Abstract
Aims: The best-established mechanism of opioid dependence is the up-regulation of adenylate cyclase (AC)/cAMP pathway, which was reported to be negatively regulated by hydrogen sulfide (H2S), a novel endogenous neuromodulator. The present study was, therefore, designed to determine whether H2S is able to attenuate the development of opioid dependence via down-regulating AC/cAMP pathway. Results: We demonstrated that application of sodium hydrosulphide (NaHS) and GYY4137, two donors of H2S, significantly alleviated naloxone-induced robust withdrawal jumping (the most sensitive and reliable index of opioid physical dependence) in morphine-treated mice. Repeated treatment with NaHS inhibited the up-regulated protein expression of AC in the striatum of morphine-dependent mice. Furthermore, NaHS also attenuated morphine/naloxone-elevated mRNA levels of AC isoform 1 and 8, production of cAMP, and phosphorylation of cAMP response element-binding protein (CREB) in mice striatum. These effects were mimicked by the application of exogenous H2S or over-expression of cystathione-β-synthase, an H2S -producing enzyme, in SH-SY5Y neuronal cells on treatment with [D-Ala2,N-Me-Phe4,Gly5-ol]-Enkephalin, a selective μ-opioid receptor agonist. Blockade of extracellular-regulated protein kinase 1/2 (ERK1/2) with its specific inhibitor attenuated naloxone-induced CREB phosphorylation. Pretreatment with NaHS or stimulation of endogenous H2S production also significantly suppressed opioid withdrawal-induced ERK1/2 activation in mice striatum or SH-SY5Y cells. Innovation: H2S treatment is important in prevention of the development of opioid dependence via suppression of cAMP pathway in both animal and cellular models. Conclusion: Our data suggest a potential role of H2S in attenuating the development of opioid dependence, and the underlying mechanism is closely related to the inhibition of AC/cAMP pathway. Antioxid. Redox Signal. 20, 31–41.
Introduction
Opioids produce analgesia in both the central nervous system (CNS) and the periphery. Repeated exposure to opioids leads to the development of dependence, which can be assessed by observing the emergence of a withdrawal syndrome after discontinuation of chronic opioid administration (spontaneous withdrawal) or the administration of a competitive opioid antagonist such as naloxone (precipitated withdrawal) (28). Withdrawal-induced symptoms are the main cause of keeping drug-dependent individuals craving for continued opioids. So, the manner of relieving withdrawal syndrome is very important to prevent the development of opioid dependence.
It has been known that the best-established mechanism underlying the development of opioid dependence and withdrawal is the up-regulation of cAMP pathway (30). Persistent activation of opioid receptors after chronic opioid exposure results in supersensitivity of the adenylate cyclase (AC) system (48). The mechanisms for AC supersensitivity include up-regulation of AC protein expression (40), AC stimulation by Gβγ (1), and AC phosphorylation by various protein kinases (2). AC supersensitivity may further induce alteration in the transcription factor cAMP response element-binding protein (CREB) in a variety of neuronal cell lines (16, 48) as well as distinct areas of the CNS (12, 31). Previous studies demonstrated that down-regulation of the cAMP pathway via infusion of its inhibitors into certain brain regions or knockout-specific AC isotypes such as AC1 and AC8 reduced opioid dependence by attenuation of the withdrawal symptoms in rodents (36, 49).
Innovation.
Hydrogen sulfide (H2S) has been demonstrated to be a novel endogenous neuromodulator, and it plays a role in the regulation of adenylate cyclase (AC)/cAMP pathway. Until now, the role of H2S in the development of opioid dependence has not been investigated. We show here that H2S significantly attenuates the development of opioid dependence via suppression of AC/cAMP/CREB pathway in both animal and cellular models. These findings provide a potential molecular target for pharmacological intervention to relieve withdrawal-induced symptoms and prevent the development of opioid dependence.
Hydrogen sulfide (H2S), a new biological gaseous transmitter alongside nitric oxide and carbon monoxide, is mainly synthesized by cystathione-β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3-MST) in the CNS (14). It presents in the brain at a relatively high level and may function as a neuromodulator (7, 15). We previously reported that sodium hydrosulphide (NaHS, a donor of H2S) significantly suppressed AC activity and, therefore, decreased forskolin-stimulated cAMP accumulation in different cell lines and tissues (25, 27, 47). It suggests that H2S plays a role in the down-regulation of AC/cAMP pathway. This prompts us to examine whether H2S produces any effect on preventing the development of opioid dependence via suppression of the AC/cAMP pathway.
In this study, we examined the role of H2S in the development of opioid dependence in both cellular and animal models. We demonstrated that administration of NaHS and GYY4137 markedly attenuated naloxone-precipitated withdrawal jumping (the most sensitive and reliable index of opioid physical dependence) in morphine-dependent mice. We also found that repeated treatment with NaHS significantly inhibited the up-regulation of AC expression, cAMP accumulation, and CREB phosphorylation in the mice striatum after chronic morphine administration. In addition, similar results were also found in SH-SY5Y neuronal cells on application of exogenous H2S or stimulation of endogenous H2S production. These results suggest a potential role of H2S in prevention of the development of opioid dependence and that is closely related to the inhibition effect of H2S on the cAMP pathway.
Results
H2S donors treatment attenuates naloxone-precipitated withdrawal jumping in morphine-dependent mice
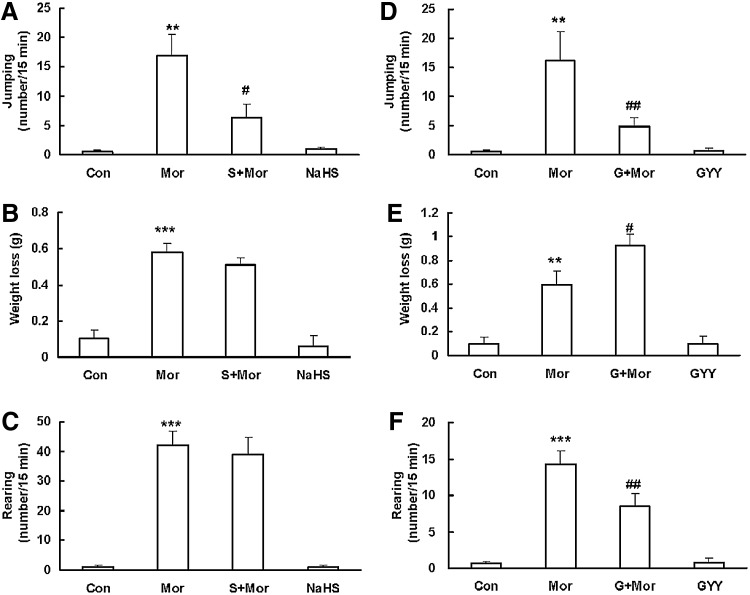
Among withdrawal behaviors in morphine-dependent rodents (especially mice), jumping is widely considered the most sensitive and reliable index, and it is commonly used to test the state of morphine physical dependence (30). To set up a chronic morphine dependence mouse model, animals received morphine (20, 30, 40, 50 mg/kg) injection twice a day for 4 days and once (20 mg/kg) on Day 5. An injection of naloxone (an opioid antagonist, 10 mg/kg) at 4 h after the last injection of morphine induced robust withdrawal jumping in morphine-dependent mice (Mor, n=11). The withdrawal jumping was obviously attenuated by the treatment with NaHS (5.6 mg/kg) 30 min before each injection of morphine (S+Mor, n=11) (Fig. 1A). We also observed other withdrawal symptoms such as weight loss (Fig. 1B) and rearing (Fig. 1C). However, we failed to find that NaHS alone produced a significant effect on these symptoms.
FIG. 1.
Effect of NaHS and GYY4137 on naloxone-induced withdrawal behaviors in morphine-dependent mice. (A, D) jumping; (B, E) weight loss; (C, F) rearing. Mean±S.E.M, n=6–11. **p<0.01, ***p<0.001, versus Con; #p<0.05, ##p<0.01, versus Mor. Con, control; Mor, morphine; S, NaHS; G, GYY4137; NaHS, sodium hydrosulphide.
We also tested the effect of GYY4137 (a slow-releasing H2S donor) on the development of morphine dependence. It was found that GYY4137 significantly alleviated naloxone-induced robust withdrawal jumping (Fig. 1D) and rearing (Fig. 1F) in morphine-treated mice (G+Mor, n=10). We also observed a clear trend that the duration of withdrawal jumping in the GYY-pretreated group (72.9±26.2 s) was much shorter than that of the morphine-treated group (233.6±77.9 s), although no significant difference was found. However, it is worth noting that GYY4137 may also produce some potential adverse effects on the body weight (Fig. 1E). GYY4137 may further decrease the body weight of mice due to diarrhea on morphine withdrawal. More studies are warranted to test the mechanisms for this gastrointestinal adverse effect of GYY4137. Nonetheless, these results further support a potential role of H2S in attenuating the development of opioid dependence and withdrawal.
NaHS treatment attenuates the upregulation of AC expression in the striatum of the morphine-dependent mice
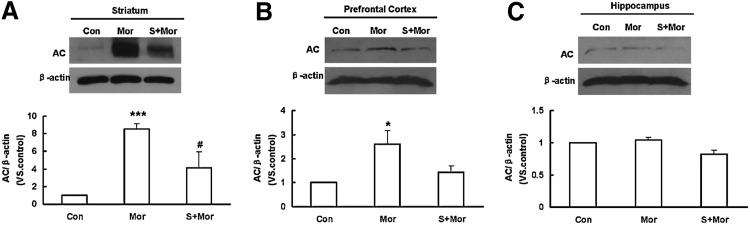
Repeated morphine treatment causes the up-regulation of AC/cAMP pathway in certain brain regions, such as striatum, prefrontal cortex, and hippocampus, and these neuroadaptive changes in the brain form the neurobiological basis for morphine dependence and withdrawal (30). In the present study, we found that chronic morphine treatment markedly elevated the expression of AC protein (150–180 KDa) in the striatum and prefrontal cortex (Fig. 2A, B), but not in the hippocampus (Fig. 2C). The up-regulation of AC in the striatum was markedly attenuated by NaHS pretreatment (Fig. 2A). Although there was the same trend in the prefrontal cortex, no significant difference was found (Fig. 2B). In a separate experiment, NaHS alone had no significant effects on the expression of AC protein in the above three brain regions (data not shown).
FIG. 2.
Effect of NaHS pretreatment on AC protein expression in the brain regions of morphine-treated mice. (A) striatum; (B) prefrontal cortex; (C) hippocampus. Mean±S.E.M, n=3–4. *p<0.05, ***p<0.001, versus Con; #p<0.05, versus Mor. Con, control; Mor, morphine; S, NaHS; AC, adenylate cyclase.
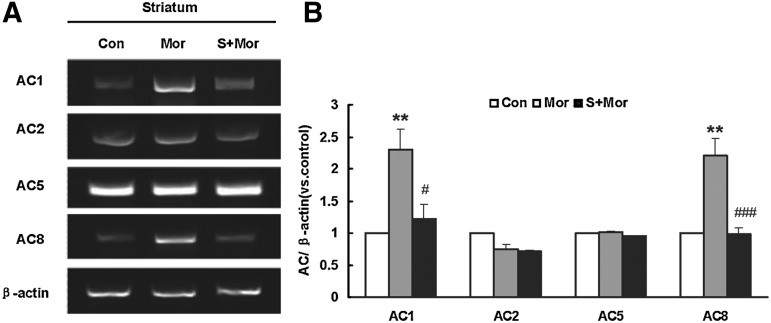
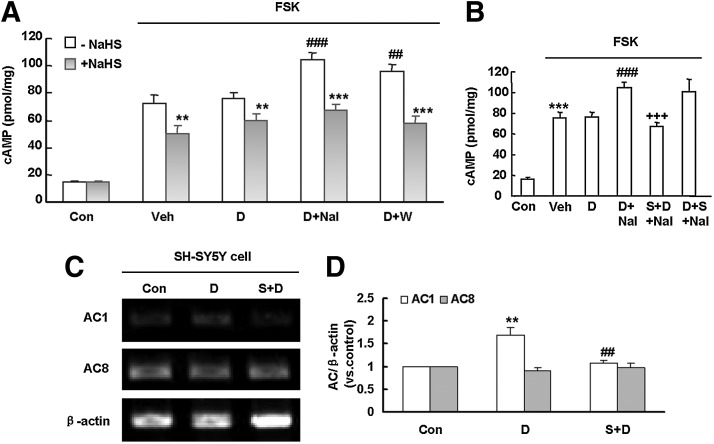
Since the effect of morphine was more obvious in the striatum, we then detected the mRNA expression of different AC isotypes in the striatum with reverse transcription-polymerase chain reaction (RT-PCR). As shown in Figure 3A and B, the mRNA expression levels for AC1 and AC8, but not AC2 and AC5, were significantly increased in the striatum of morphine-dependent mice. NaHS pretreatment almost abolished the up-regulated AC1 and AC8 expression. In a separate experiment, NaHS alone had no significant effects on the mRNA levels of all above AC isotypes (data not shown).
FIG. 3.
Effect of NaHS pretreatment on AC isoforms mRNA expression in the striatum of morphine-treated mice. (A) Representative gel images. (B) Quantifications by densitometric measurement. Mean±S.E.M, n=4. **p<0.01, versus Con; #p<0.05, ###p<0.001, versus Mor. Con, control; Mor, morphine; S, NaHS.
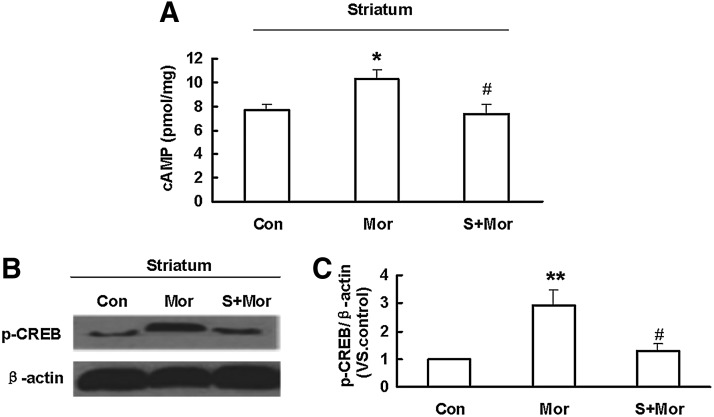
NaHS treatment inhibits cAMP accumulation and CREB phosphorylation in the striatum of morphine-dependent mice
We continued to examine the effect of H2S on cAMP accumulation in mice striatum. As shown in Figure 4A, morphine withdrawal induced cAMP elevation in the striatum of morphine-treated mice. This effect was reversed by pretreatment with NaHS. These data suggest that H2S may inhibit cAMP accumulation in special brain region via suppression of morphine-up-regulated AC expression.
FIG. 4.
NaHS treatment attenuated morphine-induced elevation of cAMP level and CREB phosphorylation in the striatum of mice. (A) Effect of NaHS on cAMP production in the striatum of morphine-treated mice. Mean±S.E.M, n=9. (B, C) Effect of NaHS pretreatment on the up-regulation of CREB phosphorylation induced by naloxone-precipitated withdrawal in mice striatum. Mean±S.E.M, n=4. *p<0.05, **p<0.01, versus Con; #p<0.05, versus Mor. Con, control; Mor, morphine; S, NaHS; CREB, cAMP response element-binding protein.
CREB is an important transcription factor in mediating the up-regulation of the cAMP pathway that is associated with opioid dependence (6, 45). Elevation of cAMP production triggers the phosphorylation of CREB. We then examined CREB phosphorylation in the striatum of morphine-dependent mice. As shown in Figure 4B and C, the expression of p-CREB protein was markedly increased in the morphine-treated group, and the effect was abolished by NaHS pretreatment.
Application of exogenous H2S or stimulation of endogenous H2S production inhibits cAMP rebound and AC up-regulation caused by DAMGO withdrawal in SH-SY5Y cells
To study the signaling mechanism, we established a cellular model with SH-SY5Y neuronal cells. SH-SY5Y cells were treated with [D-Ala2,N-Me-Phe4,Gly5-ol]-Enkephalin (DAMGO, a selective μ-opioid receptor agonist, 10 μM) for 24 h followed by the addition of naloxone (100 μM) (D+Nal) or washout (D+W). We found that both naloxone precipitation and washout rapidly and markedly elevated cAMP level (a hallmark of opioid withdrawal in the cell), and these effects were prevented by pretreatment with NaHS (10 μM) for 10 min before the addition of DAMGO (Fig. 5A). However, naloxone-induced cAMP rebound cannot be abolished by the application of NaHS 30 min before naloxone administration (D+S+Nal) (Fig. 5B). We further examined the mRNA expression of AC1 and AC8 in DAMGO-treated SH-SY5Y cells via RT-PCR. As shown in Figure 5C and D, mRNA expression of AC1 but not AC8 was markedly up-regulated after chronic DAMGO treatment (10 μM, 24 h), and this effect was prevented by NaHS pretreatment.
FIG. 5.
Effect of NaHS on cAMP rebound, AC mRNA levels in SH-SY5Y cells treated with DAMGO. (A) NaHS pretreatment attenuated cAMP rebound induced by opioid withdrawal in SH-SY5Y cells. Cells were treated with DAMGO (10 μM) for 24 h followed by addition of naloxone (100 μM) (D+Nal) or washing the cell with low-serum medium (D+W) to induce opioid withdrawal. NaHS (10 μM) was given 10 min before the addition of DAMGO. Mean±S.E.M., n=6–12. **p<0.01, ***p<0.001, versus the corresponding values without NaHS treatment in the same group; ##p<0.01, ###p<0.001, versus D (DAMGO alone without NaHS treatment). (B) Naloxone-induced cAMP rebound was abolished by NaHS (10 μM) given 10 min before the addition of DAMGO (S+D+Nal), but not by NaHS given 30 min before the addition of naloxone (D+S+Nal). Mean±S.E.M, n=6. ***p<0.001, versus Con; ###p<0.001, versus D;+++p<0.001, versus D+Nal. (C, D) NaHS pretreatment abolished DAMGO-induced up-regulation of mRNA level of AC1 and AC8 in SH-SY5Y cells. Mean±S.E.M, n=4–6. **p<0.01, versus Con; ##p<0.01, versus D. Con, control; FSK, forskolin; S, NaHS; D, DAMGO; Nal, naloxone; Veh, vehicle; DAMGO, [D-Ala2,N-Me-Phe4,Gly5-ol]-Enkephalin.
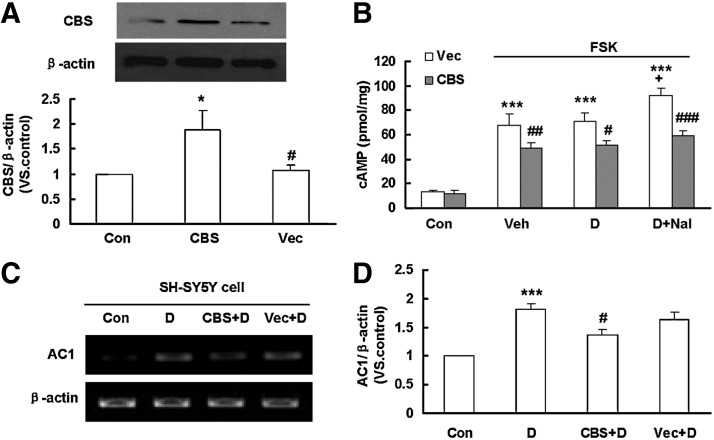
To examine the role of endogenous H2S, SH-SY5Y cells were transfected with the cDNAs of CBS, the main enzyme to produce H2S in these cells, or its empty vector (PME185-HA). The result showed that the expression of CBS in the CBS-transfected group was significantly higher than that in the control group and vector-transfected group (Fig. 6A). Over-expression of CBS, but not its vector, significantly suppressed forskolin-induced cAMP production (Fig. 6B). Interestingly, administration of naloxone failed to induce cAMP rebound in the CBS-transfected SH-SY5Y cells (Fig. 6B). These data suggest that endogenous H2S is important in the regulation of intracellular cAMP level and naloxone-induced cAMP rebound.
FIG. 6.
Effect of endogenous H2S on cAMP rebound, AC mRNA levels in SH-SY5Y cells treated with DAMGO. (A) Western blotting analysis showing the protein expression of CBS protein in SH-SY5Y cells transfected with CBS-PME185-HA vector or its empty vector. Mean±S.E.M., n=6. *p<0.05, versus Con; #p<0.05, versus CBS. (B) Over-expression of CBS, but not its empty vector, reduced cAMP production in either forskolin-stimulated, chronic DAMGO-treated, or naloxone-precipitated cells. Mean±S.E.M., n=5–14. ***p<0.001, versus Con; #p<0.05, ##p<0.01, ###p<0.001, versus the corresponding values without NaHS treatment in the same group. (C, D) Effects of CBS over-expression on DAMGO-induced AC1 expression in SH-SY5Y cells. Mean±S.E.M, n=5. ***p<0.001, versus Con; #p<0.05, versus D. Con, control; FSK, forskolin; S, NaHS; D, DAMGO; Nal, naloxone; Veh, vehicle; Vec, vector; CBS, cystathione-β-synthase; H2S, hydrogen sulfide.
Similar to what we observed earlier, we found that over-expression of CBS, but not its vector, significantly suppressed DAMGO-induced AC1 up-regulation (Fig. 6C, D). The data also confirmed the inhibitory effect of H2S on AC expression.
Application of exogenous H2S or stimulation of endogenous H2S production attenuates CREB phosphorylation in DAMGO-treated SH-SY5Y cells
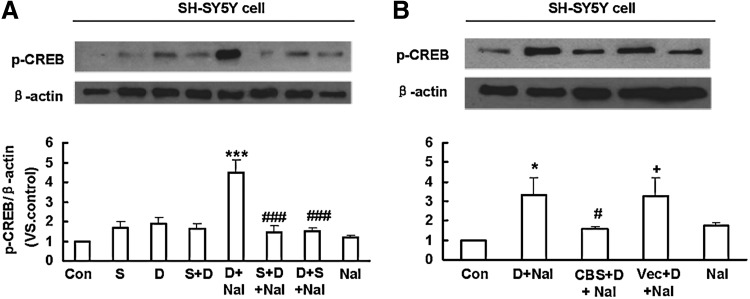
In SH-SY5Y cells, CREB was also significantly phosphorylated after naloxone-precipitated withdrawal (D+Nal) (Fig. 7A). Interestingly, the up-regulated CREB phosphorylation caused by naloxone precipitation was abolished by NaHS treatment before DAMGO administration (S+D+Nal) or before naloxone administration (D+S+Nal) (Fig. 7A). Moreover, over-expression of CBS, but not its empty vector, suppressed the up-regulated CREB phosphorylation induced by naloxone precipitation (Fig. 7B).
FIG. 7.
Western blotting analysis showing the effect of NaHS on the up-regulation of CREB phosphorylation induced by opioid withdrawal in SH-SY5Y cells. (A) The up-regulation of CREB phosphorylation caused by DAMGO withdrawal was attenuated by NaHS treatment. Mean±S.E.M, n=7. (B) DAMGO-induced CREB phosphorylation was suppressed by over-expression of CBS but not its empty vector. Mean±S.E.M., n=4. *p<0.05, ***p<0.001 versus Con; #p<0.05, ###p<0.001, versus D+Nal; +p<0.05, versus CBS+D+Nal. Con, control; S, NaHS; D, DAMGO; Nal, naloxone; Vec, vector.
Application of exogenous H2S or stimulation of endogenous H2S production suppresses ERK1/2 activation induced by opioid withdrawal in both cellular and animal models
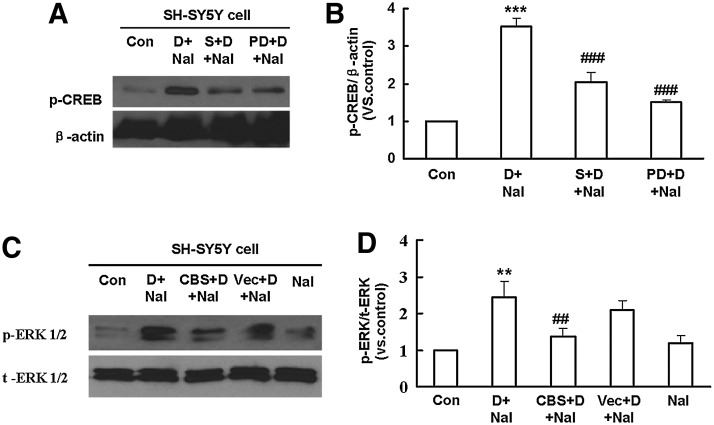
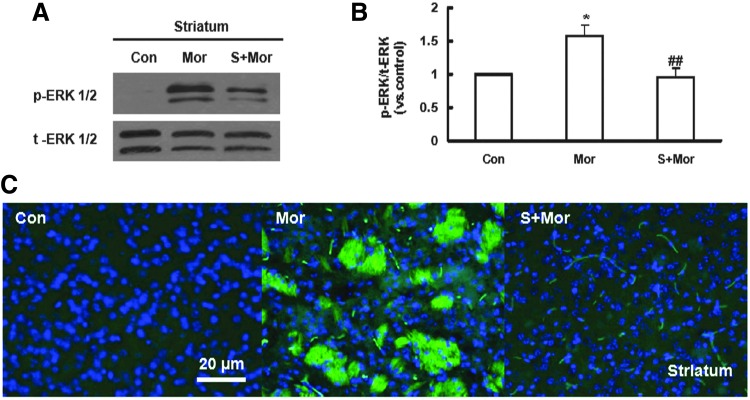
Extracellular regulated protein kinases (ERKs) are a family of serine/threonine protein kinases that play pivotal roles in opioid dependence signal transduction (33). To investigate the role of ERK1/2 in the signaling mechanism underlying the regulation of CREB phosphorylation, PD 98059, a specific ERK1/2 inhibitor, was applied into SH-SY5Y cells before administration of DAMGO. As shown in Figure 8A and B, naloxone-induced CREB phosphorylation was significantly attenuated by pretreatment with NaHS or PD 98059 (10 μM, 30 min). Furthermore, naloxone precipitation-induced up-regulation of p-ERK1/2 was blocked in CBS-transfected SH-SY5Y cells, but not in the empty vector-transfected group (Fig. 8C, D). These results suggest an inhibitory effect of endogenous H2S on ERK1/2 activation in opioid withdrawal. In addition, both Western blot and immunofluorescence analysis revealed that the phosphorylation of ERK1/2 was markedly up-regulated in the striatum of morphine-dependent mice, and this effect was significantly abolished by NaHS pretreatment (Fig. 9). However, in a separate experiment, NaHS alone failed to produce any significant effect on the expression of p-ERK1/2 in mice striatum (data not shown).
FIG. 8.
Role of ERK1/2 in CREB phosphorylation in DAMGO-treated SH-SY5Y cells. (A, B) Western blots showing the effects of NaHS and inhibition of ERK1/2 on naloxone-induced CREB phosphorylation in SH-SY5Y cells. Mean±S.E.M, n=4. ***p<0.001, versus Con; ###p<0.001, versus D+Nal. (C, D) Western blots showing the effect of CBS over-expression on the up-regulated p-ERK1/2 induced by naloxone-precipitated withdrawal in SH-SY5Y cells. Mean±S.E.M, n=4. **p<0.01, versus Con; ##p<0.01, versus D+Nal. Con, control; S, NaHS; D, DAMGO; Nal, naloxone; PD, PD98059; t-ERK, total ERK; ERK1/2, extracellular regulated protein kinase 1/2.
FIG. 9.
Effect of NaHS on ERK1/2 phosphorylation in the striatum of morphine-dependent mice. (A, B) Western blots showing the effects of NaHS pretreatment on naloxone-induced ERK1/2 phosphorylation in mice striatum. Mean±S.E.M, n=8. *p<0.05, versus Con; ##p<0.01, versus Mor. (C) Immunostaining showing the effect of NaHS administration on the expression of p-ERK1/2 in mice striatum. Green: immunostaining of p-ERK1/2; Blue: the nuclei of neurons stained with Hoechst 33342. Con, control group receiving saline; Mor, morphine-treated group; S+Mor, NaHS-pretreated morphine group. Scale bar=20 μm. Con, control; S, NaHS; Mor, morphine. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Discussion
In the present study, we demonstrated that administration of H2S donors such as NaHS and GYY4137 before a morphine injection significantly attenuated naloxone-precipitated withdrawal jumping, the most sensitive and reliable index of withdrawal intensity, in morphine-dependent mice (17). In addition to suppression of withdrawal jumping, GYY4137 also significantly attenuated rearing symptom development. These findings suggest that H2S slow-releasing compound may produce better effects. Taken together, our data suggest a potential role of H2S in attenuating the development of opioid dependence.
Opioid dependence results from adaptive changes in signal transduction networks in several brain regions, including prefrontal cortex, hippocampus, and striatum. One of the most robust adaptations to repeated opioid exposure is the supersensitivity of AC, which catabolizes ATP to cAMP (38, 45). cAMP is a ubiquitous cellular second messenger that is responsible for many biological processes. As an endogenous gaseous mediator, H2S suppresses AC activity in various cell lines and tissues (25, 27, 47) and plays important roles in regulation of various signaling pathways. Previous studies demonstrated that down-regulation of the cAMP pathway via infusion of its inhibitors into certain brain region significantly attenuated morphine withdrawal behaviors (36). These findings prompt us to investigate whether H2S is also able to block the development of opioid dependence via down-regulating the AC/cAMP pathway.
Interestingly, in the present study, we found that repeated morphine treatment significantly increased AC protein expression in the striatum and prefrontal cortex, but not in the hippocampus. These data suggest that morphine treatment may up-regulate AC expression in certain brain regions. This is consistent with previous findings (40, 43). Since μ-opioid receptor is considered crucial in the development of opioid dependence and confirmed to be most densely localized in patches in the striatum, as well as layers I and III of the cortex (42), these data imply that the region-specific response induced by morphine may be related to the different distribution of opioid receptor subtypes in the brain. In the present study, we found that pretreatment with NaHS significantly attenuated the up-regulation of AC protein, which was further confirmed by examination of cAMP level. These data indicate that H2S may inhibit the up-regulation of AC/cAMP pathway in certain brain regions caused by repeated morphine treatment.
To confirm this, we also examined the effect of H2S in SH-SY5Y neuron cells treated with DAMGO, a specific μ-receptor agonist. We found that NaHS treatment before DAMGO administration dramatically inhibited the cAMP rebound induced by naloxone-precipitated withdrawal in the cell. This is consistent with our findings in morphine-dependent mice. Moreover, over-expression of CBS, a main H2S generating enzyme in the brain, also abolished the cAMP rebound induced by naloxone-precipitation in SH-SY5Y cells. These data confirm that alteration of endogenous H2S level is important to attenuate the development of opioid dependence and withdrawal.
ACs are the family of enzymes to produce cAMP. There are nine membrane-bound and one soluble forms, each with distinct regulation and expression patterns (34). We, therefore, continued to study which isoform(s) is/are involved. To date, three specific AC isoforms such as AC1, AC5, and AC8 have been found to be involved in these adaptive processes associated with opioid dependence and withdrawal (35). In the brain, AC5 is predominantly expressed within the striatum, and it has reported that withdrawal symptoms were markedly attenuated in AC5 knockout mice (10, 18). Knockout AC1 and AC8 reduced opioid dependence by attenuation of the withdrawal symptoms, especially for withdrawal jumping (49). This was especially obvious when AC8 gene was deleted (49). These findings imply that there is a close relationship between AC/cAMP pathway and the development of withdrawal jumping symptom. This may help in explaining why NaHS produced a stronger effect to alleviate jumping over rearing symptom. Since AC2 is expressed primarily in the brain (8) and is regulated by opioid receptors (3), we also detected the expression of AC2 in mice striatum. In this study, we found that repeated treatment with morphine up-regulated the mRNA levels of AC1 and AC8 but not those of AC2 and AC5. However, in SH-SY5Y cells, chronic DAMGO treatment only up-regulated AC1 mRNA level, but not AC8. It suggests that opiate-induced AC superactivation is isozyme specific. Nonetheless, the up-regulated mRNA levels of AC isoforms either in the striatum of morphine-dependent mice or in DAMGO-treated SH-SY5Y cells were obviously attenuated by application of NaHS or stimulation of endogenous H2S in the cell. These findings offer a strong support that H2S may block opioid withdrawal by suppression of AC/cAMP pathway in the brain.
Chronic opioid administration induces changes in the expression and function of CREB, which may contribute to withdrawal behaviors and neural adaptations that are associated with opioid dependence (12, 21, 39). Moreover, there is a strong reduction of naloxone-induced withdrawal behaviors in mice lacking CREB isoforms (13). In the present study, we found that CREB was markedly phosphorylated on naloxone-precipitated withdrawal in mice striatum or in SH-SY5Y cells after chronic opioid exposure. These data suggest that modulation of CREB is an important mechanism underlying the development of opioid dependence. Furthermore, we found that the elevation of CREB phosphorylation was reversed by application of exogenous H2S or stimulation of endogenous H2S production. This result suggests that the beneficial effect of H2S on opioid withdrawal is also mediated by the down-regulation of CREB phosphorylation.
CREB is a nuclear protein that modulates the transcription of genes with cAMP responsive elements (CRE) in their promoters. On the one hand, elevation of cAMP production triggers the phosphorylation of CREB. On the other hand, CREB may also up-regulate the expression of proteins contributing to AC supersensitivity in the development of opioid dependence (21, 29). There is a putative CRE sequence in AC8 gene promoter. This may explain the induction of AC8 expression during chronic administration of morphine (4). In addition, an injection of CREB antisense oligonucleotide into specific regions of the brain, such as locus coeruleus (important to the incidence of physical dependence), attenuated the induction of AC8 and the expression of withdrawal behavior (21). In our study, we found that both AC8 expression and CREB phosphorylation were significantly elevated in the striatum of morphine-dependent mice, and NaHS administration abolished all these effects. Therefore, we presume that the reduction of withdrawal behavior by administration of NaHS might, at least partly, be attributed to the suppression of AC8 expression via down-regulation of CREB phosphorylation. Interestingly, in the present study, we found that chronic morphine treatment also up-regulated AC1, whose gene promoter lacks functional CRE elements. More experiments are warranted to study the mechanism of the up-regulation of AC1 stimulated by chronic opioid exposure.
Activation of some protein kinases, such as ERK1/2, may phosphorylate transcription factors such as CREB to regulate the target gene expression involved in the development of opioid dependence (26). ERKs are a family of serine/threonine protein kinases, and ERK1/2 is mostly expressed in the brain (33). It was demonstrated that naloxone-precipitated withdrawal may activate ERK1/2 pathway in the distinct brain regions of opioid-dependent rodents (24, 33, 37). A previous study showed that ERK inhibition or knockdown in rat spinal cord significantly reduced the phosphorylation of CREB caused by naloxone-precipitated withdrawal (11). In this study, we found that naloxone-precipitated withdrawal activated ERK1/2 in mice striatum or in SH-SY5Y cells, and these effects were obviously attenuated by NaHS pretreatment or stimulation of endogenous H2S production in the cell. Moreover, blockade of ERK1/2 significantly attenuated CREB phosphorylation induced by opioid withdrawal in SH-SY5Y cells. These data suggest that the beneficial effects of H2S on opioid withdrawal are closely related to the inhibition of protein kinases such as ERK1/2, which are important to the regulation of cAMP/CREB pathway.
However, it is interesting to note that the effects of H2S on cAMP production are varied among different cell types or tissues. Kimura reported that acute application of NaHS (5 min) increased the production of cAMP in some brain cells, but had no effect on neuroblastoma B103 and B104 cells (19). Another group also reported that H2S increased cAMP production in neural retina only when COX-2 inhibitors were given (32). In the absence of COX-2 inhibitors, H2S actually decreased cAMP production. These findings suggest that the stimulatory effect of H2S on cAMP production is secondary to other signaling pathways (e.g., COX-2). Therefore, the effect of H2S on cAMP production may be influenced by many factors, which may include different cell types, AC isoforms, AC activity status, treatment period with H2S, and activation of other signaling pathways in different pathological situations. This may also help in explaining why application of NaHS right before naloxone administration failed to prevent opioid withdrawal symptoms in morphine-treated mice (data not shown) and cAMP overshoot in SH-SY5Y cells. More studies are warranted to investigate the accurate regulatory effect of H2S on AC activity in different pathological situations.
In conclusion, in the present study, we demonstrated that H2S is important in the regulation of opioid dependence and withdrawal. This effect is mediated by inhibition of AC/cAMP/CREB pathway. Our findings suggest that H2S may be a new potential therapeutic target for the treatment of opioid addiction.
Materials and Methods
Chronic morphine dependence mouse model
Male BALB/c mice (20–25 g) were randomly divided into the following groups: control group receiving saline (Con), morphine-treated group (Mor), and NaHS-pretreated morphine group (S+Mor). Morphine sulphate (Hameln pharmaceuticals gmbh, Hameln, Germany) was injected subcutaneously twice daily at 12 h intervals (8:00 and 20:00) for 4 days with increasing doses (20, 30, 40, and 50 mg/kg) on each day. On Day 5, all animals received a single subcutaneous injection of morphine (20 mg/kg) at 8:00 (5). For the NaHS-pretreated group, animals received injections of NaHS (100 μmol/kg, equivalent to 5.6 mg/kg, i.p.; Sigma, St. Louis, MO) 30 min before each morphine injection. For GYY4137 experiments, male Kunming mice (20–25 g) were used. GYY4137 was synthesized in house as described in the previous publication (9, 23). For the GYY-pretreated group, GYY4137 (100 mg/kg, i.p.) was injected once daily for 3 days before the beginning of morphine treatment. During the morphine treatment period, GYY4137 was given 2 h before each morphine injection. The dose was chosen based on previous publication (22). To induce withdrawal behaviors, animals received a single injection of naloxone (10 mg/kg, i.p.; Sigma) 4 h after the last morphine injection on Day 5. Immediately after the naloxone injection, each mouse was placed in an acryl-glass box (30×30×40 cm) and the number of jumping was recorded over the next 15 min as the signs of development of physical dependence to morphine (46). The body weight of mice was measured just before and 30 min after naloxone injection. The animals were killed by decapitation for 1 h after the withdrawal behavioral test. The brains were rapidly dissected out on an ice-cold glass Petri dish and used for Western blot, RT-PCR, or cAMP assay. All animal experiments were approved by the Institutional Animal Care and Use Committee of the National University of Singapore and JiangXi Province People's Hospital.
Cell culture and treatment
Human neuroblastoma SH-SY5Y cells were incubated under humidified 5% CO2 and 95% air at 37°C in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) and 1% streptomycin and penicillin (Invitrogen). Cells were plated onto 35 mm dishes and incubated overnight as they grew into 80%–90% confluency. Regular medium was replaced with low-serum medium (0.5% FBS/DMEM) immediately before treatment. Opioid withdrawal was achieved by the addition of naloxone (100 μM) in the medium at the last 10 min of DAMGO (10 μM; Sigma) treatment for 24 h (D+Nal), or the cell was washed with low-serum medium to stop opioid treatment (D+W). To determine the effects of H2S on naloxone-precipitated opioid withdrawal in SH-SY5Y cells, NaHS (10 μM) was added to the cell culture medium 10 min before the addition of DAMGO, or 30 min before the addition of naloxone. To investigate the underlying mechanisms, PD 98059 (an ERK1/2 inhibitor; 10 μM; Calbiochem, Gibbstown, NJ) was given 30 min before DAMGO administration.
CBS transfection
The method of CBS transfection was described in our previous study (44). SH-SY5Y cells were plated onto a 35 mm dish and incubated overnight as they grew to 90%–95% confluency. Cells were transfected with CBS-PME185-HA vector (a gift from Dr. Hideo Kimura) or its empty vector alone as a control using lipofectamine 2000 transfection reagent (Invitrogen). After transfection for 4–6 h, cells were washed with PBS solution twice and replaced with low-serum medium (0.5% FBS/DMEM), and they then continued to be incubated at 37°C overnight. After that, the cells were treated with DAMGO (10 μM) for 24 h, followed by addition of naloxone (100 μM) for 10 min to induce opioid withdrawal. Cells were then collected for measurement of cAMP concentration or Western blot analysis.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from SH-SY5Y cells or mice brain tissues by the TRIzol extraction method (TRIzol Reagent; Invitrogen). The RNA was then used to amplify fragments of the cDNA of AC1, 2, 5, 8 by RT-PCR employing the One-step RT-PCR kit (Bio-Rad, Hercules, CA). The primers were adopted from previous publications (20, 41). A positive control was performed by using primers specific for ß-actin. One-step RT-PCR was performed with the following program. A reverse-transcription reaction was initiated at 50°C for 30 min. PCR activation at 95°C for 10 min was followed by 35 cycles; each consisted of 95°C for 30 s, 60°C for 30 s, 72°C for 1 min, and final extension time was set at 72°C for 10 min.
Western blot assay
After drug treatment, SH-SY5Y cells were washed twice with PBS and lyzed with 100 μl ice-cold lysis buffer (Cell signaling, Danvers, MA). Tissue samples were homogenized in tissue lysis buffer (1:10, w/v; Sigma). The lysate was shaken on ice for 1 h, then centrifuged at 12,000 g at 4°C for 15 min. Epitopes were exposed by boiling the protein samples at 100°C water for 5 min. Protein concentrations were determined with a NanoDrop Spectrophotometer (NanoDrop technology, Wilmington, DE). Equal amounts of the protein samples were separated by electrophoresis using a 10% sodium dodecyl sulphate/polyacrylamide gel (SDS/PAGE) and transferred onto a nitrocellulose membrane (Whatman, London, UK). After being blocked in 10% milk with Tris buffer saline-Tween 20 (TBS-T) buffer (10 mM Tris-HCl, 120 mM NaCl, 0.1% Tween-20, pH 7.4) at room temperature for 1 h, the membrane was incubated with primary antibodies of phospho-ERK1/2 (1:1000; Cell signaling), total-ERK1/2 (1:1000; Cell signaling), phospho-CREB (1:1000; Cell signaling), AC antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), or CBS antibody (1:1000; Abnova, Taipei, Taiwan) at 4°C overnight. ß-actin (1:5000; Santa Cruz Biotechnology) was used as a loading control. Membranes were washed thrice in TBS-T buffer, followed by incubation with goat anti-rabbit or goat anti-mouse secondary antibodies (1:10,000; Santa Cruz Biotechnology) at room temperature for 1 h, and washed thrice in TBS-T buffer. Visualization was carried out using ECL® (plus/advanced chemiluminescence) kit (GE Healthcare Life Sciences, Buckinghamshire, United Kingdom). The density of the bands on Western blots was quantified by Image J software.
cAMP assay
A cAMP enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) was used to examine the concentration of cAMP. To induce quick cAMP formation, forskolin (10 μM; Sigma) and 3-isobutyle-1-methylxanthine (IBMX, 0.2 mM; Sigma) were added into the cell, then incubated at 37°C for 10 min. The reaction was stopped by incubation in 0.1 M HCl for 20 min, and then centrifuged at 1000 g for 10 min. The supernatant was saved for determination of cAMP content. Fifty microliters of samples were added into a 96-well plate followed by incubation with cAMP acetylcholine esterase tracer and cAMP antiserum for 18 h at 4°C. Each sample was developed by Ellman's reagent, and the plate was read at a wavelength of 405 nm (27). cAMP concentration was calculated according to the cAMP standard, and the protein was examined by Bradford Assay.
Immunofluorescence staining
The tissue of mouse brain was fixed with 4% paraformaldehyde (Sigma) for over 24 h, and then cryoprotected in 30% sucrose followed by cutting with a freezing microtome (Leica, Germany) at 30 μm. After blocking with 0.5% bovine serum albumin (BSA; Sigma) for 1 h, the slices were incubated for 2 h at room temperature with the rabbit anti-phospho-ERK1/2 antibody diluted in 0.5% BSA (1:100). The slices were then washed thrice with PBS and further exposed to the fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (1:300; Invitrogen) at room temperature for 1 h. After three additional PBS washes, the slices were mounted by Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen), and images were acquired using a Nikon Eclipse 80i digital imaging microscope system (Tokyo, Japan). Negative controls were performed with the primary antibody omitted.
Statistical analysis
All data are presented as mean±S.E.M. Statistical significance was assessed with one-way analysis of variance followed by LSD test. For comparison between two groups, student's T-test was used. Differences with p-values less than 0.05 were considered statistically significant.
Abbreviations Used
- 3-MST
3-mercaptopyruvate sulfurtransferase
- AC
adenylate cyclase
- BSA
bovine serum albumin
- CBS
cystathione-β-synthase
- CNS
central nervous system
- CRE
cAMP responsive elements
- CREB
cAMP response element-binding protein
- DAMGO
[D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco's modified Eagle's medium
- ERK1/2
extracellular regulated protein kinase 1/2
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- H2S
hydrogen sulfide
- IBMX
3-isobutyle-1-methylxanthine
- NaHS
sodium hydrosulphide
- RT-PCR
reverse transcription-polymerase chain reaction
- SDS/PAGE
sodium dodecyl sulphate/polyacrylamide gel
- TBS-T
Tris buffer saline-Tween 20
Acknowledgment
This work is supported by research grants from the Singapore National Medical Research Council (NMRC1183/2008).
Author Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Chakrabarti S, Rivera M, Yan SZ, Tang WJ, and Gintzler AR. Chronic morphine augments G(beta)(gamma)/Gs(alpha) stimulation of adenylyl cyclase: relevance to opioid tolerance. Mol Pharmacol 54: 655–662, 1998 [PubMed] [Google Scholar]
- 2.Chakrabarti S, Wang L, Tang WJ, and Gintzler AR. Chronic morphine augments adenylyl cyclase phosphorylation: relevance to altered signaling during tolerance/dependence. Mol Pharmacol 54: 949–953, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Chan JS, Chiu TT, and Wong YH. Activation of type II adenylyl cyclase by the cloned mu-opioid receptor: coupling to multiple G proteins. J Neurochem 65: 2682–2689, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Chao JR, Ni YG, Bolanos CA, Rahman Z, DiLeone RJ, and Nestler EJ. Characterization of the mouse adenylyl cyclase type VIII gene promoter: regulation by cAMP and CREB. Eur J Neurosci 16: 1284–1294, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Crain SM. and Shen KF. Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci U S A 92: 10540–10544, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deisseroth K, Bito H, and Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16: 89–101, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, and Kimura H. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J 19: 1854–1856, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Feinstein PG, Schrader KA, Bakalyar HA, Tang WJ, Krupinski J, Gilman AG, and Reed RR. Molecular cloning and characterization of a Ca2+/calmodulin-insensitive adenylyl cyclase from rat brain. Proc Natl Acad Sci U S A 88: 10173–10177, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox B, Schantz JT, Haigh R, Wood ME, Moore PK, Viner N, Spencer JP, Winyard PG, and Whiteman M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: is H2S a novel cytoprotective mediator in the inflamed joint? J Cell Mol Med 16: 896–910, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glatt CE. and Snyder SH. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature 361: 536–538, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Grewal SS, York RD, and Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol 9: 544–553, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Guitart X, Thompson MA, Mirante CK, Greenberg ME, and Nestler EJ. Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem 58: 1168–1171, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, and Nestler EJ. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci 26: 4624–4629, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu LF, Lu M, Hon Wong PT, and Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal 15: 405–419, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, and Kimura H. Cystathionine beta-synthase is enriched in the brains of Down's patients. Biochem Biophys Res Commun 338: 1547–1550, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Johnson SM. and Fleming WW. Mechanisms of cellular adaptive sensitivity changes: applications to opioid tolerance and dependence. Pharmacol Rev 41: 435–488, 1989 [PubMed] [Google Scholar]
- 17.Kest B, Palmese CA, Hopkins E, Adler M, Juni A, and Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience 115: 463–469, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW, Lee JK, Nestler EJ, and Han PL. Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci U S A 103: 3908–3913, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267: 129–133, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kolachala V, Asamoah V, Wang L, Srinivasan S, Merlin D, and Sitaraman SV. Interferon-gamma down-regulates adenosine 2b receptor-mediated signaling and short circuit current in the intestinal epithelia by inhibiting the expression of adenylate cyclase. J Biol Chem 280: 4048–4057, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, and Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci 17: 7890–7901, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, and Deng LW. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One 6: e21077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, and Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117: 2351–2360, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Li T, Hou Y, Cao W, Yan CX, Chen T, and Li SB. Naloxone-precipitated withdrawal enhances ERK phosphorylation in prefrontal association cortex and accumbens nucleus of morphine-dependent mice. Neurosci Lett 468: 348–352, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Lim JJ, Liu YH, Khin ES, and Bian JS. Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am J Physiol Cell Physiol 295: C1261–C1270, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Liu JG. and Anand KJ. Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev 38: 1–19, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lu M, Liu YH, Goh HS, Wang JJ, Yong QC, Wang R, and Bian JS. Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol 21: 993–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldonado R. and Koob GF. Destruction of the locus coeruleus decreases physical signs of opiate withdrawal. Brain Res 605: 128–138, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka I, Maldonado R, Defer N, Noel F, Hanoune J, and Roques BP. Chronic morphine administration causes region-specific increase of brain type VIII adenylyl cyclase mRNA. Eur J Pharmacol 268: 215–221, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Nestler EJ. and Aghajanian GK. Molecular and cellular basis of addiction. Science 278: 58–63, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Nestler EJ. and Tallman JF. Chronic morphine treatment increases cyclic AMP-dependent protein kinase activity in the rat locus coeruleus. Mol Pharmacol 33: 127–132, 1988 [PubMed] [Google Scholar]
- 32.Njie-Mbye YF, Kulkarni M, Opere CA, and Ohia SE. Mechanism of action of hydrogen sulfide on cyclic AMP formation in rat retinal pigment epithelial cells. Exp Eye Res 98: 16–22, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, and Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci 15: 1285–1297, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel TB, Du Z, Pierre S, Cartin L, and Scholich K. Molecular biological approaches to unravel adenylyl cyclase signaling and function. Gene 269: 13–25, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Pierre S, Eschenhagen T, Geisslinger G, and Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov 8: 321–335, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Punch LJ, Self DW, Nestler EJ, and Taylor JR. Opposite modulation of opiate withdrawal behaviors on microinfusion of a protein kinase A inhibitor versus activator into the locus coeruleus or periaqueductal gray. J Neurosci 17: 8520–8527, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz S. and Hollt V. Opioid withdrawal activates MAP kinase in locus coeruleus neurons in morphine-dependent rats in vivo. Eur J Neurosci 10: 1196–1201, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Sharma SK, Klee WA, and Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci U S A 74: 3365–3369, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, and Nestler EJ. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci 22: 3663–3672, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shijun H, Liping Z, Yongqiang Q, Zhen L, Yonghe Z, and Lihua L. Morphine-induced changes of adenylate and guanylate cyclase in locus ceruleus, periaqueductal gray, and substantia nigra in rats. Am J Drug Alcohol Abuse 35: 133–137, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Strait KA, Stricklett PK, Chapman M, and Kohan DE. Characterization of vasopressin-responsive collecting duct adenylyl cyclases in the mouse. Am J Physiol Renal Physiol 298: F859–F867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tempel A. and Zukin RS. Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci U S A 84: 4308–4312, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, and Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res 548: 100–110, 1991 [DOI] [PubMed] [Google Scholar]
- 44.Tiong CX, Lu M, and Bian JS. Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/Akt pathway. Br J Pharmacol 161: 467–480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams JT, Christie MJ, and Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81: 299–343, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Yang HY. and Pu XP. Chronic morphine administration induces over-expression of aldolase C with reduction of CREB phosphorylation in the mouse hippocampus. Eur J Pharmacol 609: 51–57, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Yong QC, Pan TT, Hu LF, and Bian JS. Negative regulation of beta-adrenergic function by hydrogen sulphide in the rat hearts. J Mol Cell Cardiol 44: 701–710, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Yu VC, Eiger S, Duan DS, Lameh J, and Sadee W. Regulation of cyclic AMP by the mu-opioid receptor in human neuroblastoma SH-SY5Y cells. J Neurochem 55: 1390–1396, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Zachariou V, Liu R, LaPlant Q, Xiao G, Renthal W, Chan GC, Storm DR, Aghajanian G, and Nestler EJ. Distinct roles of adenylyl cyclases 1 and 8 in opiate dependence: behavioral, electrophysiological, and molecular studies. Biol Psychiatry 63: 1013–1021, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]