Abstract
Norway rats (Rattus norvegicus) are reservoir hosts for zoonotic pathogens that cause significant morbidity and mortality in humans. Studies evaluating the prevalence of zoonotic pathogens in tropical Norway rat populations are rare, and data on co-infection with multiple pathogens are nonexistent. Herein, we describe the prevalence of leptospiral carriage, Seoul virus (SEOV), and Bartonella spp. infection independently, in addition to the rates of co-infection among urban, slum-dwelling Norway rats in Salvador, Brazil, trapped during the rainy season from June to August of 2010. These data were complemented with previously unpublished Leptospira and SEOV prevalence information collected in 1998. Immunofluorescence staining of kidney impressions was used to identify Leptospira interrogans in 2010, whereas isolation was used in 1998, and western blotting was used to detect SEOV antibodies in 2010, whereas enzyme-linked immunosorbent assay (ELISA) was used in 1998: in 2010, Bartonella spp. were isolated from a subsample of rats. The most common pathogen in both years was Leptospira spp. (83%, n=142 in 1998, 63%, n=84 in 2010). SEOV was detected in 18% of individuals in both 1998 and 2010 (n=78 in 1998; n=73 in 2010), and two species of Bartonella were isolated from 5 of 26 rats (19%) tested in 2010. The prevalence of all agents increased significantly with rat mass/age. Acquisition of Leptospira spp. occurred at a younger mass/age than SEOV and Bartonella spp. infection, suggesting differences in the transmission dynamics of these pathogens. These data indicate that Norway rats in Salvador serve as reservoir hosts for all three of these zoonotic pathogens and that the high prevalence of leptospiral carriage in Salvador rats poses a high degree of risk to human health.
Key Words: : Leptospira interrogans, Seoul virus, Bartonella spp., Norway rats (Rattus norvegicus), Brazil
Introduction
The Norway rat (Rattus norvegicus) is one of the most successful invasive vertebrates, inhabiting both urban and rural habitats. High densities of Norway rats are often found in low-income neighborhoods in populous cities (Childs et al. 1998, Glass et al. 2009). In addition to their importance as a commensal pest species, Norway rats serve as reservoir hosts for a number of zoonotic, bacterial, and viral pathogens that cause disease in humans, including Leptospira spp., Seoul virus (SEOV), and Bartonella spp. (Gratz 1994, Mills 1999, Kosoy et al. 2010). Although the prevalence and patterns of acquisition of these zoonotic pathogens in Norway rat populations have been described from a handful of urban locations in the United States and Europe (Webster et al. 1995, Macdonald 1999, Battersby et al. 2002, Easterbrook et al. 2007, Antoniou et al. 2010), similar reports on these co-circulating pathogens from tropical, developing countries are unavailable.
Over 30% of the world's urban population lives in slums. Projections estimate that this number will increase to two billion people over the next 30 years (UN-HABITAT 2003). In Brazil, 37% of the population reside in slums ( favelas) (UN-HABITAT 2003), where poverty, overcrowding, and sanitary deficiencies provide an ideal environment for Norway rats and promote close interactions with humans. In this setting, the risk for transmission of zoonotic pathogens is enhanced (Meyer 2003, Taylor et al. 2008).
The most frequently reported zoonotic disease within Brazilian slums is leptospirosis (Ko et al. 1999, Sarkar et al. 2002, Gouveia et al. 2008, Reis et al. 2008) with an annual incidence of reported and confirmed cases of exceeding 10,000 per year (Health Surveillance Secretary 2007); the true morbidity and mortality far exceeds this figure because many infections go unreported (Reis et al. 2008). Spirochetes of the genus Leptospira colonize the renal tubules of Norway rats (Ko et al. 2009) and are shed in urine. Human infection is most often related to contact with rat urine-contaminated water or soil. Many tropical cities areas experience epidemics of leptospirosis during periods of high rainfall and flooding; outbreaks occur annually during the winter rainy season in Salvador (Ko et al. 1999, Sarkar et al. 2002). Although leptospirosis has been monitored for decades within Salvador, other zoonotic pathogens of rats capable of causing human disease such as SEOV and Bartonella spp, have received little or no attention, and no information on co-infection within Norway rats in Brazil exists.
There is no formal surveillance for SEOV, and the lack of medical awareness, in addition to the inability to confirm cases on site by field or local laboratory diagnosis, precludes any estimates of disease burden (Santos and Garrett 2005). SEOV virus has been detected in Norway rats from Asia, Europe, and the Americas (Sanfeliu et al. 2011), including Brazil (LeDuc et al. 1985). However, laboratory-confirmed human cases outside of Asia are not frequent (Sanfeliu et al. 2011), and few cases have been reported in Brazil (Clement et al. 1999).
Diseases caused by Bartonella spp. are rarely investigated in Brazil, and only sparse data on human infections caused by a few of the pathogenic species of Bartonella have been published. Rat-associated Bartonella spp. have not been identified in Brazil (Lamas et al. 2008, Lamas et al. 2010).
Herein, we describe the results from field and laboratory studies of slum-dwelling Norway rats, conducted in Salvador, documenting the prevalence and occurrence of co-infections caused by Leptospira spp., SEOV, and Bartonella spp. Of note, we demonstrate that the patterns of infection by leptospires and SEOV conducted in 1998 and 2010 were nearly identical over 12 years; we describe the first Bartonella spp. isolated from Brazilian rats and show that different pathogen/antibody detection methods provided indistinguishable results.
Materials and Methods
Study sites
Salvador, Brazil, with more than 2.7 million inhabitants is the third most populous city in Brazil (Instituto Brasileiro de Geografia e Estatística 2007). Study sites for rat sampling targeted three slum areas within communities participating in an active leptospirosis surveillance program; Pau da Lima (13°32'53.47'' S; 38°43'51.10'' W), Valeria (13°26'19.65''S; 38°44'03.90''W), and Sete de Abril (13°31'19.63'' S; 38°43'24.69'' W) (Ko et al. 1999). Each area experienced high annual incidences leptospirosis in 2010 (10.4, 10.1, and 23.3/100,000, respectively) (M. Reis and A.I. Ko, unpublished data).
Data collection
Live R. norvegicus were captured in June, July, and August of 2010 by placing three to five Tomahawk traps at each of six to eight contiguous households; ten trap sites, six in Pau da lima, three in Sete de Abril, and one in Valeria, were selected (Porter et al., submitted). Traps were set before sundown and collected at sunrise. Traps containing rats were double-bagged and transported to an outdoor processing site where animals were handled and tissues obtained according to Centers for Disease Control and Prevention (CDC) guidelines (Mills et al. 1995).
Animals were euthanized with thiopental solution and then weighed, sexed, and examined for sexual maturity and for scars and wounds, which were scored on a five-point qualitative scale, as previously described (Glass et al. 1988). Blood was obtained by cardiac puncture, and kidneys were removed for pathogen determinations as described below. All animal procedures and methods were approved by the Institutional Animal Care and Use Committee (IACUC) committee at the Oswaldo Cruz Foundation (Salvador, Brazil).
Pathogen survey
In 2010, kidney imprints were obtained by exerting pressure of the cut surface of a kidney onto poly-l-lysine-coated glass slides, as previously described (Chagas-Junior et al. 2009). Slides were dried at room temperature and fixed in acetone (3 min) prior to incubation (1 h) with a primary rabbit polyclonal anti-leptospiral antibody at a dilution of 1:200. Following three phosphate-buffered saline (PBS) washes, the slides were incubated (1 h) with goat anti-rabbit immunoglobulin G–fluorescein isothiocyanate (IgG–FITC) conjugate at a 1:500 dilution. After final washings, the slides were dried and examined for leptospires using fluorescent microscopy. Positive samples were determined by microscopic observation of intact leptospires. The results obtained were compared to findings from the 1998 study, which employed isolation of leptospires on Ellinghausen–McCullough–Johnson–Harris (EMJH) medium (Difco, USA), as previously described (de Faria et al. 2008).
In 2010, serum antibodies to SEOV were detected using a commercially available modified western blotting procedure (Hjelle et al. 1997) in which differential antibody binding to three hantaviral antigens (SEOV, Andes virus, and Sin Nombre virus), previously adsorbed on the strips, was determined by visualization of the density of band staining. Using an eight-well western blot, rodent sera were diluted (1:200) in 1 mL of 5% PET blocking reagent in a Blotto solution, prior to adding antigen strips. After a 4-h incubation, strips were washed three times in 1 mL of washing buffer (0.1 M saline, 10 mM NaPO4 pH, 7.4, 0.1% Triton/DOC). Strips were left in Blotto solution for 10 min. This solution was then replaced with a Blotto solution containing 1:1000 anti-rat IgG, alkaline phosphatase (AP) labeled, and allowed to incubate for 1 h, after which strips were washed three times using 1 mL of washing buffer. Antigen–antibody bands were visualized by addition of the developer solution blue tetrazolium chloride–5-bromo-4-chloro-3-indolyl phosphate (NBT-BCIP), and reactive samples were identified by color density compared to standard controls consisting of a SEOV-positive and -negative Norway rat sera. The previously unpublished SEOV antibody results from the 1998 study, determined by enzyme-linked immunosorbent assay (ELISA) (described in detail in Easterbrook et al. 2007), were compared with regard to prevalence and co-infection infection and mass/age-specific acquisition of infection.
Bartonella spp. were isolated and characterized at the CDC Fort Collins campus using methods previously described (Kosoy et al. 2004). Briefly, 0.1 mL of whole blood, diluted 1:4 in brain–heart infusion (BHI) medium (BBL, Becton Dickinson Microbiology System), was placed on agar plates and incubated at 35°C in an aerobic atmosphere of 5% CO2. Plates were held for 8–20 days, and viable Bartonella-like colonies were identified. Confirmation and characterization of the infecting Bartonella spp. was determined by sequencing the partial citrate synthase gene gltA obtained by PCR, as previously described (Kosoy et al. 1997).
Inclusion of additional animals
In a previous 1998 study conducted in Salvador (de Faria et al. 2008), 114 isolates of Leptospira were obtained from 142 (80.3% positive) Norway rats sampled. These rats were captured from households in which an individual with severe leptospirosis had resided. The results of SEOV testing of sera from these rats by ELISA (Feldmann et al. 1993) were not reported at that time and are included herein. No rats were tested for Bartonella in 1998.
Statistical analysis
To assess whether the observed co-infection prevalence was different from expected values, we multiplied the prevalence of each individual pathogen by its prevalence in co-infected individuals and evaluated the outcomes for significance by using two-sided chi-squared or Fisher exact tests. Correlation analyses of pathogen prevalence by mass were assessed by Pearson product moment tests after binning rat mass/age into three 200-gram intervals (juveniles were classified <200 grams, young adults were 200–399 grams, and adults were ≥400 grams). This stratification of body mass/age facilitated comparison with previously published results from Baltimore, MD (Easterbrook et al. 2007) and was only used after confirming that these same maturation categories held for rats trapped within Salvador (Porter et al., submitted).
A logistic regression model of infection status by various co-variates included only the 2010 data, because the 1998 database was missing values (wounding, reproductive status, and Bartonella infection). After stratification of the data using demographic features of the rodent population, including mass/age, sex, and reproductive status (pregnancy for females and scrotal testes for males), we computed bivariate odds ratios (ORs) and adjusted ORs (after multivariable logistic regression) using EpiInfo (version 3.5.1, CDC, Atlanta, GA). Variable associations with outcomes were considered statistically significant when 95% confidence intervals (CI) did not include the value 1. Following the bivariate analysis, the variables meeting our inclusion criteria were retained for analysis by a multivariable logistic regression using a backward elimination strategy to develop our final model by excluding co-variates determined not to be significant at the p<0.05 value. In the final model, mass was evaluated as a continuous variable.
Results
Prevalence and co-infection of zoonotic pathogens
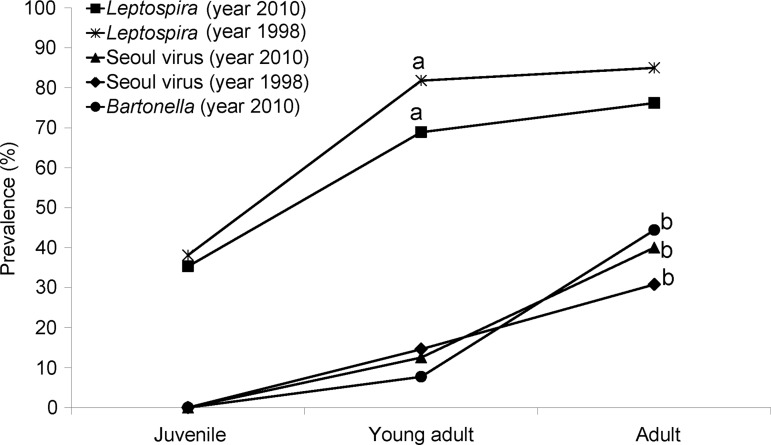
Leptospira spp. were the most prevalent pathogens among rats in 2010 (53/84; 63.1%; Table 1). The overall prevalence of leptospiral isolation in 1998 (114/142; 83%) was significantly higher when compared with indirect fluorescent antibody (IFA) results from 2010 (χ2=7.76, p<0.005), although this difference was not significant after adjustments for mass/age. Although culture isolation of leptospires was not performed in 2010, data from 1998 indicate that Leptospira interrogans serovar Copenhageni was the only serovar circulating in Norway rat populations in Salvador at that time. It is unlikely that other serovars occur at any frequency because Copenhageni is the serovar isolated from humans within our study areas.
Table 1.
Prevalence of Zoonotic Pathogens in Rattus norvegicus from Slum Areas in Salvador, Brazil, 2010
| Zoonotic pathogen | Prevalence | Number of positive rats | Total no. of ratsa |
|---|---|---|---|
|
Leptospira spp. |
63.1 (80.3) |
53 (114) |
84 (142) |
| Seoul virus (SEOV) |
17.8 (17.9) |
13 (14) |
73 (78) |
|
Bartonella spp. |
19.2 (NAb) |
5 (NA) |
26 (NA) |
|
Co-infectionsc | |||
|
Leptospira spp. and SEOV | |||
| Observed |
13.8 (15.3) |
10 (12) |
72 (78) |
| Expected |
11.2 (14.3) |
|
|
|
Leptospira spp. and Bartonella spp. | |||
| Observed |
7.6 (NA) |
2 (NA) |
26 (NA) |
| Expected |
12.1 (NA) |
|
|
| SEOV and Bartonella spp. | |||
| Observed |
3.8 (NA) |
1 (NA) |
26 (NA) |
| Expected |
3.4 (NA) |
|
|
| Leptospira spp., SEOV and Bartonella spp. | 0 (NA) | 0 (NA) | 26 (NA) |
Findings from the 1998 survey are shown in bold type within parentheses, immediately after the 2010 results.
Unequal sample sizes due to sample availability.
Not available.
No significant differences between observed and expected co-infection prevalences.
Antibodies against SEOV were detected in 13 of 73 rats (17.8%) in 2010 by western blotting, whereas SEOV was detected by ELISA in 17.9% (14/78) of rats captured in 1998. Infection with Bartonella spp. was confirmed in five of 26 rats (19.2%) in 2010, whereas no data are available from the 2008 sample. Isolates from four of the five positive rats from 2010 were identified as B. queenslandensis; the other isolate was B. tribocorum.
Leptospira spp.-positive animals were found in all 10 trap locations, in all three neighborhoods sampled in 2010; carriage prevalence varied between 50% and 100%. SEOV-positive rats were found in three of 10 trap locations. Among SEOV-positive sites, the prevalence was 14% (2/14) in Pau da Lima and 60% (6/10) in Sete de Abril. Bartonella-infected animals were found in two out of five trap sites from which rat blood clots were tested in the neighborhood of Pau da Lima.
There were no significant differences between observed and expected prevalence for co-infection by Leptospira spp. or SEOV (1998 and 2010 data included), or for Leptospira spp. and SEOV with Bartonella spp. (2010 only), or a three-way co-infection (Table 1).
Prevalence by sex
Comparable numbers of males (n=40) and females (n=44) were captured in 2010 and 1998 (76 males and 65 females) (χ2=0.83, p>0.36), and there were no significant difference in the number of males or females trapped by age class (juvenile, young adult, adult) in either sampling year (p>0.05 for each matched comparison). There was no significant difference in the prevalence of leptospiral carriage (χ2=0.71, p>0.05), prevalence of SEOV antibodies (χ2=2.01, p>0.05) or isolation of Bartonella spp. (χ2=0.09, p>0.05) by sex in the 2010 study. However, female rats from the 1998 sample had a significantly higher prevalence of leptospiral carriage than males (OR 4.8; CI 1.7—13.8) (de Faria et al. 2008). More male rats from 2010 were seropositive for SEOV (25.6%, 10/39) than females (10.5%, 4/38), but this difference was not statistically significant (χ2=2.95, p=0.08). Female and male rats from 1998 had equivalent SEOV prevalence (25%, 10/40 and 26%, 10/38, respectively).
Body size and sexual maturity
In both the 2010 and 1998 samples, prevalence of leptospiral carriage increased by mass/age class and the infection acquisition curves were notably convex (Fig. 1). Prevalence was significantly higher in adults and young adults compared to juveniles (χ2=8.71, p<0.005; 2010) and (χ2=12.56, p<0.005; 1998). After stratification by mass/age class, there were no significant differences in prevalence among rats captured in 2010 and 1998 (p>0.05 for each matched comparison). As the patterns of acquisition and age-stratified prevalence of leptospiral infection did not differ between 2010 and 1998, we combined these data (N=226) to derive a finer scale sex-age-mass stratification (Fig. 2).
FIG. 1.
Prevalence of Leptospira spp. and Seoul virus in 2010 and 1998; and Bartonella spp. In 2010, for three age groups of rats—juveniles (<200 grams), young adults (200–399 grams), and adults >400 grams). aSeroprevalence young adult and adult >juvenile; bseroprevalence adult >young adult and juvenile; p<0.05.
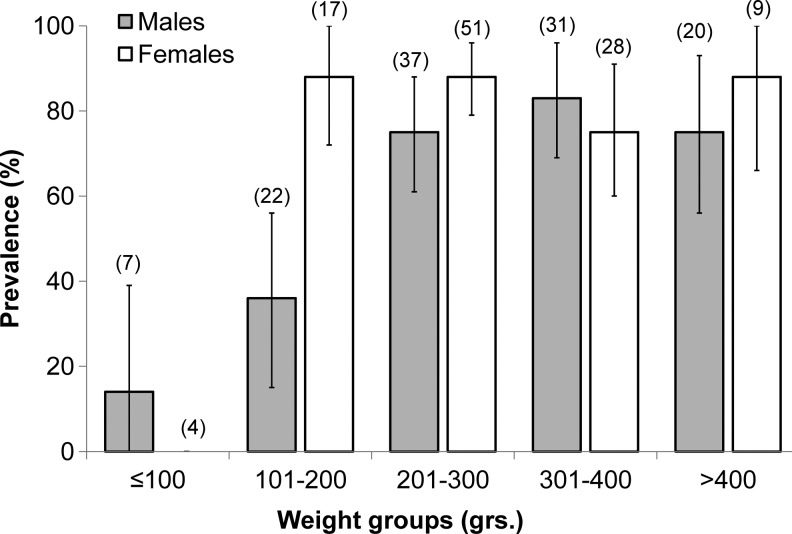
FIG. 2.
Leptospiral carriage prevalence and 95% confidence intervals for the combined samples from 2012 and 1998 (n=226 rats) stratified by mass and sex.
As anticipated by the mass/age association with leptospiral prevalence (Fig. 1), leptospiral carriage in 2010 was higher in males with coiled epididymis (67.7%, 21/31) compared to animals with uncoiled epididymis (22.2%, 2/9: χ2=5.91, p≤0.05), and pregnant subadult or adult females had a higher prevalence (80.0%; n=20) of leptospiral carriage compared to nonpregnant females (61.15%; n=13), although this association was not significant (χ2=1.35, p=0.242) (Table 2).
Table 2.
Risk Factors for Pathogen Identification among Rats Captured During 2010 in Three Neighborhoods of High Incidence of Human Severe Leptospirosis in Salvador, Brazil
| |
Leptospiral carriage (n=84) |
SEOV antibodies (n=73) |
Bartonella spp. infection (n=26) |
|||
|---|---|---|---|---|---|---|
| |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
| Variables | OR (95% CI)a | |||||
| Female |
1.46 (0.60–3.60) |
NS |
0.42 (0.12–1.43) |
NS |
0.70 (0.10–5.30) |
NS |
| Body mass |
1.00 (1.00–1.01) |
1.004 (1.001–1.009) |
1.01 (1.00–1.02) |
1.01 (1.00–1.01) |
1.01 (1.00–1.02) |
1.01 (1.00–1.02) |
| Reproductive status |
4.00 (0.92–17.34) |
NS |
1.25 (0.78–1.47) |
NS |
0.20 (0.01–3.90) |
NS |
| Wounding score |
3.35 (1.33–8.45) |
2.16 (0.78–6.00) |
10.50 (1.28–85.93) |
5.65 (0.64–49.44) |
3.00 (0.20–31.6) |
NS |
| SEOV antibodiesb |
1.98 (0.49–7.98) |
NS |
NA |
NA |
5.00 (0.20–97.70) |
NS |
|
Bartonella spp.c |
0.41 (0.56–3.01) |
NS |
5.00 (0.26–97.70) |
NS |
NA |
NA |
| Leptospira spp.c | NA | NA | 1.98 (0.50–0.33) | NS | 0.40 (0.10–3.00) | NS |
Odds ratios (OR) and 95% confidence intervals (CI) are shown for risk factor analyses. Logistic regression was performed to obtain estimates for ORs, which were adjusted for co-variates in the final model.
SEOV antibodies were available for 73 rats.
Bartonella spp. infection status was available for 26 rats.
NA, not applicable; NS, not significant.
The prevalence of SEOV was significantly higher in adult rats compared to juveniles and young adults (χ2=9.01, p<0.005) for pooled data from 2010 and 1998. Prevalence of Bartonella sp. was also significantly higher in adults compared to juveniles and young adults (Fischer exact test, p<0.034) (Table 2).
Wounding
Wounds were identified in 55% of rats; 45% presented minor to moderate wounding and 10% were classified as having extensive wounds. Males and females presented similar wounding rates (54% and 57%, respectively). For the 2010 sample, the prevalence of leptospiral carriage and presence of SEOV antibodies increased with wounding score (OR=3.4, 95% CI=1.3–8.4 and OR=10.5, 95% CI=1.3–85.9, respectively). Bartonella prevalence was not associated with wounding (OR=3.0, CI=0.2–31.6) (Table 2). Data on wounding score was not collected for the 1998 sample.
Mulivariate analysis
Due to co-linearity between reproductive status and mass in both male and female rats, only body mass was included in the final logistic regression model. Body mass and wounding were independently associated with both the prevalence of leptospiral carriage and antibodies to SEOV, whereas only body mass was significantly associated with Bartonella spp. infection (Table 2).
Discussion
Before a broader discussion, we highlight three major findings and conclusions obtained from the data analyses of zoonotic pathogen prevalence and co-infection among Norway rats described in this article. First, the prevalence of Leptospira spp. was greater than that of SEOV antibodies, and similar infection patterns were obtained in 1998 and 2010. Although the overall infection prevalence of leptopires was higher in 1998, possibly due to the targeted trapping of rats from households in which a human case of leptospirosis had occurred, this difference disappeared after stratifying by the three mass/age groups. The prevalence of antibodies to SEOV was identical in 2010 and 1998. These findings suggest that these zoonotic pathogens were maintained at a stable frequency over this 12-year interval. Second, infection prevalence of all three pathogens increased with host mass/age. However, prevalence estimates suggest different dynamics of pathogen acquisition, with leptospiral infection occurring early in life and continuing into adulthood (convex acquisition curve), whereas SEOV and Bartonella spp. infections were absent among juveniles and rare in subadults (concave acquisition curves). Finally, results obtained in 2010 using assays different than those employed in 1998 were consistent and indistinguishable after mass/age stratification, indicating these different methods may be interchanged without concern of obtaining different results.
The three pathogens described in this study have each been demonstrated to cause serious, sometimes fatal, disease in humans (Iversson et al. 1992, Ko et al. 1999, Kosoy et al. 2010), but data on co-infection among tropical rat populations was unavailable. Although Norway rats are reservoir hosts for many other zoonotic pathogens, these three agents were selected because similar co-infection studies have been conducted in Baltimore, Maryland, making a comparison of temperate and tropical urban rat populations possible (Easterbrook et al. 2007). To our knowledge, our results are not only the first data on co-infection among urban tropical Norway rats but also the first study to compare outcomes to values obtained from a temperate-zone city.
The prevalence of leptospiral carriage in 2010 ranged from 50% to 100% among sites, and 80.3% of rats sampled in 1998 were positive. An early study of leptospiral carriage in Norway rats of Salvador, as determined by silver impregnation of kidney samples (Andrade 1954), reported 29% positive rats. This difference could indicate an increase in infected rodents over the last 50 years, but variation in sampling and diagnostic procedures, or in the demographic profile of rats tested preclude any conclusions.
The mass/age-specific prevalence relationships for leptospiral and SEOV infection were identical between rats sampled in 2010 and 1998 (Fig. 1). Infection acquisition with Leptospira spp. among rats occurred earlier in life (present among juveniles <200 grams) than infection by SEOV or Bartonella spp., suggesting that the dynamics of pathogen acquisition varies with the agent. Wounding grade was positively associated with higher prevalence of leptospiral carriage and SEOV antibody in Salvador rats (Table 2). This pattern is well established for SEOV infection among North American Norway rats, although no data for such an association for leptospires have previously been published to our knowledge. Intraspecific aggression has been hypothesized as a mechanism of SEOV transmission due to the exposure to infected saliva in bite wounding (Kawamata 1987, Klein 2002). Although leptospiral shedding in saliva has not been demonstrated, the presence of wounds could increase the risk for acquisition through abraded skin, as has been reported for humans (Phraisuwan et al. 2002).
Increasing mass/age-related acquisition of one or more pathogens among Norway rats has been previously documented by other studies in Brazil and in temperate-zone cities in Argentina (Vanasco et al. 2003, de Faria et al. 2008). This consistent pattern of infection acquisition has been hypothesized to be the result of the continual risk of infection over time, starting when young rats (<50 grams) leave the nest environment and disperse from natal sites (Klein 2002, Vanasco et al. 2003, Easterbrook et al. 2007). Although the mass/age-specific prevalence curves from Salvador suggest different mass/age dynamics of infection for Leptospira spp. and SEOV when compared to results from Baltimore, female-biased increased infection prevalence with Leptospira spp. was observed in our study (Fig. 2) and was previously found in Baltimore (Easterbrook et al. 2007).
Rats infected with SEOV have been found worldwide (LeDuc et al. 1986). Sporadic cases of human disease caused by SEOV have been documented in several countries outside of Korea (Clement et al. 1994, Clement et al. 1997, Chow et al. 2005), and human disease has been identified in Salvador (A.I. Ko, unpublished data) and other Brazilian cities (LeDuc et al. 1985, Iversson et al. 1992, Katz et al. 2001). Antibodies to SEOV were previously documented among R. norvegicus and R. rattus captured in the Brazilian cities of Recife (6%), Sao Paulo (14%), and Belen (56%) (LeDuc et al. 1985). The seroprevalence of SEOV estimated in this study (17.3%) was similar to previous findings from Brazil but represented far lower figures than from the United States (Hinson 2004, Easterbrook et al. 2005, Easterbrook et al. 2007), where overall, 50% of rats were infected. However, the absence of sex differences in infection prevalence with SEOV found in Salvador was consistent with previous reports from Baltimore (Childs et al. 1988, Childs et al. 1998, Klein 2002, Easterbrook et al. 2007).
Although human disease caused by rat-associated Bartonella has not been described from Salvador, rat-associated Bartonella are potential human pathogens elsewhere (Kosoy et al. 2010). This is the first report of the isolation of Bartonella spp. from Norway rats in Brazil. Although only five isolates of Bartonella were obtained, the finding of two different species, B. queenslandensis and B. tribocorum, among our small sample of rats from Salvador was unexpected. Infection with B. queenslandensis has been demonstrated from rats in Australia (Gundi et al. 2009), while B. tribocorum is associated with rats from Thailand (Bai et al. 2009). Although both species were found in the same rat population sampled from Los Angeles, California (Gundi et al. 2012), only one rat was infected with B. queenslandensis compared to 54 rats infected with B. tribocorum. In Salvador, four rats were infected with B. queenslandensis and a single rat with B. tribocorum. Infection in Salvador rats mirrored SEOV antibody prevalence and suggested a mass/age-specific increase in Bartonella spp. infection (Fig. 1). However, data on Bartonella spp. infection from Baltimore suggests no relationship between mass/age and prevalence (Easterbrook et al. 2007). As such, the conclusions drawn from our results are premature as the number of rats tested for Bartonella spp. infection in Salvador, n=26, was too small to identify reliable trends.
Cross-sectional studies have repeatedly demonstrated that Norway rats serve as important reservoirs of Leptospira spp. in Salvador (Ko et al. 1999, Pereira et al. 2000, Sarkar et al. 2002, Matthias et al. 2008, Reis et al. 2008). However, all of these studies, including our own, were short studies that were not designed to capture the temporal or spatial dynamics of pathogen transmission and acquisition. Human leptospirosis is strongly associated with the rainy winter season in Salvador (Ko et al. 1999), and seasonally related demographic shifts in Norway rat population structure could influence the dynamics of pathogen acquisition, carriage prevalence, and shedding. Thereby, changes in rat demography, pathogen populations, and environmental factors could alter the risk for human infection. Future studies examining and linking observations obtained from studying the complex ecology of leptospiral carriage in Norway rats will not only clarify our understanding of the epidemiology of human disease but should provide spatio-temporal information to better inform control efforts.
Acknowledgments
We would like to thank the staff of Centro de Controle de Zoonoses from Salvador for their assistance in conducting the study and Julia Schoen for assisting with database management; Ying Bai for the Bartonella spp. sequencing; and Kate Hacker for her critical advice during the preparation of the manuscript. This work was supported by the Secretariat of Health Surveillance, the Oswaldo Cruz Foundation and the National Institutes of Health (grants R01 AI052473, U01 AI088752, R01 TW009504, R24 TW007988, R25 TW009338 and D43 TW00919) and CAPES (Coordination for the Improvement of Higher Education Personnel/Ministry of Education/Brazil).
Author Disclosure Statement
No competing financial interests exist.
References
- Andrade ZA, Oliveira J.C. Studies on leptospirosis in Bahia. Boletim da Fundação Gonçalo Moniz 1954; 3:1–36 [Google Scholar]
- Antoniou M, Psaroulaki A. Toumazos P. Rats as indicators of the presence and dispersal of pathogens in Cyprus: Ectoparasites, parasitic helminths, enteric bacteria, and encephalomyocarditis virus. Vector Borne Zoonotic Dis 2010; 10:867–873 [DOI] [PubMed] [Google Scholar]
- Bai Y, Kosoy MY, Lerdthusnee K, et al. . Prevalence and genetic heterogeneity of Bartonella strains cultured from rodents from 17 provinces in Thailand. Am J Trop Med Hyg 2009; 81:811–816 [DOI] [PubMed] [Google Scholar]
- Battersby S, Parson R, Webster JP. Urban rat infestations and the risk to public health. J Environ Health Res 2002; 1:4–12 [Google Scholar]
- Chagas-Junior AD, McBride AJ, Athanazio DA, et al. . An imprint method for detecting leptospires in the hamster model of vaccine-mediated immunity for leptospirosis. J Med Microbiol 2009; 58:1632–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs JE, Glass GE, Korch GW, et al. . The ecology and epizootiology of hantaviral infections in small mammal communities of Baltimore: A review and synthesis. Bull Soc Vector Ecol 1988; 13:113–122 [Google Scholar]
- Childs JE, McLafferty SL, Sadek R, et al. . Epidemiology of rodent bites and prediction of rat infestation in New York City. Am J Epidemiol 1998; 148:78–87 [DOI] [PubMed] [Google Scholar]
- Chow L, Shu PY, Huang JH, et al. . A retrospective study of hantavirus infection in Kinmen, Taiwan. J Microbiol Immunol Infect 2005; 38:343–349 [PubMed] [Google Scholar]
- Clement J, Mc Kenna P, Avsic-Zupanc T, et al. . Rat transmitted hantavirus diseases in Sarajevo. Lancet 1994; 344:131. [DOI] [PubMed] [Google Scholar]
- Clement J, Heyman P, Mc Kenna P, et al. . The hantaviruses of Europe: From the beside to the bench. Emerg Infect Dis. 1997; 3:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J, Neild G, Hinrichsen S, et al. . Urban leptospirosis versus urban hantavirus infection in Brazil. Lancet 1999; 354:2003–2004 [DOI] [PubMed] [Google Scholar]
- de Faria MT, Calderwood MS, Athanazio DA, et al. . Carriage of Leptospira interrogans among domestic rats from a high endemic urban setting for leptospirosis in Brazil. Acta Trop 2008; 108:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook JD, Shields T, Klein SL, et al. . Norway rat population in Baltimore, Maryland, 2004. Vector Borne Zoonotic Dis 2005; 5:296–299 [DOI] [PubMed] [Google Scholar]
- Easterbrook JD, Kaplan JB, Vanasco NB, et al. . A survey of zoonotic pathogens carried by Norway rats in Baltimore, Maryland, USA. Epidemiol Infect 2007; 135:1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H, Sanchez A, Morzunov S, et al. . Utilization of autopsy RNA for the synthesis of the nucleocapsid antigen of a newly recognized virus associated with hantavirus pulmonary syndrome. Virus Res 1993; 30:351–367 [DOI] [PubMed] [Google Scholar]
- Glass G, Childs J, Korch G, et al. . Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus). Epidemiol Infect 1988:459–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass GE, Gardner-Santana LC, Holt RD, et al. . Trophic garnishes: Cat–rat interactions in an urban environment. PLoS One 2009; 4:e5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia EL, Metcalfe JMD, de Carvalho ALF, et al. . Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerg Infect Dis 2008; 14:[serial on the Internet] Available from http://www.cdc.gov/EID/content/14/3/505.htm [DOI] [PMC free article] [PubMed]
- Gratz NG. Rodents as carriers of diseases In: Buckle AP.Smith RH. eds. Rodent Pests and Their Control. Wallingford, UK: CAB International; 1994:85–108 [Google Scholar]
- Gundi VA, Taylor C, Raoult D, et al. . Bartonella rattaustraliani sp. nov., Bartonella queenslandensis sp. nov. and Bartonella coopersplainsensis sp. nov., identified in Australian rats. Int J Syst Evol Microbiol 2009; 59:2956–2961 [DOI] [PubMed] [Google Scholar]
- Gundi VA, Billeter SA, Rood MP, et al. . Bartonella spp. in rats and zoonoses, Los Angeles, California, USA. Emerg Infect Dis 2012; 18:631–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Surveillance Secretary, BMoH. [Leptospirosis case notification records, Brazil] 2007
- Hinson ER. Wounding: The primary mode of Seoul virus transmission among male Norway rats. Am J Trop Med Hygiene 2004; 70:310–317 [PubMed] [Google Scholar]
- Hjelle B, Jenison S, Torrez-Martinez N, et al. . Rapid and specific detection of Sin Nombre virus antibodies in patients with hantavirus pulmonary syndrome by a strip immunoblot assay suitable for field diagnosis. J Clin Microbiol 1997; 35:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística Contagem da População 2007. Tabela 1.1.16, População recenseada e estimada, segundo os municípios, Bahia, 2007. 2007 [Google Scholar]
- Iversson LB, Travassos da Rosa APA, Rosa MDB, et al. . Infecçäo humana por Hantavirus no sul e sudeste do Brasil/Human infection by Hantavirus in southern and southeastern Brazil. Rev Assoc Med Bras 1992; 40:85–92 [PubMed] [Google Scholar]
- Katz G, Williams RJ, Burt MS, et al. . Hantavirus pulmonary syndrome in the State of Sao Paulo, Brazil, 1993–1998. Vector Borne Zoonotic Dis 2001; 1:181–90 [DOI] [PubMed] [Google Scholar]
- Kawamata J. Control of laboratory acquired hemorrhagic fever with renal syndrome (HFRS) in Japan. Lab Anim Sci 1987; 37:431–436 [PubMed] [Google Scholar]
- Klein SL. Environmental and physiological factors associated with Seoul virus infection among urban populations of Norway rats. J Mammal 2002; 83:478–488 [Google Scholar]
- Ko AI, Galvao Reis M, Ribeiro Dourado CM, et al. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet 1999; 354:820–825 [DOI] [PubMed] [Google Scholar]
- Ko AI, Goarant C, Picardeau M. Leptospira: The dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 2009; 7:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy MY, Regnery RL, Tzianabos T, et al. . Distribution, diversity, and host specificity of Bartonella in rodents from the Southeastern United States. Am J Trop Med Hyg 1997; 57:578–588 [DOI] [PubMed] [Google Scholar]
- Kosoy M, Mandel E, Green D, et al. . Prospective studies of Bartonella of rodents. Part I. Demographic and temporal patterns in population dynamics. Vector Borne Zoonotic Dis 2004; 4:285–295 [DOI] [PubMed] [Google Scholar]
- Kosoy M, Bai Y, Sheff K, et al. . Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg 2010; 82:1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas C, Curi A, Boia M, et al. . Human bartonellosis: Seroepidemiological and clinical features with an emphasis on data from Brazil—a review. Mem Inst Oswaldo Cruz 2008; 103:221–235 [DOI] [PubMed] [Google Scholar]
- Lamas C, Koppe K, Azevedo Tl. Bartonella spp infections diagnosed between 2005 and 2009 by the National Rickettsial Reference Laboratory in Rio de Janeiro, Brazil. Int J Infect Dis 2010; 14:E373–E37320594886 [Google Scholar]
- LeDuc JW, Smith GA, Pinheiro FP, et al. . Isolation of a Hantaan-related virus from Brazilian rats and serologic evidence of its widespread distribution in South America. Am J Trop Med Hyg 1985; 34:810–815 [DOI] [PubMed] [Google Scholar]
- LeDuc JW, Smith GA, Childs JE, et al. . Global survey of antibody to Hantaan-related viruses among peridomestic rodents. Bull World Health Org 1986; 64:139–144 [PMC free article] [PubMed] [Google Scholar]
- Macdonald DW, Mathews F, Berdoy ML. The behaviour and ecology of Rattus norvegicus: From opportunism to kamikaze tendencies. In: Singleton GR HL, Leirs H, Zhang Z, eds. Ecologically-Based Management of Rodent Pests. Canberra: Aciar Monograph No. 59; 1999 [Google Scholar]
- Matthias MA, Ricaldi JN, Cespedes M, et al. . Human leptospirosis caused by a new, antigenically unique leptospira associated with a rattus species reservoir in the peruvian Amazon. PLoS Negl Trop Dis 2008; 2:e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. Urban commensal rodent control: Fact or fiction? In: Singleton GR HL, Krebs CJ, Spratt DM, eds. Rats, Mice and People: Rodent Biology and Management. Canberra: Australian Centre for International Agricultural Research monograph no. 96, ACIAR2003:446–450 [Google Scholar]
- Mills JM. The role of rodents in emerging human disease: Examples from the hantaviruses, the arenaviruses. In: Singleton GR.Hinds LA, Leirs H, Zhang Z, eds. Ecologically-Based Management of Rodent Pests. Canberra: Australian Centre for International Agricultural Research; 1999:134–160 [Google Scholar]
- Mills JN, Childs JE, Ksiazek TG, et al. . Methods for trapping and sampling small mammals for virologic testing. In: Services USDoHaH, ed. Atlanta, 1995 [Google Scholar]
- Pereira MM, Matsuo MGS, Bauab AR, et al. . A clonal subpopulation of Leptospira interrogans sensu stricto is the major cause of leptospirosis outbreaks in Brazil. J Clin Microbiol 2000; 38:450–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phraisuwan P, Whitney EA, Tharmaphornpilas P, et al. . Leptospirosis: Skin wounds and control strategies, Thailand, 1999. Emerg Infect Dis 2002; 8:1455–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter FH, Costa F, Rodrigue G, et al. Demographic differences between tropical and temperate Norway rats. Submitted [Google Scholar]
- Reis RB, Ribeiro GS, Felzemburgh RDM, et al. . Impact of environment and social gradient on leptospira infection in urban slums. PLoS Neglected Trop Dis 2008; 2:e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfeliu I, Nogueras MM, Gegúndez MI, et al. . Seroepidemiological survey of hantavirus infection in healthy people in Valle`s Occidental, Barcelona. Vector Borne Zoonotic Dis 2011; 11:697–700 [DOI] [PubMed] [Google Scholar]
- Santos ED, Garrett DO. Evaluation of the hantavirus surveillance system in Brazil. Epidemiologia e Serviços de Saúde 2005; 14:15–31 [Google Scholar]
- Sarkar U, Nascimento SF, Barbosa R, et al. . Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am J Trop Medi Hyg 2002; 66:605–610 [DOI] [PubMed] [Google Scholar]
- Taylor PJ, Arntzen L, Hayter M, et al. Understanding and managing sanitary risks due to rodent zoonoses in an African city: Beyond the Boston Model. Integr Zool 2008; 3:38–50 [DOI] [PubMed] [Google Scholar]
- UN-HABITAT Slums of the world: The face of urban poverty in the new millennium? Nairobi: UN-HABITAT, 2003:94 pp [Google Scholar]
- Vanasco NB, Sequeira MD, Sequeira G, et al. . Associations between leptospiral infection and seropositivity in rodents and environmental characteristics in Argentina. Prev Vet Med 2003; 60:227–235 [DOI] [PubMed] [Google Scholar]
- Webster JP, Ellis WA. MacDonald DW. Prevalence of Leptospira and other zoonoses in wild brown rats on UK farms. Mammalia 1995; 59:645–622 [DOI] [PMC free article] [PubMed] [Google Scholar]