Abstract
Background and Purpose: Positron-emission tomography/computed tomography (PET/CT) with fluorine-18 fluorodeoxyglucose (FDG) is used as first-line staging for patients with newly diagnosed non-small cell lung cancer (NSCLC). Our purpose was to review the accuracy of FDG-PET/CT to predict adrenal gland metastasis, explain the causes for false-positive PET, and provide a diagnostic algorithm.
Patients and Methods: Two patients with incidentally discovered lung masses were found to have hypermetabolic adrenal activity by FDG-PET/CT with maximal standard uptake value (SUV) of 4.5 and 6.5. A MEDLINE search was performed on the topic of FDG-PET/CT, adrenal gland metastasis, and NSCLC. Literature was reviewed with regard to diagnosis, accuracy, outcomes, and alternative imaging or diagnostic strategies.
Results: Both patients underwent transabdominal laparoscopic adrenalectomy and were found to have nodular hyperplasia without evidence of adrenal tumor. A total of seven articles containing 343 patients were identified as having pertinent oncologic information for NSCLC patients with adrenal lesions. Sensitivity and specificity of PET/CT for distant metastasis was 94% and 85%, respectively, but only 13% (44/343) of these patients had histologically confirmed adrenal diagnoses. Based on this, a diagnostic algorithm was created to aid in decision making.
Conclusions: Although PET/CT has high sensitivity and specificity for adrenal metastasis in the setting of NSCLC, adrenal biopsy or other secondary imaging should be considered to confirm the finding. Adrenalectomy in lieu of biopsy may have both diagnostic and therapeutic benefit in cases where the adrenal mass is ≥10 mm with high PET maximum SUV (≥3.1) and SUV ratios (>2.5), where washout CT or chemical shift MRI is positive, or where percutaneous biopsy is deemed too difficult or unsafe.
Introduction
The 2013 National Comprehensive Care Network (NCCN) guidelines recommend integrated positron-emission tomography/computed tomography (PET/CT) with the glucose analogue fluorine-18 fluorodeoxyglucose (FDG) as first-line staging of patients with almost all stages of lymphoma, mesothelioma, head and neck tumors, cervical, esophageal, and lung cancers.1–4 These recommendations have evolved because of FDG-PET/CT's ability to integrate both anatomic and functional assessments of potential metastasis, improving not only staging accuracy but also surveillance and restaging accuracy for a variety of cancer types.5 When one considers that more than 200,000 new cases of non-small cell lung cancer (NSCLC) alone are diagnosed in the United States annually and up to 20% of these patients have adrenal gland lesions, minimally invasive urologists will increasingly be called on to evaluate and potentially operate on patients with suspicious PET/CT adrenal findings.2–4
This review examines two cases of NSCLC with positive adrenal PET/CT, summarizes the existing literature, explains the possible causes for false-positive PET findings, and provides a treatment algorithm that may be helpful in PET/CT interpretation and in decision making.
PET Adrenal Case Reports
Patient #1
A 63-year-old Caucasian man with a 120-pack year smoking history and previous five-vessel coronary artery bypass grafting with stent placement was being evaluated for in-stent stenosis. Routine chest radiography revealed a 1.4 cm left upper lobe lung mass, suspicious for malignancy. FDG-PET/CT revealed a 1.2×1.4×1.4 cm hypermetabolic lung nodule with maximal standard uptake value (SUVmax) of 14.70. The left adrenal gland was found to be without masses, but hypermetabolic PET activity was present with a SUVmax of 4.46 (Fig. 1A), suspicious for occult metastatic disease. Because the patient was considered high risk for thoracotomy due to his previous bypass graft, he was referred to the urology department for an adrenalectomy.
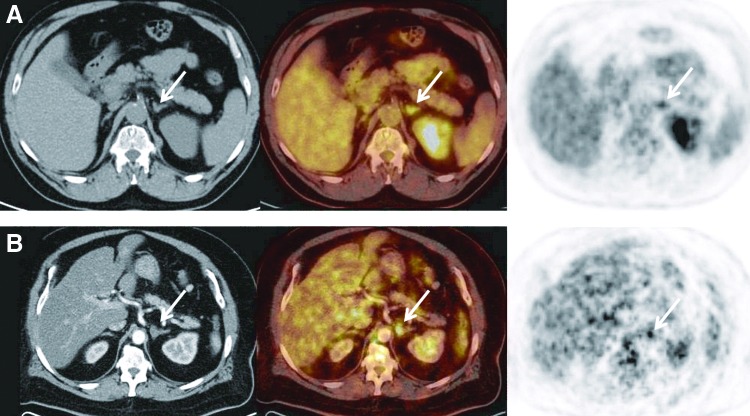
FIG. 1.
Axial computed tomography (CT), coregistered, and positron emission tomography (PET) images of adrenal lesions (arrows). (A) Axial images for Patient #1 include unenhanced CT (left) demonstrating no discrete adrenal nodule and PET (right). Coregistered images (middle) show increased FDG uptake with standard uptake value (SUV)max of 4.46. (B) Axial images of Patient #2 include enhanced CT (left) demonstrating 1.2 cm adrenal nodule and PET (right). Coregistered images (middle) show increased FDG uptake with SUVmax of 6.5. This patient also had attenuation correction CT demonstrating a relative washout value of 40% (not shown).
A left-sided transabdominal laparoscopic adrenalectomy was performed without complications. Pathologic examination of the adrenal gland revealed adrenal hyperplasia with focal incidental pigmented adrenal nodules without adenoma or carcinoma. The patient later underwent left lobectomy and received a diagnosis of moderately differentiated adenocarcinoma of the lung.
Patient #2
A 60-year-old man with a 140-pack year smoking history was found by chest radiography and subsequent chest CT to have a 5-cm lobulated right chest mass with several subcentimeter mediastinal nodules. Fine needle aspiration (FNA) of the lymph nodes by bronchoscopy showed poorly differentiated NSCLC. FDG-PET/CT scan was performed and, in addition to the hypermetabolic right lobe mass (SUVmax=15.7), the left adrenal gland was noted to contain a metabolically active 1.2 cm nodule with SUVmax of 6.5 (Fig. 1B). Attenuation correction CT demonstrated a relative washout value of ∼40%.
As in Patient #1, the patient was considered a borderline surgical candidate (stage IIIA) because of the possibility of distant metastasis and was referred to the urology department. Pathologic examination of the left adrenal after an unremarkable transabdominal laparoscopic adrenalectomy revealed nodular cortical hyperplasia without tumor. The patient subsequently elected for radiotherapy and systemic chemotherapy for his lung cancer.
Literature Review Methods and Results
Relevant studies were searched from electronic databases including Cochrane Central Register of Controlled Trials (The Cochrane Library), MEDLINE, and EMBASE. Reference lists were also made from thoracic textbooks and review articles. Search terms included all forms and abbreviations of lung carcinoma, non-small cell lung carcinoma, bronchogenic and adenocarcinoma of the lung, adrenal mass, adrenal nodule, PET, and PET/CT. Of the 104 articles identified, 24 contained clinical information dealing with metastasis to the adrenal gland while 80 used PET nonspecifically or specifically to other organs (thorax, mediastinum, brain, liver, etc). Of these 24 studies, 8 were identified to contain reportable pertinent oncologic information, including at least 50% of the study population with NSCLC and concomitant adrenal lesions evaluated by FDG-PET/CT6–12 or FDG-PET.13 The FDG-PET only study was excluded because PET-only literature is considered obsolete compared with PET/CT.
All seven studies were retrospective in nature (Table 1) with independent PET review performed by “blinded” nuclear medicine physicians. No prospective clinical trials or cohort studies were identified nor were any FDG-PET protocols before 1995. A total of 343 patients were identified, with the majority of these patients having pathologically confirmed NSCLC. A number of studies excluded small (<1 cm) indeterminate masses, questionable lesions with insufficient follow-up, or metabolically active adrenal glands that did not contain an adrenal mass. Mean adrenal nodule size was 2.2 cm (range 0.4 cm–10.4 cm) with three studies including adrenal lesions smaller than 1 cm or hypermetabolic adrenal glands.8,9,11 More than 85% of the adrenal diagnoses were made by serial CT imaging follow-up and not by tissue diagnosis. In total, the average weighted sensitivity of FDG-PET/CT for adrenal metastasis in the setting of NSCLC was 94% with specificity of 85%. SUVmax and ratio criteria were included in studies published after 2008 and are further reviewed below.
Table 1.
Results of Fluorine-18 Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Studies in Patients with Histologically Confirmed Non-small Cell Lung Cancer and Adrenal Lesions
| Study | # NSCLC/patients (%) | Mean size in cm (range) | Histologic diagnosisa | Sensitivity | Specificity | Miscellaneous |
|---|---|---|---|---|---|---|
| Erasmus 19976 |
24/27 (89%) |
3.0 (1.0–9.0) |
11 |
100 % |
80% |
PET/CT accurate in 23/23 true-positives; 2 false+patients had FDG uptake of 3.0 and 3.7 |
| Gupta 20017 |
27/30 (90%) |
NR |
7 |
94.4% |
91.6% |
PET/CT accurate in 17/18 true-positives, 11/12 true-negatives; 1 false-negative+adrenal schwannoma |
| Sung 20088 |
39/42 (87%) |
1.6 (0.5–10.4) |
9 |
80% |
89% |
PET/CT accurate in 28/35 true-positives and 23/26 true-negatives |
| Brady 20099 |
NR/76b |
2.2±1.4c |
12 |
97% |
86% |
92 excluded masses; PET/CT using strict SUVmax and ratio criteria accurate in 22/37 true-positives and 58/58 true-negatives |
| Okada 200910 |
23/30 (77%) |
2.0±0.85c |
0 |
89% |
94% |
PET/CT using strict SUVmax and ratio criteria accurate in accurate in 18/19 true-positives and 16/16 true-negatives |
| Lu 201011 |
NR/87 |
1.8 (0.4–5.8) |
3 |
97% |
94% |
PET/CT accurate in 74/77 true-positives and 31/33 true-negatives |
| Cho 201112 |
43/51 (84%) |
2.8 (1.0–7.2) |
2 |
97% |
81% |
PET/CT accurate in 43/45 true-positives and 13/16 true-negatives using strict SUVmax and ratio criteria |
| Total | NC/343 | 2.2 (0.4–10.4) | 44 | 94% | 85% | Limitations: Many studies exclude indeterminate masses or questionable lesions with insufficient follow-up |
Nonhistologic adrenal mass determination made by CT change in nodule at ≥6 follow-up (malignancy=nodule growth; benign=stable or reduced nodule size).
Some patients had “suspected” lung cancer.
Mean lesion size±standard deviation.
NSCLC=non-small cell lung cancer; PET/CT=positron-emission tomography/computed tomography; FDG=fluorine-18 fluorodeoxyglucose; NR=not reported; SUV=standard uptake value; NC=not calculable because of missing variables.
Discussion
What is PET scanning?
PET scanning has been heavily used in oncologic imaging since the 1990s. While there are a number of PET radionuclides, 18F-FDG is the only one used in clinical oncologic practice. Based on the principle that malignant cells have increased glucose utilization, PET can detect malignancy in organs that do not yet show morphologic change—an obvious advantage over traditional anatomic imaging. One of PET's largest limitations, lack of spatial imaging and localization, was overcome in 2001 with the clinical introduction of the first hybrid PET/CT scanner. Twelve years later, PET/CT's ability to localize potential metastasis has led to increased utilization in the staging, restaging, recurrence, and therapeutic setting for a variety of solid organ malignancies, making it one of the fastest growing imaging modalities in the United States.5,14–16
Despite its advantages, PET/CT is most limited by its false-positive rate, because nonspecific FDG-labeled glucose uptake is known to occur in the setting of inflammation or infection.15,17,18 For example, a retrospective review of more than 1000 cancer patients determined that 25% of PET/CT positive lesions were benign, and more than 75% of this was because of inflammation.17 Because these “false-positives” have clinical implications for our patients, a brief review of FDG uptake mechanisms is appropriate to better understand why false-positives occur and how radiologists may limit them.
How does PET work and what can cause a false-positive adrenal PET/CT?
PET scan functional information is obtained by measuring the biodistribution of radiolabeled drugs or other ligands.19–21 FDG, the most common radiolabeled ligand, accumulates within highly metabolically active cells and may indicate a malignancy (primary or metastasis), inflammation, infection, or other hypermetabolic processes. Malignant cells upregulate hexokinase activity, increasing their utilization of both normal glucose and radiolabeled FDG. When FGD-labeled and nonlabeled glucose enter cells via the glucose transporter, they are phosphorylated to form glucose-6-phosphate and FDG-6-phosphate. FDG-6-phosphate lacks the ability to be further metabolized via glycolysis and therefore accumulates within the cell, resulting in positive PET findings.19,21,22 Accumulation of FDG-6-phosphate within cells on PET scan is typically identified by visual inspection and is quantified using the SUV, calculated based on tissue tracer activity, injected radiotracer dose, and the patient's weight.21 Maximum FDG uptake can also be compared with surrounding normal organs, usually the liver, termed lesion SUVmax/liver SUVmax or the SUV “ratio.”
FDG uptake typically depends on organ-specific glucose metabolism, ranging from high (brain) to moderate (liver, spleen, adrenal, gut) to low (myocardium). In addition to infection, tissues affected by autoimmune or granulomatous disease may also demonstrate increased FDG uptake, because activated granulocytes, lymphocytes, and macrophages all need high glucose turnover. For instances other than carcinoma or metastasis, intense FDG uptake of the adrenal has been reported to occur in the setting of benign hyperplasia, believed because of rapid glucose turnover from endocrine hyperfunction. Two such case reports include symmetric bilateral adrenal uptake in a patient with severe Cushing's syndrome23 and an unilateral uptake in a patient with subclinical Cushing's syndrome.24 Thus, in addition to malignancy, infection, and autoimmune diseases, hyperfunctioning adenomas should also be in the differential in patients with increased adrenal FDG uptake.
How common are adrenal masses?
The adrenal gland poses a unique challenge in imaging, because incidental findings are common. The incidental adrenal mass or “incidentaloma” is defined as a 1-cm adrenal mass that is discovered by imaging for indications unrelated to adrenal gland—not if imaging was performed for cancer staging purposes or in patients with a cancer history (our cases). The frequency of these masses has been determined to be approximately 5% by autopsy study and 4% by abdominal CT imaging, with increasing incidence with age.25
The consensus by most radiologists is that small adrenal lesions with ≤10 Hounsfield units (HU) on noncontrast phases can be considered benign “lipid-rich” adrenal cortical adenomas and need no further imaging. Based on an NIH guidelines panel in 2002, all patients with an incidentaloma should have a functional work-up, including 1-mg dexamethasone suppression test and measurement of plasma-free metanephrines.26 If hypertensive, patients should also have serum potassium and plasma aldosterone concentration/plasma renin activity ratio.26 Masses that do not fit this criteria, however, could represent other diagnoses, such as lipid-poor adenomas (an estimated 30% of all adenomas27), pheochromocytomas, primary adrenocortical carcinomas, or metastasis.28 For patients with primary solid organ malignancies, the prevalence of adrenal metastasis by postmortem autopsy ranges from 10% to 27% while CT estimates range from 25% to 75%, depending on the type and size of the primary tumor.25,29,30 Overall, given the high prevalence of adrenal adenomas in the general population, an adrenal mass discovered even in an oncology patient is most likely benign. Larger, more complex, and/or hypermetabolic masses within an adrenal gland, however, should prompt further imaging as well as functional work-up.
Lung cancer and adrenal metastasis
The American Cancer Society estimates that approximately 230,000 new cases (respectively) of breast, prostate, and lung cancer will be diagnosed this year. Of these, lung cancer leads all causes of cancer-related mortality with 5-year survival rates varying depending on the stage at diagnosis, from 49% to 16% to 2% for patients with local, regional, and distant stage, respectively.31 Because of the large discrepancy in staged-based survival and the superiority of PET/CT compared with CT alone, the 2013 NCCN guidelines now recommend all patients with a new diagnosis of lung cancer undergo integrated FDG-PET/CT as first-line cancer staging.1 Although whole-body PET/CT improves staging accuracy, incidental findings of FDG-avid lesions unrelated to the NSCLC can mimic distant metastases and lead to misinterpretation that can affect oncologic treatments. Because of this, a brief review of NSCLC metastasis is appropriate.
Of the patients who develop NSCLC, ∼20% to 50% will present with extrapulmonary metastasis to brain, bone, liver, and/or adrenal.32 The majority of these metastatic lesions are not amenable to curative treatment with an overall median patient survival time of 7 to 11 months.33 Specific involvement of the adrenal gland has been reported to range from 18% to 42% in three large NSCLC necrospy series,34–36 but the incidence of a solitary, potentially curable NSCLC metastasis to the adrenal gland is much less, around 2% to 4%.37,38 When one considers that a considerable portion of the general population has adrenal adenomas, the importance of histopathologic confirmation of suspected adrenal masses before lung resection is critical.
What further imaging work-up should be performed in the setting of NSCLC and an adrenal mass?
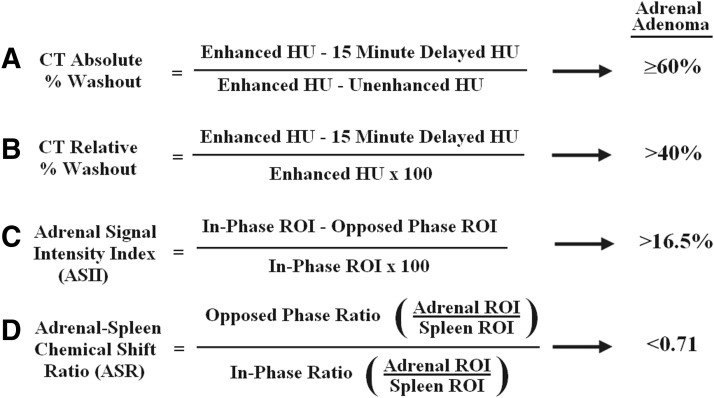
Any adrenal mass found on a preoperative PET/CT scan in a patient with NSCLC should undergo further evaluation. This can include diagnostic imaging using adrenal washout CT or chemical-shift magnetic resonance imaging (MRI) or tissue sampling via biopsy or adrenalectomy. Although not the focus of this review, further imaging, both adrenal washout CT and chemical-shift MRI, is commonly used to further characterize the adrenal in the setting of a potential metastasis. Adenomas and metastases both enhance rapidly after injection of iodinated contrast or gadolinium, but contrast material typically washes out more rapidly in adenomas than metastases, allowing for washout value calculations.39 For CT, absolute percentage washout values (noncontrast compared with delayed) ≥60% or relative percentage washout values (enhanced compared with delayed) >40% are considered diagnostic for adrenal adenoma (Fig. 2), with reported sensitivity of 83% to 93% and specificity of 93% to 98%.40,41 Limitations of this technique include an additional patient visit to radiology, exposure to further multiphasic scanning, radiation, and intravenous contrast as well as the potential of under- or overstaging by improper adrenal characterization.
FIG. 2.
Calculation formulas and generally accepted values for adrenal adenoma using absolute (A) and relative (B) percentage washout by computed tomography (CT) or chemical shift adrenal signal intensity index (ASII) (C) or adrenal-to-spleen ratio (ASR) (D) magnetic resonance imaging. HU=Hounsfield unit; ROI=region of interest.
Finally, the majority of the adrenal washout literature is centered on the common incidental adrenal tumors. In circumstances where timely diagnosis and treatment are important, adrenalectomy or tissue biopsy may be of more value. Readers who wish to further review recommendations on incidental adrenal masses are encouraged to review the “White Paper” recently published by the American College of Radiology.42
MRI using in-phase and opposed-phase images, known as “chemical-shift MRI,” is an alternative modality to CT for adrenal mass characterization. Based on the presumption that benign adrenal masses contain more intracytoplasmic lipid than malignant, quantitative analysis can be made solely using different adrenal phases over large regions of interest, known as Adrenal Signal Intensity Index or ASII. Despite reports of sensitivity as high as 97% and specificity of 93% with ASII techniques (16.5% threshold value, Fig. 2), this MRI technique can be limited in adenomas that are lipid-poor.43 More modern quantitative techniques compare adrenal to signal intensities of the spleen, known as Adrenal-Spleen Chemical Shift Ratio (ASR), adding slightly more specificity using 0.71 threshold (Fig. 2).43 Better studies in this area are needed because, like PET/CT, many of these series lack pathologic data,43–45 have limited follow-up,43–45 or include patients with portal hypertension that can affect iron content and confound subsequent ASR values within the spleen.45
Percutaneous needle biopsy or FNA of the adrenal can be technically challenging because of the close proximity of the adrenal to the great vessels in the abdomen. Two of the larger series in this area report that about 25% of adrenal sampling cases are not feasible because of anatomic constraints while nondiagnostic biopsies, usually FNA, occur in 8% to 37% of the tissues retrieved.35,46 These nondiagnostic FNA concerns were recently addressed in a study of more than 200 adrenal core biopsies.47 In this selected population of cancer patients, adrenal biopsies positive for metastatic carcinoma were almost always accurate (positive predictive value of 96%). “Benign” biopsies, however, had a negative predictive value of 58%, which led the authors to conclude that a negative large core biopsy may not always rule out malignancy.
Feliciotti and associates48 and others49 have suggested that diagnostic laparoscopy has potential benefits over biopsy alone because it allows the exploration of the entire peritoneal cavity with examination of suspected nodules as well as therapeutic opportunity for resection during the same procedure. Indeed, local resection of a solitary adrenal metastasis has produced a number of long-term survivors in three large trials over the last 30 years.50–52 In these settings, the adrenal metastasis was usually solitary, and the lung lesions were usually at a curable stage I or II. The recent 2013 NCCN panel gave adrenal “metastatectomy” a category 2B rating, stating that “based on the lower level of evidence…the intervention is appropriate.”1 Obviously, a noninvasive, reliable test such as PET-FDG could aide in the preoperative management of patients with a diagnosis of NSCLC by potentially alleviating unnecessary biopsy or abdominal exploration.
How accurate is PET/CT for adrenal NSCLC metastasis?
Pooled results of studies evaluating accuracy of FDG-PET, presented in Table 1, demonstrate high sensitivity and specificity. All seven studies contained cases of adrenal metastasis secondary to NSCLC with 6/7 studies specifying the total number of patients with NSCLC (the lowest was 77%10). Two studies9,11 did not report a specific number of NSCLC cases but focused studies on “lung cancer patients” only. All studies reported interpretation of imaging by board certified radiologists, but all the studies admit to excluding a number of adrenal masses that were either indeterminate over time or lost to radiologic follow-up (selection bias). The more recent studies9,10,12 evaluated adrenal findings with quantitative analysis of SUVmax and SUVratio as well as attenuation in HU. Each of these studies report increased lesion predictability by including SUVmax and SUVratio.
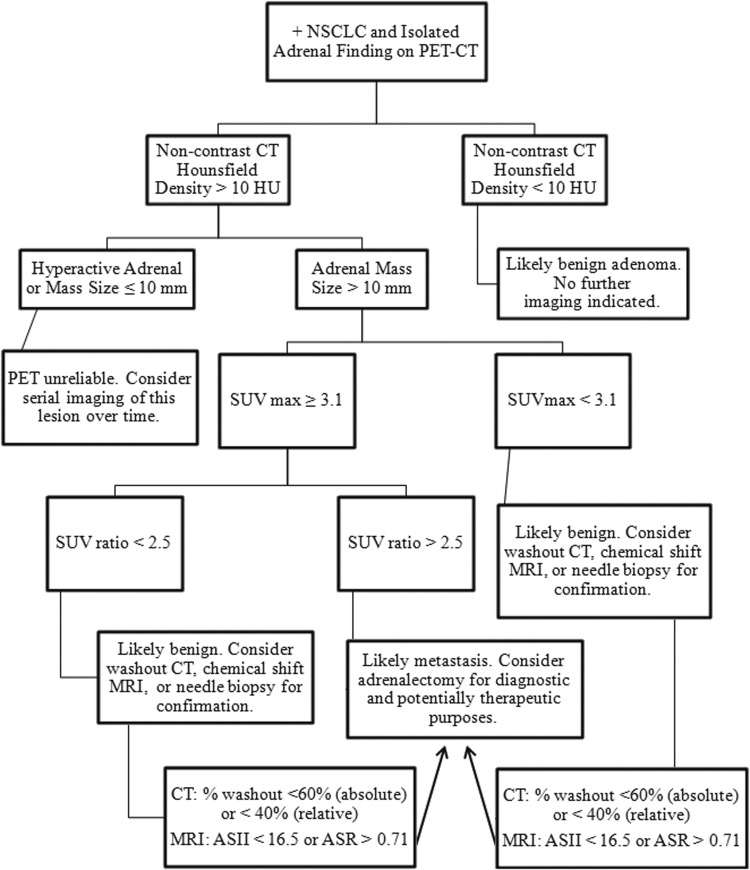
In 2009, Brady and colleagues9 proposed a diagnostic algorithm for adrenal nodules in patients with known or suspected lung cancer. In this study, 187 adrenal nodules were evaluated by diameter, mean attenuation in HU, SUVmax, and SUVratio (nodule SUVmax/liver SUVmax). CT attenuation criteria <10 HU was used to exclude benign, lipid-rich adenomas. In lesions with attenuation greater than 10 HU, PET SUVmax greater than 3.1 and SUVratios greater than 2.5 were further used as cutoffs to identify malignant lesions in their population, maintaining high sensitivity (97.3%) but increasing their sensitivity by more than 30%.9 Similarly, both Okada and coworkers10 and Cho and associates12 reported an increased PET/CT accuracy by using strict SUVmax and SUVratio criteria.
Using these studies, we propose a systematic approach algorithm (Fig. 3) based on HU mean attenuation, SUVmax, and SUVratio thresholds. It is important to note that this algorithm represents pooled studies with false positive and negative rates around 10% to 15%. In addition, all the authors noted that standardization of radiology protocols is extremely important to limit test variability, because SUV itself is a semi-quantitative measure.
FIG. 3.
Systematic algorithm to determine adrenal status in patients with non-small cell lung cancer (NSCLC) based on positron emission tomography/computed tomography (PET/CT) Hounsfield unit (HU) mean attenuation, standard uptake value (SUV)max, and SUVratio thresholds. Other imaging modalities such as washout CT and chemical shift magnetic resonance imaging (MRI) are not a part of the National Comprehensive Care Network guideline staging recommendations but are common clinical radiology practice. All patients should have an endocrine functional work-up of the mass in addition to imaging. Supportive literature is found within the text. ASII=Adrenal Signal Intensity Index; ASR=Adrenal-Spleen Chemical Shift Ratio.
Would this algorithm have changed our patients' care?
Applying this algorithm to our cases, patient #1 (hyperactive adrenal without discrete mass) would have been recommended to have serial imaging of the lesion over time because of the unreliability of a PET scan in masses <10 mm. In patient #2 (12-mm discrete hyperactive mass with SUVmax=6.5), the calculated SUVratio was 2.2. Based on the algorithm, this patient would have been recommended to undergo further characterization (washout CT was performed and mass was indeterminate) or needle biopsy. Interestingly, both of these cases were presented at a multidisciplinary tumor board. Based on the high chance of a nondiagnostic adrenal biopsy and high suspicion of early metastatic detection in Patient #1, the decision was made to proceed with adrenalectomy. The opinion of our interventional radiologist for Patient #2 was that the mass was not amenable to biopsy, and adrenalectomy was recommended—reinforcing the fact that treatment decisions are inevitably tailored to each patient's unique anatomy and circumstance with independent judgment coming from the primary provider and other members of the healthcare team.
Conclusion
FDG-PET/CT is a powerful tool in the field of oncology, improving the clinician's ability to stage a variety of cancer types. For the urologist who is asked to provide tissue diagnosis for patients with NSCLC and adrenal lesions, the literature clearly states that PET/CT testing can be highly sensitive and specific in selected cases. In addition to a good multidisciplinary team, the use of PET/CT with HU, SUVmax, and SUVratio, washout CT, and/or chemical shift MRI can aid in assessing potential cancer risk and in surgical decision making.
Abbreviations Used
- ASII
adrenal signal intensity index
- ASR
adrenal-spleen chemical shift ratio
- FDG
fluorine-18 fluorodeoxyglucose
- FNA
fine needle aspiration
- HU
Hounsfield unit
- MRI
magnetic resonance imaging
- NCCN
National Comprehensive Care Network
- NSCLC
non-small cell lung cancer
- PET/CT
positron-emission tomography/computed tomography
- SUV
standard uptake value
- SUVmax
maximal standard uptake value
- SUVratio
standard uptake value ratio
Disclosure Statement
No competing financial interests exist.
References
- 1.Ettinger DS, Akerley W, Borghaei H, et al. Non-Small Cell Lung Cancer Version 2.2013 2013. 2013 National Comprehensive Care Network Guidelines. Available at: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf Accessed: June12, 2013 [DOI] [PubMed]
- 2.Maziak DE, Darling GE, Inculet RI, et al. Positron emission tomography in staging early lung cancer: A randomized trial. Ann Intern Med 2009;151:221–228 [DOI] [PubMed] [Google Scholar]
- 3.Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med 2009;361:32–39 [DOI] [PubMed] [Google Scholar]
- 4.De Wever W, Stroobants S, Coolen J, et al. Integrated PET/CT in the staging of nonsmall cell lung cancer: Technical aspects and clinical integration. Eur Respir J 2009;33:201–212 [DOI] [PubMed] [Google Scholar]
- 5.Bockisch A, Freudenberg LS, Schmidt D, et al. Hybrid imaging by SPECT/CT and PET/CT: Proven outcomes in cancer imaging. Semin Nucl Med 2009;39:276–289 [DOI] [PubMed] [Google Scholar]
- 6.Erasmus JJ, Patz EF, Jr, McAdams HP, et al. Evaluation of adrenal masses in patients with bronchogenic carcinoma using 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol 1997;168:1357–1360 [DOI] [PubMed] [Google Scholar]
- 7.Gupta NC, Graeber GM, Tamim WJ, et al. Clinical utility of PET-FDG imaging in differentiation of benign from malignant adrenal masses in lung cancer. Clin Lung Cancer 2001;3:59–64 [DOI] [PubMed] [Google Scholar]
- 8.Sung YM, Lee KS, Kim BT, et al. (18)F-FDG PET versus (18)F-FDG PET/CT for adrenal gland lesion characterization: A comparison of diagnostic efficacy in lung cancer patients. Korean J Radiol 2008;9:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady MJ, Thomas J, Wong TZ, et al. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: A proposal for an efficient diagnostic algorithm. Radiology 2009;250:523–530 [DOI] [PubMed] [Google Scholar]
- 10.Okada M, Shimono T, Komeya Y, et al. Adrenal masses: The value of additional fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) in differentiating between benign and malignant lesions. Ann Nucl Med 2009;23:349–354 [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Xie D, Huang W, et al. 18F-FDG PET/CT in the evaluation of adrenal masses in lung cancer patients. Neoplasma 2010;57:129–134 [DOI] [PubMed] [Google Scholar]
- 12.Cho AR, Lim I, Na II, et al. Evaluation of adrenal masses in lung cancer patients using F-18 FDG PET/CT. Nucl Med Mol Imaging 2011;45:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R, Xiu Y, Yu JQ, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med 2004;45:2058–2062 [PubMed] [Google Scholar]
- 14.Hany TF, Steinert HC, Goerres GW, et al. PET diagnostic accuracy: Improvement with in-line PET-CT system: Initial results. Radiology 2002;225:575–581 [DOI] [PubMed] [Google Scholar]
- 15.Blodgett TM, Meltzer CC, Townsend DW. PET/CT: Form and function. Radiology 2007;242:360–385 [DOI] [PubMed] [Google Scholar]
- 16.von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: Current applications and future directions. Radiology 2006;238:405–422 [DOI] [PubMed] [Google Scholar]
- 17.Metser U, Miller E, Lerman H, Even-Sapir E. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: Characterization and incidence. AJR Am J Roentgenol 2007;189:1203–1210 [DOI] [PubMed] [Google Scholar]
- 18.Culverwell AD, Scarsbrook AF, Chowdhury FU. False-positive uptake on 2-[(1)(8)F]-fluoro-2-deoxy-D-glucose (FDG) positron-emission tomography/computed tomography (PET/CT) in oncological imaging. Clin Radiol 2011;66:366–382 [DOI] [PubMed] [Google Scholar]
- 19.Histed SN, Lindenberg ML, Mena E, et al. Review of functional/anatomical imaging in oncology. Nucl Med Commun 2012;33:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechalekar K, Sharma B, Cook G. PET/CT in oncology—a major advance. Clin Radiol 2005;60:1143–1155 [DOI] [PubMed] [Google Scholar]
- 21.Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics 2004;24:523–543 [DOI] [PubMed] [Google Scholar]
- 22.Pauwels EK, Sturm EJ, Bombardieri E, et al. Positron-emission tomography with [18F]fluorodeoxyglucose. Part I. Biochemical uptake mechanism and its implication for clinical studies. J Cancer Res Clin Oncol 2000;126:549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin EC, Helgans R. Adrenal hyperplasia in Cushing's syndrome demonstrated by FDG positron emission tomographic imaging. Clin Nucl Med 2002;27:516–517 [DOI] [PubMed] [Google Scholar]
- 24.Shimizu A, Oriuchi N, Tsushima Y, et al. High [18F] 2-fluoro-2-deoxy-D-glucose (FDG) uptake of adrenocortical adenoma showing subclinical Cushing's syndrome. Ann Nucl Med 2003;17:403–406 [DOI] [PubMed] [Google Scholar]
- 25.Sancho JJ, Triponez F, Montet X, Sitges-Serra A. Surgical management of adrenal metastases. Langenbecks Arch Surg 2012;397:179–194 [DOI] [PubMed] [Google Scholar]
- 26.NIH State-of-the-Science Statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consens State Sci Statements 2002;19:1–23 [PubMed] [Google Scholar]
- 27.Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: An analysis of the CT literature. AJR Am J Roentgenol 1998;171:201–204 [DOI] [PubMed] [Google Scholar]
- 28.Young WF., , Jr.Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007;356:601–610 [DOI] [PubMed] [Google Scholar]
- 29.Chong S, Lee KS, Kim HY, et al. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: Diagnostic efficacy and interpretation pitfalls. Radiographics 2006;26:1811–1826 [DOI] [PubMed] [Google Scholar]
- 30.McLean K, Lilienfeld H, Caracciolo JT, et al. Management of isolated adrenal lesions in cancer patients. Cancer Control 2011;18:113–126 [DOI] [PubMed] [Google Scholar]
- 31.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29 [DOI] [PubMed] [Google Scholar]
- 32.Quint LE, Tummala S, Brisson LJ, et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg 1996;62:246–250 [DOI] [PubMed] [Google Scholar]
- 33.Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: Recent advances and future directions. Oncologist 2008;13(suppl 1):5–13 [DOI] [PubMed] [Google Scholar]
- 34.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74–85 [DOI] [PubMed] [Google Scholar]
- 35.Burt M, Heelan RT, Coit D, et al. Prospective evaluation of unilateral adrenal masses in patients with operable non-small cell lung cancer. Impact of magnetic resonance imaging. J Thorac Cardiovasc Surg 1994;107:584–589 [PubMed] [Google Scholar]
- 36.Marabella P, Takita H. Adenocarcinoma of the lung: Clinicopathological study. J Surg Oncol 1975;7:205–212 [DOI] [PubMed] [Google Scholar]
- 37.Porte HL, Roumilhac D, Graziana JP, et al. Adrenalectomy for a solitary adrenal metastasis from lung cancer. Ann Thorac Surg 1998;65:331–335 [DOI] [PubMed] [Google Scholar]
- 38.Ettinghausen SE, Burt ME. Prospective evaluation of unilateral adrenal masses in patients with operable non-small-cell lung cancer. J Clin Oncol 1991;9:1462–1466 [DOI] [PubMed] [Google Scholar]
- 39.Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol 1998;170:747–752 [DOI] [PubMed] [Google Scholar]
- 40.Caoili EM, Korobkin M, Francis IR, et al. Delayed enhanced CT of lipid-poor adrenal adenomas. AJR Am J Roentgenol 2000;175:1411–1415 [DOI] [PubMed] [Google Scholar]
- 41.Caoili EM, Korobkin M, Francis IR, et al. Adrenal masses: Characterization with combined unenhanced and delayed enhanced CT. Radiology 2002;222:629–633 [DOI] [PubMed] [Google Scholar]
- 42.Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: White paper of the ACR incidental findings committee. J Am Coll Radiol 2010;7:754–773 [DOI] [PubMed] [Google Scholar]
- 43.Halefoglu AM, Yasar A, Bas N, et al. Comparison of computed tomography histogram analysis and chemical-shift magnetic resonance imaging for adrenal mass characterization. Acta Radiol 2009;50:1071–1079 [DOI] [PubMed] [Google Scholar]
- 44.Fujiyoshi F, Nakajo M, Fukukura Y, Tsuchimochi S. Characterization of adrenal tumors by chemical shift fast low-angle shot MR imaging: Comparison of four methods of quantitative evaluation. AJR Am J Roentgenol 2003;180:1649–1657 [DOI] [PubMed] [Google Scholar]
- 45.Sandrasegaran K, Patel AA, Ramaswamy R, et al. Characterization of adrenal masses with diffusion-weighted imaging. AJR Am J Roentgenol 2011;197:132–138 [DOI] [PubMed] [Google Scholar]
- 46.Fassina AS, Borsato S, Fedeli U. Fine needle aspiration cytology (FNAC) of adrenal masses. Cytopathology 2000;11:302–311 [DOI] [PubMed] [Google Scholar]
- 47.Villelli NW, Jayanti MK, Zynger DL. Use and usefulness of adrenal core biopsies without FNA or on-site evaluation of adequacy: A study of 204 cases for a 12-year period. Am J Clin Pathol 2012;137:124–131 [DOI] [PubMed] [Google Scholar]
- 48.Feliciotti F, Paganini AM, Guerrieri M, et al. Laparoscopic anterior adrenalectomy for the treatment of adrenal metastases. Surg Laparosc Endosc Percutan Tech 2003;13:328–333 [DOI] [PubMed] [Google Scholar]
- 49.Beitler AL, Urschel JD, Velagapudi SR, Takita H. Surgical management of adrenal metastases from lung cancer. J Surg Oncol 1998;69:54–57 [DOI] [PubMed] [Google Scholar]
- 50.Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: A systematic review and pooled analysis. J Clin Oncol 2008;26:1142–1147 [DOI] [PubMed] [Google Scholar]
- 51.Raviv G, Klein E, Yellin A, et al. Surgical treatment of solitary adrenal metastases from lung carcinoma. J Surg Oncol 1990;43:123–124 [DOI] [PubMed] [Google Scholar]
- 52.Reyes L, Parvez Z, Nemoto T, et al. Adrenalectomy for adrenal metastasis from lung carcinoma. J Surg Oncol 1990;44:32–34 [DOI] [PubMed] [Google Scholar]