Abstract
Background: HIV-infected individuals may be at increased risk of poor physical function. Chronic inflammation has been associated with decreased physical function in the elderly and may also influence physical function in HIV-infected individuals.
Methods: This cross-sectional study assessed physical function in 65 HIV-infected women aged 40 and older on stable antiretroviral treatment using the Short Physical Performance Battery (SPPB): a standardized test of balance, walking speed, and lower- extremity strength developed for elderly populations. The relationship between low SPPB score, selected demographic and medical characteristics, and high inflammatory biomarker profile was analyzed using Fisher's exact test and Wilcoxon rank sum test.
Results: The median age of subjects was 49 years (interquartile range [IQR] 45–55), and the median CD4 T-cell count was 675 cells/mm3 (IQR 436–828). Thirteen subjects (20%) had a low SPPB score. Subjects with a low SPPB score were more likely to be cigarette smokers (p=0.03), had more medical comorbidities (p=0.01), and had higher levels of interleukin-6 (IL-6) (p<0.05). They also tended to be older (median age 55 vs. 48, p=0.06), more likely to have diabetes (p=0.07), and have higher levels of soluble tumor necrosis factor-1 (p=0.09).
Conclusions: Twenty percent of women aged 40 and older with well-treated HIV had poor physical-function performance, which was associated with the high burden of comorbidities in this population and with increased IL-6. However, it is unclear from this cross-sectional study whether increased inflammation was related to poor physical function or to other factors, such as age and medical comorbidities.
Introduction
For HIV-infected individuals receiving antiretroviral therapy (ART), HIV infection has been transformed from a deadly disease to a chronic condition. A growing number of individuals with HIV are now living into older age—about half of the 1.5 million people with HIV in the United States will be aged 50 or older in 2015.1 With the advent of ART, the incidence of previously common opportunistic infections has decreased.2 However, HIV-infected individuals remain at higher risk for a variety of noninfectious comorbidities, including cardiovascular disease, lipid abnormalities, and diabetes.3–8 Multiple medical comorbidities are common in ART-treated individuals,3,8,9 and older HIV-infected individuals are at increased risk for multiple comorbidities compared to the general population.3,8
HIV-infected individuals are also at increased risk for poor physical function, including the “frailty phenotype,” defined by the presence of three or more of the following: unintentional weight loss, self-reported exhaustion, weakness, slow walking speed, and low physical activity.10 HIV-infected individuals appear to have worse physical function compared to those without HIV11–15; however, poor physical function in HIV-infected individuals often appears related to low CD4 T-cell count11,12,14–17 or presence of other comorbidities, such as hypertension, diabetes, and chronic pulmonary disease.13,18–20 Worse physical function was associated with non-HIV-related comorbidities in studies conducted predominantly in HIV-infected men,13,17,19,20 although not in a study predominantly of women.14 In contrast to one study in South Africa, in which frailty risk was decreased in individuals with higher body mass index (BMI),14 poor physical function in HIV-infected individuals in the United States has frequently been associated with obesity and truncal obesity.18,20,21 HIV-infected men may have a frailty prevalence comparable to uninfected men 10 years older12 and aerobic capacity more similar to HIV-uninfected men 20 years older.22
The reasons behind the apparent decreased physical function in HIV-infected individuals are unclear. Several studies have reported associations between higher inflammatory biomarker levels, including interleukin-6 (IL-6), high-sensitivity C-reactive protein (CRP), hyaluronic acid (HA), D-dimer, soluble CD14 (sCD14), and soluble tumor necrosis factor receptor-1 (sTNFR1) and sTNFR2, and increased mortality in ART-treated individuals.23–26 Higher levels of inflammatory and coagulation biomarkers, such as IL-6, D-dimer, sCD14, and CRP, are seen in HIV-infected individuals compared to HIV-uninfected controls, despite treatment with ART and adjustment for other comorbidities27,28; however, one study suggested that these elevated biomarker levels in ART-treated individuals were seen primarily in those with low CD4 counts or elevated HIV viral loads.28 A recent study of HIV-infected individuals found that poor physical function was associated with immune activation and increased levels of IL-6.29 Higher levels of IL-6 and CRP are also associated with aging in the general population30,31 and have been associated with worse physical function in the elderly.32–36 Worse scores on the Short Physical Performance Battery (SPPB), an assessment of physical function, have been associated with higher levels of IL-6 and CRP in individuals aged 55 years or older with chronic conditions, including chronic obstructive lung disease, congestive heart failure, high cardiovascular disease risk, and self-reported disability.37 As HIV infection is associated with both increased inflammation and declines in physical function compared to the general population, it is possible that increased inflammation contributes to the declines in physical function seen in HIV-infected individuals despite apparent viral control with ART.
The primary goal of this cross-sectional study was to assess the level of physical function of HIV-infected women on ART. Our goal was to describe differences in women with lower physical function compared to those with high function and to test the hypothesis that higher levels of inflammatory biomarkers are associated with poor physical function in this population. We chose to perform this study with HIV-infected women because of the limited physical- function research in this group and the increasing global burden of HIV infection in racial and ethnic minority women.
Material and Methods
Population enrolled
This study was conducted at the Center for Infectious Diseases (CID) at Boston Medical Center in Boston, MA. The CID cares for approximately 1,400 HIV-infected patients, including about 40% women, and is one of the largest HIV providers in New England. English-speaking HIV-infected women aged 40 and older on a stable ART regimen for at least 6 months with a most recent HIV viral load less than 1,000 copies/mL were eligible to participate. Women were recruited during medical appointments or through self-referral from study fliers in the clinic. After written informed consent was obtained, each woman completed a questionnaire that included information about demographic characteristics and medical history. ART regimen, nadir CD4 count, most recent CD4 and HIV viral load values, and date of HIV diagnosis were abstracted from the electronic medical record. We also collected information about hepatitis C, coronary artery disease, and comorbidities associated with cardiovascular disease, including hypertension, hyperlipidemia, and diabetes mellitus, as these conditions have been shown to be associated with increased risk of inflammation.28,38–41 Medical comorbidities were defined by inclusion as an active problem in the electronic medical record. Serum was collected for biomarker quantification, and each woman underwent a physical-function assessment as described below. Body weight and height were recorded from the medical record and used to calculate BMI. The Boston University Medical Center Institutional Review Board approved this protocol.
Physical-function testing
Participants underwent a physical-function assessment consisting of the SPPB, which was conducted at the Boston Medical Center Laboratory for Exercise Physiology and Physical Performance. The SPPB was developed by the National Institute of Aging to assess physical function in individuals over 65 years of age and includes three measures: balance (defined by length of time able to stand with feet together, feet semitandem [big toe of one foot next to heel of the other], and tandem [heel to toe]), walking speed (best of two timed attempts at walking usual speed over a 4 meter distance), and chair stand (time to complete five transitions from sitting to standing without using arms).42 Each component of the SPPB is scored on a scale of 0 to 4, based on ability to complete the task and time required for completion, using the standardized scoring published previously for a maximum subscore of 4 for each component and a maximum overall score of 12.42 Although the SPPB was developed primarily for geriatric populations, we chose to use it as a function measure in our study because of the increased prevalence of frailty and diminished physical function that occur at an earlier age in HIV-infected individuals.11,12,14,16,17,22 The physical-function assessment was scheduled on the same day as the questionnaire and blood draw when possible; however, the physical-function assessment was performed by different study staff in another building and thus could be scheduled for a separate visit if necessary.
Inflammatory biomarkers
A single blood draw was obtained from each subject for serum collection. As this was a cross-sectional study, we chose a variety of inflammatory and fibrotic markers implicated in prior studies of inflammation and HIV23–27 for possible inclusion in a future longitudinal study. The following tests were performed: enzyme-linked immunosorbent assays (ELISAs): IL-6 (R&D Systems D6050, Minneapolis, MN), sCD14 (a marker of monocyte activation) (R&D Systems DC140), soluble tumor necrosis factor receptor 1 (sTNFR1) (R&D Systems DRT100), transforming growth factor beta 1 (TGFb1) (R&D Systems DB100B), hyaluronic acid (a marker for tissue turnover and fibrosis) (Corgenix, Broomfield, CO), and lipopolysaccharide (LPS) (a marker of bacterial translocation) (Lonza QCL-1000 LALAssay 50-647U, Walkersville, MD).
Statistical analysis
We analyzed demographics, general medical and HIV-related information, SPPB score, and inflammatory biomarker results, using descriptive statistics, including medians for continuous variables and proportions for categorical variables. We dichotomized our primary outcome, SPPB score, into low and high categories, using a cut-off score of 9 or lower to denote poor physical function, because scores in this range have been shown to be associated with increased risk of subsequent disability compared to scores of 10 to 12.43,44 Relationships between SPPB, inflammatory biomarkers, and potentially important covariates were assessed by Fisher's exact test for categorical data and Wilcoxon rank sum test for continuous data. We created a medical-comorbidities variable that included hypertension, hepatitis C, hyperlipidemia, diabetes, and coronary artery disease. We also assessed SPPB as a continuous variable, using Spearman correlation testing. All analyses were conducted in SAS, Version 9.1 (Cary, ID).
Results
Seventy-two women were enrolled in the study between February and August 2011, and 65 subjects (90%) completed the SPPB and are included in this analysis. The median age was 49 years (range 40–66 years), and 59 (91%) were racial and/or ethnic minorities, including 44 (68%) black and 11 (17%) Hispanic women (Table 1). Of 25 non-U.S.-born women, the majority were born in Haiti or on the African continent. The median BMI was 28.0 (IQR 24.2–33.7), and 26 subjects (40%) were obese (BMI≥30 kg/m2). Medical comorbidities included hypertension (49%), hepatitis C (28%), hyperlipidemia (28%), diabetes (25%), and coronary artery disease (5%). The seven excluded subjects did not attend SPPB testing. No subjects were excluded for safety reasons or because they were unable to complete SPPB testing. Excluded subjects were more likely to report active drug use (43% vs. 9% of subjects who underwent SPPB testing); otherwise, there were no significant differences in demographic or medical variables between subjects included in this analysis and excluded individuals.
Table 1.
Baseline Demographic and Medical Information (n=65)
| Characteristic | Result |
|---|---|
| Demographic information | |
| Age (years) |
49 (45–55) |
| Race/ethnicity | |
| White, not Hispanic |
6 (9) |
| Black, not Hispanic |
44 (68) |
| Hispanic |
11 (17) |
| Other or mixed race |
4 (6) |
| Born in United States |
40 (62) |
| Married or living with partner |
20 (31) |
| High school graduate or higher education |
38 (58) |
| Unemployed |
41 (63) |
| Illicit drug use (n=64) | |
| Current |
6 (9) |
| Former |
25 (39) |
| Smoking status | |
| Current |
22 (34) |
| Former |
14 (22) |
| General medical information | |
| BMI category | |
| Less than 25 |
18 (28) |
| 25 to less than 30 |
21 (32) |
| 30 to less than 35 |
17 (26) |
| ≥35 |
9 (14) |
| Hypertension |
32 (49) |
| Hepatitis C infection |
18 (28) |
| Hyperlipidemia |
18 (28) |
| Diabetes |
16 (25) |
| Coronary artery disease |
3 (5) |
| Number of comorbid medical conditionsa | |
| 0 |
16 (25) |
| 1 |
26 (40) |
| 2 or more |
23 (35) |
| HIV history | |
| Years since HIV diagnosis |
13 (8–16) |
| Nadir CD4 T-cell count (cells/mm3) (n=61) |
202 (96–269) |
| Current CD4 T-cell count (cells/mm3) |
675 (436–828) |
| Current HIV viral load ≤75 copies/mL | 58 (89) |
Data are presented as number (percent) or median (interquartile range).
Includes diagnosis of hypertension, hepatitis C, hyperlipidemia, diabetes, and coronary artery disease.
BMI, body mass index.
HIV history is reported in Table 1. Although 49% of women with a known nadir CD4 count had a nadir less than 200 CD4 cells/mm3, 43 subjects (66%) had a current CD4 count of ≥500 cells mm3, and 58 (89%) had a current HIV viral load ≤75 copies/mL. The levels of inflammatory biomarkers for the cohort are shown in Table 2.
Table 2.
Inflammatory Biomarker Levels for the Entire Cohort
| Inflammatory biomarkers | Median (interquartile range) |
|---|---|
| sTNFR1 (ng/mL) (n=62) |
1.7 (1.3–2.0) |
| sCD14 (ug/mL) (n=62) |
1.7 (1.6–2.2) |
| TGFB1 (ng/mL) (n=59) |
28.6 (17.5–43.3) |
| IL-6 (pg/mL) (n=62) |
0 (0–2) |
| HA (ng/mL) (n=61) |
0.6 (0–2.9) |
| LPS (endotoxin unit) (n=61) | 0.8 (0.4–1.2) |
HA, hyaluronic acid; IL-6, interleukin-6; LPS, lipopolysaccharide; sCD14, soluble CD14; sTNFR1, soluble tumor necrosis factor receptor-1; TGFB1, transforming growth factor beta 1.
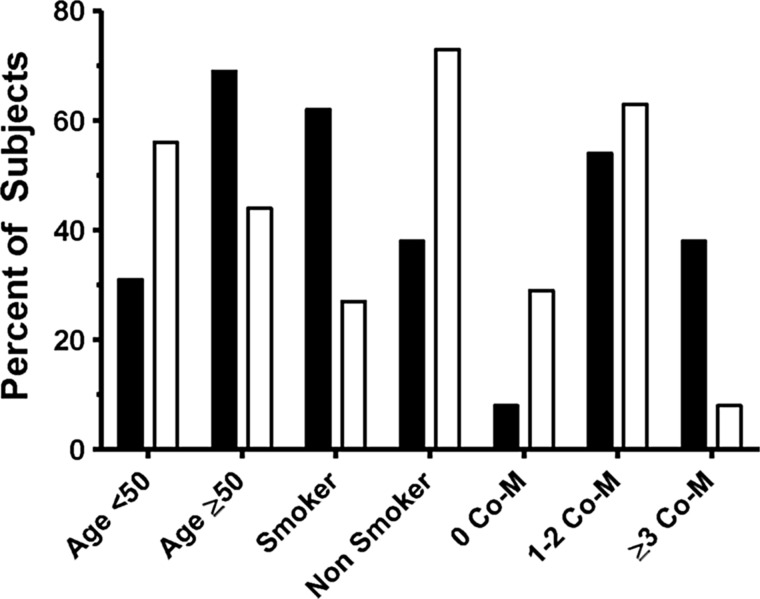
The median SPPB score was 11 out of 12 (range 6–12). Thirteen of 65 subjects (20%) had an SPPB score of 9 or lower (Table 3). There were greater differences in walking speed and chair-stand time (a surrogate for leg power) between the low- and high-scoring SPPB groups compared to balance score. Women with a low SPPB score were more likely to be current cigarette smokers and had more medical comorbidities, including hypertension, hepatitis C, hyperlipidemia, diabetes, and coronary artery disease (Table 3). (See Fig. 1.) Women in the low-SPPB group tended to be older (p=0.06) and be more likely to have diabetes (p=0.07). There were no apparent differences between women in the low- and high-SPPB groups in terms of current or nadir CD4 count or current viral load.
Table 3.
Factors Associated with Poor Short Physical Performance Battery Score
| Characteristic | SPPB≤9 n=13 | SPPB>9 n=52 | p-value |
|---|---|---|---|
| Demographic variables | |||
| Age (years) |
55 (48–56) |
48 (44–54) |
0.06 |
| Race/ethnicity | |||
| Black |
9 (69) |
35 (67) |
1.0 |
| White |
1 (8) |
5 (10) |
|
| Hispanic |
2 (15) |
9 (17) |
|
| Other |
1 (8) |
3 (6) |
|
| Current cigarette smoker |
8 (62) |
14 (27) |
0.03 |
| Current illicit drug use (n=64) |
0 |
6 (12) |
0.33 |
| General medical variables | |||
| Body mass index |
30.5 (25.6–34.5) |
27.9 (23.8–32.4) |
0.38 |
| Hypertension |
9 (69) |
23 (44) |
0.13 |
| Hepatitis C infection |
6 (46) |
12 (23) |
0.16 |
| Hyperlipidemia |
4 (31) |
14 (27) |
0.74 |
| Diabetes |
6 (46) |
10 (19) |
0.07 |
| Number of comorbid medical conditionsa | |||
| 0 |
1 (8) |
15 (29) |
0.01 |
| 1–2 |
7 (54) |
33 (63) |
|
| 3 or more |
5 (38) |
4 (8) |
|
| HIV variables | |||
| Nadir CD4 count (cells/mm3) (n=61) |
202 (70–223) |
200.5 (125–269.5) |
0.51 |
| Current CD4 count (cells/mm3) |
595 (471–826) |
678.5 (427–831.5) |
0.95 |
| Current HIV viral load ≤75 copies/mL |
10 (77) |
48 (92) |
0.14 |
| Component scores of Short Physical Performance Battery | |||
| Balance |
4 (3–4) |
4 (4–4) |
0.054 |
| Walking speed |
3 (3–3) |
4 (4–4) |
<0.0001 |
| Chair stand time |
2 (1–3) |
4 (3.5–4) |
<0.0001 |
| Inflammatory Variables | |||
| sTNFR1 (ng/mL) (n=62) |
2.0 (1.7–2.5) |
1.6 (1.3–1.8) |
0.09 |
| sCD14 (ug/mL) (n=62) |
2.0 (1.5–2.2) |
1.7 (1.6–2.0) |
0.84 |
| TGFB1 (ng/mL) (n=59) |
25.4 (19.1–47.8) |
32.2 (17.5–40.7) |
0.98 |
| IL-6 (pg/mL) (n=62) |
1 (0–3) |
0 (0–1) |
0.048 |
| HA (ng/mL) (n=61) |
1.5 (0–12.6) |
0.2 (0–2.6) |
0.17 |
| LPS (endotoxin unit) (n=61) | 0.8 (0.3–1.0) | 0.8 (0.4–1.2) | 0.54 |
Results presented as number (percent) or median (interquartile range).
Includes diagnosis of hypertension, hepatitis C, hyperlipidemia, diabetes, and coronary artery disease.
CD4, CD4 T-cell.
FIG. 1.
Percentage of subjects with Short Physical Performance Battery (SPPB) scores ≤9 (solid bars) and SPPB >9 (open bars) for variables associated with performance on SPPB. Comorbidities (Co-M) include hypertension, hepatitis C, hyperlipidemia, diabetes, and coronary artery disease.
Women in the low-SPPB group had higher IL-6 levels and a trend toward higher sTNFR1 levels (Table 3). More than 50% of our subjects had IL-6 levels below the limit of detection; when IL-6 was analyzed as a dichotomous variable, 9 subjects (69%) in the low-SPPB group had a detectable IL-6 level compared to 19 subjects (39%) in the higher-SPPB group (p=0.06) (results not shown). In Spearman correlation testing, SPPB score was inversely associated with sTNFR1 (correlation coefficient −0.29, p=0.02) but not any other inflammatory biomarker (results not shown).
Discussion
We found that 20% of HIV-infected women in this study had an SPPB score of 9 or lower, despite the fact that ART was effective in improving markers of HIV infection in the majority of subjects. Given the levels of immune reconstitution and viral suppression in these subjects, the SPPB scores were still low in 20% of our relatively young subjects on a test designed to assess decreased physical function in elderly individuals aged 65 years and older.42 It is concerning that one-fifth of our sample of relatively young participants with well-controlled HIV scored in a SPPB range associated with increased risk of developing disabilities.43,44 For comparison, in a study of men and women aged 65 years and older in the general population, 26% scored 9 or lower on the SPPB.45 Over half (53%) of community-dwelling adults aged 71 or older had an SPPB score of 9 or lower at baseline, and individuals with a baseline SPPB of 7 to 9 (the scores for the majority of our subjects with poor physical function) had 1.6 times the risk of disability in activities of daily living by 4 years compared to individuals with an SPPB of 10 to 12.43 In an additional analysis, the risk of subsequent disability for those with poor physical function was 1.8 to 2.1 times higher at 1 year and 1.5 to 1.9 times higher at 4 years after follow-up for elderly individuals with a baseline SPPB score of 7 to 9.44 The authors of that study projected from their results that about 10% of women aged 65 to 69 years with an SPPB score of 9 or lower would be disabled by one 1 year, increasing to 39% of women aged 85 to 89 years with an SPPB score of 9 or lower.44 This portends that younger HIV-infected women with low SPPB scores may have higher utilization of healthcare resources and risk of disability in the future. As the number of HIV-infected individuals living into older age will only continue to increase, additional research on physical function and disability in this population is needed.
A recently published study that assessed physical function in HIV-infected individuals aged 45 to 65 years on ART for at least 6 months20 found that, whereas a similar proportion of subjects had poor function, as defined by an SPPB score less than 9 in that study (7% in their population vs. 9% in our population), significantly more subjects in that cohort had no physical-function deficits compared to our cohort (62% vs. 45%, p=0.01). Although that study population20 is similar to our cohort in terms of age, median current CD4 >500 cells/mm3, and proportion of current smokers, that cohort consisted primarily of Caucasian (74%) men (85%) who have sex with men as their HIV risk factor (65%), which is starkly different compared to our study population. Women in the general population, as well as those with HIV infection, appear to be at increased risk for poor physical function10,14,15,20; however, the population in the study by Erlandson et al. and our cohort are too different to directly compare whether the difference in SPPB performance between the two groups is related to gender. To our knowledge, only two previous studies have assessed physical function and HIV infection in predominantly female populations.11,14 Terzian et al. found that the overall frailty prevalence in a younger cohort of HIV-infected women (median age 41 years, IQR 36–47 years) was less than 10%, which was similar to an HIV-uninfected comparison group.11 In a study of South Africans consisting of 74% women, HIV-infected individuals were at increased risk for frailty compared to HIV-uninfected subjects; however, less than 20% of that cohort was aged 50 or older, and increased risk of frailty was significantly associated with increasing age in women but not men.14 As the subjects in that study were relatively young, further study is needed to see how HIV infection impacts physical function as women age.
Forty percent of subjects in our study had one medical comorbidity (including hypertension, hepatitis C, hyperlipidemia, diabetes, and coronary artery disease), and 35% had two or more medical comorbidities. Women with greater numbers of medical comorbidities were more likely to have poor SPPB performance. This raises the question whether there is truly a connection between poor physical function and HIV infection or whether poor physical function in HIV-infected individuals is due primarily to comorbidities, such as hypertension and diabetes. A greater proportion of women in the low-SPPB-score group had diabetes, hypertension, and hepatitis C. However, these differences were not statistically significant when compared to women with an SPPB score greater than 9. Since this study was not powered to assess differences in prevalence of individual comorbid conditions, these findings must be replicated in larger studies. Because we were interested in the link between inflammatory biomarkers and poor physical function, we chose to focus this study on cardiovascular comorbidities and hepatitis C, which have previously been shown to be associated with increased inflammation.28,38–41 Since we were focused on inflammatory-associated comorbidities, we did not collect information on chronic obstructive pulmonary disease (COPD) or anemia, which have been associated with self-reported fatigue and poor function.18,46 Additionally, we felt that COPD diagnosis was likely to be underdiagnosed and reported in the clinical records of this patient population. The high frequency of comorbidities in our cohort composed of 91% racial and/or ethnic minority women appears consistent with what is observed in minority women of a similar age in the general U.S. population with regard to high BMI,47 hypertension,48 hyperlipidemia,49 and diabetes49 and the consequent increased risk for obesity and related complications.47 The association between non-HIV-related comorbidities and poor physical function seen in our study is in line with prior studies focused largely on men.13,15,17,19 Women with poor SPPB performance were also more likely to be current smokers. Cigarette smoking has been associated with worse physical function in some studies of HIV-infected and uninfected individuals13,20,50 but not others11,20; however, it is unclear from our analysis whether cigarette smoking is a risk factor for other medical comorbidities or is directly associated with poor physical function.
The median BMI (30.5 vs. 27.9) was higher in the poor-physical-function group, and a larger proportion of women in that group were obese as defined by BMI of 30 or greater (54% vs. 37%); however, these differences were not statistically significant. This is in contrast to a previous study of 40 HIV-infected individuals aged 50 or older on stable ART, which found that poor physical function was associated with higher BMI, truncal obesity, and fat mass.21 Notably, sarcopenic obesity has been described as a condition of increasing body weight coupled with decreasing body mass51 and has been associated with increased risk of declines in physical function.52,53 We did not collect information on body composition, and the phenomenon of frailty and decreased muscle mass in obese HIV-infected individuals warrants further exploration in future studies.
Although lower CD4 count has been associated with increased risk of frailty in multiple studies,11,12,14,16,17 current or nadir CD4 count was not statistically different between the low- and high-SPPB groups. A smaller proportion of women in the low-SPPB-score group had a viral load ≤75 copies/mL; however, this study was underpowered to assess the relationship between low SPPB score and CD4 count or HIV viral load. We did not collect information on prior opportunistic infection history or AIDS-defining events other than nadir CD4 count. In order to control for higher levels of inflammation due to untreated HIV, all participants in this study were on stable ART for at least 6 months. Two-thirds had a CD4 count ≥500 cells/mm3, and 89% had a viral load ≤75 copies/mL; therefore, our population likely has better physical function compared to the group of HIV-infected women over age 40 as a whole. In addition, only one subject who completed the physical-function assessment was older than 65. Thus, the burden of poor physical function in HIV-infected individuals is likely to increase as this population continues to age.
As seen in studies of the elderly and individuals with other chronic conditions,32–37,54 higher levels of IL-6 and sTNFR1 were associated with worse physical-function performance in HIV-infected women in our study. It is unclear from this cross-sectional study whether these inflammatory-profile differences are truly related to worse physical-function performance or simply a marker of the increased burden of comorbidities seen in the low-SPPB-score group. One study has looked at the association between inflammation and immune activation and functional impairment in HIV-infected individuals and found that after adjustment for CD4 count, tobacco use, and hepatitis B and C, poor physical function was associated with low CD4 count, high CD8 count, low CD4:CD8 ratio, immune activation as evidenced by percent of CD38/HLA-DR/CD8 T-cells, and increased level of IL-6 but not markers of microbial translocation (including LPS) or immune senescence.29 TNF-alpha and sCD14 were associated with increased risk of poor function in unadjusted, but not adjusted, analysis in that study, which was conducted in a predominantly male and Caucasian population.29 As both elderly and HIV-infected individuals have increased levels of inflammatory biomarkers, it makes sense that higher inflammatory profile could be a contributor to the diminished physical function seen in those with HIV. One study showed that elevated inflammatory biomarkers in ART-treated individuals were associated with an HIV viral load ≥500 copies/mL or CD4 count <200 cells/mm3,28; however, almost none of our subjects met these criteria.
Strengths of our study included that it was conducted in a population of predominantly minority women, with a racial and ethnic breakdown representative of HIV-infected women in the United States as a whole.55 To our knowledge, only one other study of physical function has focused solely on women with HIV.11 In addition, the physical-function assessment was conducted by trained exercise physiologists under standardized conditions.
There are some limitations to this study. Although 20% of subjects performed poorly on the SPPB, the SPPB was designed for elderly populations. The median SPPB score of 11 out of 12 in our study suggests that there is likely a ceiling effect to the SPPB in that some of our subjects who performed well on the SPPB may have physical-function limitations that would be apparent on a more rigorous test of physical function. The benefit of using the SPPB, however, is that it can easily and quickly be conducted in a clinical setting with minimal training for staff. Although we felt that it was important to limit the study to women with well-controlled HIV in order to limit variation in inflammation due to untreated HIV infection, the fact that all subjects were on stable ART and that the majority had a high CD4 T-cell count and HIV viral load of 75 copies/mL or lower means that our findings are likely not generalizable to the entire population of HIV-infected women over age 40. In addition, our study population was relatively young, with only five women aged 60 years or older; however, the age distribution of our cohort is representative of English-speaking women over 40 years of age on ART in our large female patient population. Further research will need to be done to assess the rate of decline in physical function as the population of HIV-infected women grows older. Finally, although we were able to compare the prevalence of poor SPPB score in our population with studies of older individuals in the general population and a largely male HIV-infected cohort, we were unable to include an age- and race/ethnicity-matched HIV seronegative comparison group, and an appropriate HIV-negative comparison group will be important for future research.
Conclusions
Our cross-sectional study identified 20% of relatively young HIV-infected women with poor physical function despite treatment with ART and good levels of immune reconstitution and viral suppression. Women with inflammatory comorbidities and higher systemic inflammation were at increased risk for poor physical function. A better understanding of the relationship between chronic inflammation and poor physical function in HIV-infected individuals will be necessary in order to decrease risk for disability at a younger age in this population. These issues warrant exploration in a larger longitudinal study.
Acknowledgments
The authors gratefully acknowledge Michal Naisteter and Elizabeth Meuser, who recruited the study subjects and provided study coordination.
This study was funded by pilot funding from the Section of Infectious Diseases, Boston University School of Medicine, the Claude D. Pepper Older Americans Independence Center, and NIH grants R01 AR05515 (M.M.) and 5 K12 HD043444-07 (A.S.B.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Luther VP, Wilkin AM. HIV Infection in older adults. Clin Geriatr Med 2007;23:567–583 [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338:853–860 [DOI] [PubMed] [Google Scholar]
- 3.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120–1126 [DOI] [PubMed] [Google Scholar]
- 4.Guaraldi G, Zona S, Alexopoulos N, et al. Coronary aging in HIV-infected patients. Clin Infect Dis 2009;49:1756–1762 [DOI] [PubMed] [Google Scholar]
- 5.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–1184 [DOI] [PubMed] [Google Scholar]
- 6.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–2003 [DOI] [PubMed] [Google Scholar]
- 7.Riddler SA, Li X, Chu H, et al. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med 2007;8:280–287 [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: Increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDs 2013;27:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchacz K, Baker RK, Palella FJ Jr., et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther 2013;18:65–75 [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–156 [DOI] [PubMed] [Google Scholar]
- 11.Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health 2009;18:1965–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007;62A:1279–1286 [DOI] [PubMed] [Google Scholar]
- 13.Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: Results from the Veterans Aging Cohort Study. AIDS Patient Care STDs 2011;25:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathai S, Gilbert C, Weiss HA, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr 2012;26:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desquilbet L, Margolick JB, Fried LP, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr 2009;50:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect 2009;59:346–352 [DOI] [PubMed] [Google Scholar]
- 18.Oursler KK, Goulet JL, Leaf DA, et al. Association of comorbidity with physical disability in older HIV-infected adults. AIDS Patient Care STDs 2006;20:782–791 [DOI] [PubMed] [Google Scholar]
- 19.Oursler KK, Katzel LI, Smith BA, Scott WB, Russ DW, Sorkin JD. Prediction of cardiorespiratory fitness in older men infected with the human immunodeficiency virus: Clinical factors and value of the six-minute walk distance. J Am Geriatr Soc 2009;57:2055–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erlandson KM, Allshouse AA, Jankowski CM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012;13:324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A new frailty syndrome: Central obesity and frailty in older adults with the human immunodeficiency virus. J Am Geriatr Soc 2012;60:545–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses 2006;22:1113–1121 [DOI] [PubMed] [Google Scholar]
- 23.Boulware DR, Huppler Hullsiek K, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011;203:1637–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis 2010;201:1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuhaus J, Jacobs DR, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010;201:1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease and biomarkers of inflammation, altered coagulation and monocyte activation. Clin Infect Dis 2012;55:126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erlandson KM, Allshouse AA, Jankowski CM, et al. Association of functional impairment with inflammation and immune activation in HIV-1-infected adults on effective antiretroviral therapy. J Infect Dis 2013;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leng SX, Xue Q-L, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc 2007;55:864–871 [DOI] [PubMed] [Google Scholar]
- 31.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities. Arch Intern Med 2002;162:2333–2341 [DOI] [PubMed] [Google Scholar]
- 32.Tiainen K, Hurme M, Hervonen A, Luukkaala T, Jylha M. Inflammatory markers and physical performance among nonagenarians. J Gerontol A Biol Sci Med Sci 2010;65:658–663 [DOI] [PubMed] [Google Scholar]
- 33.Taekema DG, Westendorp RGJ, Frolich M, Gussekloo J. High innate production capacity of tumor necrosis factor-a and decline of handgrip strength in old age. Mech Ageing Dev 2007;128:517–521 [DOI] [PubMed] [Google Scholar]
- 34.Stenholm S, Maggio M, Lauretani F, et al. Anabolic and catabolic biomarkers as predictors of muscle strength decline: The InCHIANTI Study. Rejuvenation Res 2010;13:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J Gerontol A Biol Sci Med Sci 2002;57:M326–332 [DOI] [PubMed] [Google Scholar]
- 36.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J Gerontol A Biol Sci Med Sci 2004;59:242–248 [DOI] [PubMed] [Google Scholar]
- 37.Brinkley TE, Leng X, Miller ME, et al. Chronic infl ammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci 2009;64A:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011;141:1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cushman M, Lemaitre RN, Kuller LH, et al. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 1999;19:493–498 [DOI] [PubMed] [Google Scholar]
- 40.Luc G, Bard JM, Juhan-Vague I, et al. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: The PRIME Study. Arterioscler Thromb Vasc Biol 2003;23:1255–1261 [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000;101:1767–1772 [DOI] [PubMed] [Google Scholar]
- 42.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Biol Sci Med Sci 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 43.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: Analysis from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 2009;64A:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semba RD, Martin BK, Kempen JH, Thorne JE, Wu AW. The impact of anemia on energy and physical functioning in individuals with AIDS. Arch Intern Med 2005;165:2229–2236 [DOI] [PubMed] [Google Scholar]
- 47.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US Adults, 1999–2010. JAMA 2012;307:491–497 [DOI] [PubMed] [Google Scholar]
- 48.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010;303:2043–2050 [DOI] [PubMed] [Google Scholar]
- 49.Crawford AG, Cote C, Couto J, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: Findings from the GE Centricity Electronic Medical Record Database. Popul Health Manag 2010;13:151–161 [DOI] [PubMed] [Google Scholar]
- 50.Erlandson KM, Allshouse AA, Jankowski CM, Mawhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV-infection. J Acquir Immune Defic Syndr 2013;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: Definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008;11:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rolland Y, Lauwers-Cances V, Cristini C, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: The EPIDOS (EPIDemiologie de l'OSteoporose) Study. Am J Clin Nutr 2009;89:1895–1900 [DOI] [PubMed] [Google Scholar]
- 53.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12:1995–2004 [DOI] [PubMed] [Google Scholar]
- 54.Schaap LA, Pluijm SMF, Deeg DJH, et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 2009;64A:1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention HIV surveillance in women (through 2011). http://www.cdc.gov/hiv/library/slideSets/index.html Accessed May10, 2013