Hippel-Lindau families: Comparison with sporadic pheochromocytoma gives insight into natural history of pheochromocytoma. J Urol 1999;162:659–664 [DOI] [PubMed] [Google Scholar]
- 3.Telenius-Berg M, et al. . Quality of life after bilateral adrenalectomy in MEN 2. Henry Ford Hosp Med J 1989;37:160–163 [PubMed] [Google Scholar]
- 4.Lairmore TC, et al. . Management of pheochromocytomas in patients with multiple endocrine neoplasia type 2 syndromes. Ann Surg 1993;217:595–601; discussion 601–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaye DR, et al. . Partial adrenalectomy: Underused first line therapy for small adrenal tumors. J Urol 2010;184:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanford TH, et al. . Outcomes and timing for intervention of partial adrenalectomy in patients with a solitary adrenal remnant and history of bilateral phaeochromocytomas. BJU Int 2011;107:571–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boris RS, et al. . Robot assisted laparoscopic partial adrenalectomy: Initial experience. Urology 2011;77:775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asher KP, et al. . Robot-assisted laparoscopic partial adrenalectomy for pheochromocytoma: The National Cancer Institute technique. Eur Urol 2011;60:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diner EK, et al. . Partial adrenalectomy: The National Cancer Institute experience. Urology 2005;66:19–23 [DOI] [PubMed] [Google Scholar]
- 10.Benhammou JN, et al. . Functional and oncologic outcomes of partial adrenalectomy for pheochromocytoma in patients with von Hippel-Lindau syndrome after at least 5 years of followup. J Urol 2010;184:1855–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munver R, Del Pizzo JJ, Sosa RE. Adrenal-preserving minimally invasive surgery: The role of laparoscopic partial adrenalectomy, cryosurgery, and radiofrequency ablation of the adrenal gland. Curr Urol Rep 2003;4:87–92 [DOI] [PubMed] [Google Scholar]
- 12.Pavlovich CP, Linehan WM, Walther MM. Partial adrenalectomy in patients with multiple adrenal tumors. Curr Urol Rep 2001;2:19–23 [DOI] [PubMed] [Google Scholar]
- 13.Liao CH, et al. . Laparoscopic simultaneous bilateral partial and total adrenalectomy: A longer follow-up. BJU Int 2009;104:1269–1273 [DOI] [PubMed] [Google Scholar]
- 14.Walz MK, et al. . Laparoscopic and retroperitoneoscopic treatment of pheochromocytomas and retroperitoneal paragangliomas: Results of 161 tumors in 126 patients. World J Surg 2006;30:899–908 [DOI] [PubMed] [Google Scholar]
- 15.Walther MM, et al. . Laparoscopic partial adrenalectomy in patients with hereditary forms of pheochromocytoma. J Urol 2000;164:14–17 [PubMed] [Google Scholar]