Abstract
Background: Oral contraceptive (OC) use seems to have little effect on weight change in normal weight women. Most previous studies have excluded obese women, so the effect of OC use on weight change in obese women is unknown.
Methods: This analysis evaluates weight and body composition change with OC use among obese (body mass index [BMI] 30.0–39.9) and normal weight (BMI 19.0–24.9) women who were randomly assigned to two OC doses: 20 μg ethinyl estradiol (EE) and 100 μg levonorgestrel (LNG) OCs or 30 μg EE and 150 μg LNG OCs. Follow-up occurred after three to four OC cycles. Weight and body composition were measured at baseline and at follow-up using a bioelectrical impedance analyzer.
Results: Among 150 women (54 obese and 96 normal weight) who used OCs for 3 to 4 months, there were no clinically or statistically significant weight or body composition changes in the overall group or by BMI or OC formulation group.
Conclusions: These findings add to evidence that EE/LNG OCs are not associated with short term weight or body composition change for normal weight women and suggest that OCs are also are not associated with short term weight or body composition change in obese women.
Introduction
Many women and clinicians believe that weight gain is a common side effect of oral contraceptive (OC) use.1,2,3 In a report from the 2006–2010 National Survey of Family Growth, 63% of women who had ever used OCs and discontinued use due to dissatisfaction cited side effects as a reason for discontinuation,4 and weight gain is one of the most common side effects reported by OC users.1,5,6,7 OC users who attribute weight gain to OCs are less likely to continue OC use.8 This belief has the potential to have a significant public health impact, as unintended pregnancies among women who discontinue OCs significantly contributes to the number of unintended pregnancies in the United States.9 In the 2006–2010 National Survey of Family Growth, concerns about side effects of birth control was cited as a reason for not using contraception at time of conception for 19% of unwanted births and 12% of mistimed births.10
Numerous studies have examined the effect of OCs on weight. In 2011, the Cochrane Collaboration conducted a meta-analysis of 49 trials,2 and the authors concluded that the current literature is insufficient to draw a definite conclusion about the effect of OCs on weight gain, but that there is no evidence of a large effect.2
Although over 30% of women in the United States are obese,11 most previous studies of the effect of OC use on weight gain have excluded obese women. It is possible that the effect of OCs on weight gain may differ among women with obesity compared to normal weight women. Most previous studies lack standardized measurement procedures, which could introduce random error and attenuate a small effect of OC use on weight change. Most previous studies have examined only the effect of OCs on weight, but if OC use is associated with weight gain, then body composition changes (changes in total body water, fat free mass, and fat mass) might indicate the causal pathway through which OC use leads to weight gain.
Hypothesized pathways through which OC use could cause weight gain include water retention2,12; an estrogen-mediated increase in subcutaneous fat2,13; an effect on satiety and appetite, leading to an increase in food intake2,13; and an androgen-mediated increase in muscle mass.2,13 If OC use does have an effect on weight gain, then a higher dose OC might have a greater effect than a lower dose OC of the same estrogen and progestin types.2
The objective of this analysis was to examine weight and body composition change after three months of OC use in obese and normal weight women and to examine whether this relation differs between obese versus normal weight women, as well as between two widely marketed OC doses.
Materials and Methods
Study design and participants
This was a planned secondary analysis from a prospective randomized trial that evaluated whether obese women are at a higher risk of OC failure than normal weight women due to less contraceptive-mediated ovarian suppression.14 The present analysis tested two hypotheses: (1) short term OC use is not associated with weight or body composition change among obese or normal weight women and (2) similarly, OC use is not associated with weight or body composition change for 20 μg ethinyl estradiol (EE) and 100 μg levonorgestrel (LNG) OCs or 30 μg EE and 150 μg LNG OCs. The primary outcome is weight change (kg) and the secondary outcomes are changes in (1) total body water (kg), (2) percent body fat, (3) fat mass (kg), and (4) fat free mass (kg). Change in body mass index (BMI) (kg/m2) is captured by weight change, as BMI was calculated using the baseline height measurement for each study participant; thus, BMI change is not reported separately.
Details of the study procedures have been described in detail elsewhere.14 Briefly, between July 2006 and December 2008, 226 women (128 normal weight and 98 obese women) participated in this study. Participants were recruited in two groups: normal weight (BMI 19.0–24.9) and obese (BMI 30.0–39.9). Overweight women were not included to create a clear distinction between BMI groups. Participants were aged 18–35 and had no contraindications to OC use. Participants were stratified by BMI class and randomly assigned to one of the two 21-day monophasic OC formulations (1:1 allocation): 30 μg EE and 150 μg LNG, or 20 μg EE and 100 μg LNG (Portia and Lessina, respectively, Barr Laboratories, Inc.). Henceforth, we refer to these formulations as 30/150 and 20/100, respectively. Both OC formulations were FDA-approved and widely marketed. Follow-up occurred after three to four OC cycles. This length of follow up was selected based on the design of the parent trial, which specified biweekly blood draws and sonograms during the third or fourth cycle of OC use.
To evaluate weight and body composition change with OC use, the sample for the present analysis includes women who remained in the study for at least three OC cycles and were determined to be consistent OC users based on LNG assay, as previously described.14
All study visits took place at the Columbia University Medical Center in New York City. This trial was approved by the Columbia University Medical Center Institutional Review Board and was registered with ClinicalTrials.gov (NCT00827632).
Outcome measures
At baseline and follow-up, the research coordinator measured the height, weight, and body composition of each participant. For each participant, weight and body composition change was calculated as the difference between the measurements from baseline to follow-up. Standing height was measured to the nearest 1.0 cm using a stadiometer. Weight and body composition [total body water (kg), percent body fat, fat mass (kg), and fat free mass (kg)] were measured by bioelectrical impedance analysis (BIA) using the BC-418 (Tanita Corp., Tokyo, Japan), an eight-contact electrode single frequency 50-kHz body composition analyzer. To avoid inter-instrument variation, all BIA measurements were taken with the same machine.
BIA was chosen as the primary method of body composition assessment because it is easy to use, fast, safe, and relatively inexpensive.15 Due to the large sample size and because body composition change was not the primary outcome of the parent trial, this method was chosen over more advanced techniques, such as dual-energy x-ray absorptiometry (DXA), which is less accessible and more costly. BIA measures the impedance of a small electrical current traveling through the body.16 Based on impedance measurements, total body water is calculated, and subsequently, fat-free mass and fat mass are calculated.15,16,17 BIA has been shown to provide reliable estimates of body composition in healthy adults.16,18
Statistical analysis
Characteristics of obese and normal weight participants were compared using t-tests for continuous variables and chi-square and Fisher's exact tests for categorical variables. We used t-tests to compare weight and body composition change from baseline to follow-up in the overall sample, within each BMI subgroup and each OC dose subgroup, as well as between BMI subgroups and OC dose subgroups. Multiple linear regression was used to examine BMI subgroup and OC dose subgroup together, and the following variables were considered as potential confounders: age, race/ethnicity, education, previous birth, smoking, OC use at baseline, and length of follow-up.
The sample size was selected based on power calculations for the primary objective of the parent study, which was to evaluate differences in follicular suppression during OC use in obese and normal weight women. Retrospective power analysis using observed sample size and variance for weight change showed that the present analysis had 90% power to detect a mean weight change of 1.05 kg in the obese group and 0.56 kg in the normal weight group as well as 90% power to detect a difference in weight change of 1.08 kg between BMI groups and 1.04 kg between OC randomization groups. Thus, the achieved sample size was sufficient to detect small changes in weight. Statistical analyses were performed using SAS statistical software, versions 9.1 and 9.2 (SAS Institute).
Results
A participant was included in the present analysis if she completed at least one follow-up visit and was a consistent OC user during the study, as defined by LNG assay.14 Out of the 226 enrolled participants, 45 (19.9% overall, 17.2% in the normal weight group, 23.5% in the obese group) were lost to follow-up or withdrew from the study prior to the follow-up examinations. Of the 181 women who participated in follow-up examinations, 31 (17.1% overall, 9.4% in the normal weight group, 28.0% in the obese group) were not consistent OC users based on LNG assays. Thus, the present analysis includes 150 (66.4%) of the 226 enrolled participants: 96 (75.0%) of the 128 normal weight and 54 (55.1%) of the 98 obese participants; 70 (61.4%) of the 114 participants assigned to 20/100 OCs, 80 (71.4%) of the 112 participants assigned to 30/150 OCs. Follow-up occurred during the third or fourth study cycle for 145 (96.7%) of the 150 participants and during the fifth to seventh study cycle for the five remaining participants due scheduling of the follow-up visits for the parent study, which required bi-weekly exams during the follow-up OC cycle.
Baseline characteristics of the analytic sample, stratified by BMI group, are presented in Table 1. Obese and normal weight participants differed with respect to race/ethnicity and reproductive history.
Table 1.
Baseline Characteristics of Consistent Oral Contraceptive Users by Body Mass Index Group
| Variable | Normal weight (n=96) | Obese (n=54) | p1 |
|---|---|---|---|
| Height (cm) |
164.1±6.4 |
163.8±6.6 |
0.83 |
| Weight (kg) |
59.6±6.7 |
91.9±9.3 |
<0.01 |
| BMI (kg/m2) |
22.1±1.5 |
34.2±2.6 |
<0.01 |
| Percent body fat (%)2 |
29.2±3.7 |
47.4±3.5 |
<0.01 |
| Fat mass (kg)2 |
17.6±3.7 |
43.8±6.9 |
<0.01 |
| Fat free mass (kg)2 |
42.1±3.9 |
48.2±4.0 |
<0.01 |
| Total body water (kg)2 |
29.4±2.9 |
36.8±3.6 |
<0.01 |
| Age |
24.7±4.5 |
25.7±4.0 |
0.17 |
| Race/ethnicity | |||
| Hispanic |
19 (19.8) |
19 (35.2) |
<0.01 |
| Non-Hispanic black |
30 (31.3) |
23 (42.6) |
|
| Non-Hispanic white |
33 (34.4) |
11 (20.4) |
|
| Non-Hispanic Asian |
14 (14.6) |
1 (1.9) |
|
| Education | |||
| Less than a bachelor's degree |
43 (44.8) |
31 (57.4) |
0.14 |
| Bachelor's degree or more |
53 (55.2) |
23 (42.6) |
|
| Previous pregnancy | |||
| Yes |
29 (30.2) |
28 (51.9) |
<0.01 |
| No |
67 (69.8) |
26 (48.2) |
|
| Previous birth | |||
| Yes |
9 (9.4) |
20 (37.0) |
<0.01 |
| No |
87 (90.6) |
34 (63.0) |
|
| Using OC at enrollment | |||
| Yes |
22 (22.9) |
12 (22.2) |
0.92 |
| No |
74 (77.1) |
42 (77.8) |
|
| Smokes cigarettes | |||
| Yes |
11 (11.5) |
10 (18.5) |
0.23 |
| No |
85 (88.5) |
44 (81.5) |
|
| OC dose | |||
| 20 μg EE/100 μg LNG |
47 (49.0) |
23 (42.6) |
0.45 |
| 30 μg EE/150 μg LNG | 49 (51.0) | 31 (57.4) | |
Values are shown as mean±standard deviation or n (%).
t-test for continuous variables and chi-square test or Fisher's exact test for categorical variables.
Baseline measurements for these variables were unavailable for one normal weight participant due to machine malfunction.
EE, ethinyl estradiol; LNG, levonorgestrel; OC, oral contraceptive.
All 150 participants are included in analysis of weight change, but 5 participants were excluded from analyses of BIA measurements. Baseline BIA measurements were not taken for one normal weight participant (30/150 group). Four [n=3 normal weight (all 20/100) and n=1 obese (30/150)] BIA change observations were extreme outliers. For all four of these participants, measured fat mass change was disproportionate to or in the opposite direction of weight change. Due to conditions known to affect BIA values (hydration, consumption of food and beverages, and changes in the menstrual cycle)16,18 that were not standardized for this study, it was concluded that these extreme outliers were due to measurement error, and not due to true changes in body composition. The resulting sample size for BIA analyses was 145 participants (n=92 normal weight, n=53 obese; n=67 20/100, n=78 30/150).
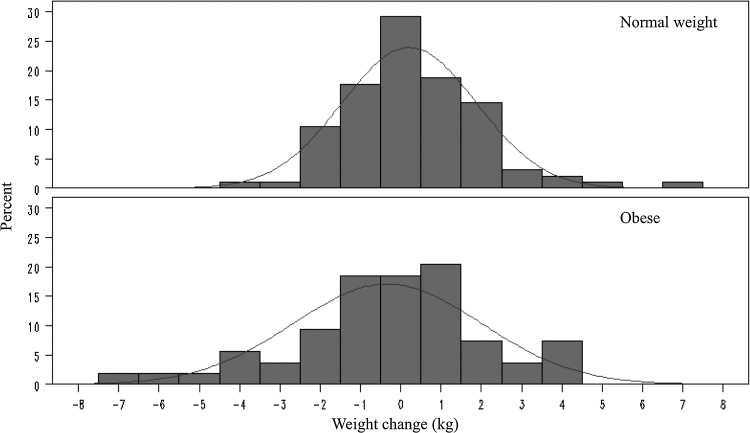
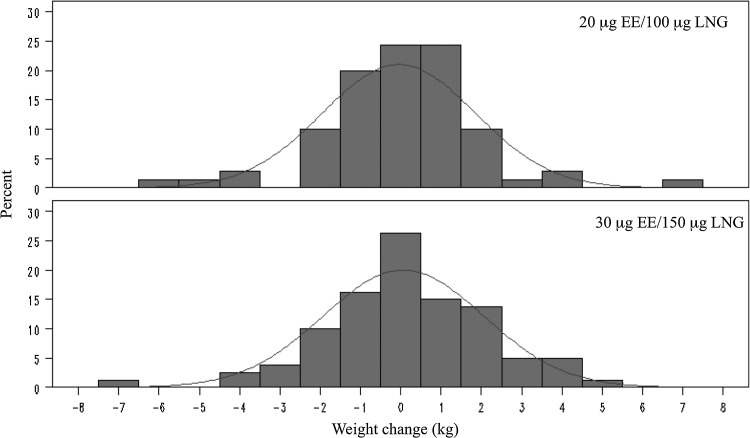
The results support our hypotheses that on average, short term OC use has little effect on weight and body composition for both normal weight and obese women and for both 20/100 and 30/150 OC formulations. No significant changes in weight or body composition were observed in the overall sample (Table 2), in either BMI group (Fig. 1), or in either OC group (Fig. 2). Additionally, no significant differences in weight or body composition change were observed between BMI groups (Table 3) or between OC formulation groups (Table 4). Multiple linear regression analyses adjusted for potential confounders showed that BMI group (obese vs. normal weight b=−0.63 kg, p=0.08), OC formulation (30/150 vs. 20/100, b=−0.036 kg, p=0.92), and their interaction (p=0.76) were not significantly associated with weight change.
Table 2.
Weight and Body Composition Change After Three Months Among All Consistent Oral Contraceptive Users
| Variable | Difference (follow-up – baseline) | p |
|---|---|---|
| Weight (kg) (n=150) |
0.016±1.95 |
0.92 |
| Total body water (kg) (n=145) |
0.080±1.15 |
0.40 |
| Fat free mass (kg) (n=145) |
0.035±0.84 |
0.61 |
| Fat mass (kg) (n=145) |
0.008±1.60 |
0.95 |
| Percent body fat (n=145) |
0.018±1.35 | 0.87 |
Values are shown as mean±standard deviation.
FIG. 1.
Weight change (kg) after 3 months of oral contraceptive use by body mass index group.
FIG. 2.
Weight change (kg) after 3 months of oral contraceptive use by oral contraceptive group.
Table 3.
Weight and Body Composition Change After Three Months Among Consistent Oral Contraceptive Users by Body Mass Index Group
| Variable | Normal weight Difference (follow-up – baseline) | Obese Difference (follow-up – baseline) | p |
|---|---|---|---|
| Weight (kg) (n=150) |
0.21±1.67 |
−0.33±2.34 |
0.11 |
| Total body water (kg) (n=145) |
0.16±1.18 |
−0.051±1.11 |
0.30 |
| Fat free mass (kg) (n=145) |
0.11±0.98 |
−0.096±0.48 |
0.15 |
| Fat mass (kg) (n=145) |
0.12±1.32 |
−0.19±1.98 |
0.25 |
| Percent body fat (n=145) |
0.10±1.53 | −0.13±0.95 | 0.33 |
Values are shown as mean±standard deviation.
Table 4.
Weight and Body Composition Change After Three Months Among Consistent Oral Contraceptive Users by Oral Contraceptive Pill Dose
| |
20 μg EE/100 μg LNG |
30 μg EE/150 μg LNG |
|
|---|---|---|---|
| Variable | Difference (follow-up – baseline) | Difference (follow-up – baseline) | p |
| Weight (kg) (n=150) |
−0.044±1.90 |
0.069±2.00 |
0.72 |
| Total body water (kg) (n=145) |
0.128±1.13 |
0.038±1.18 |
0.64 |
| Fat free mass (kg) (n=145) |
0.013±0.82 |
0.054±0.85 |
0.77 |
| Fat mass (kg) (n=145) |
−0.046±1.61 |
0.054±1.60 |
0.71 |
| Percent body fat (n=145) |
−0.007±1.50 | 0.040±1.21 | 0.83 |
Values are shown as mean±standard deviation.
Discussion
In this planned secondary analysis from a prospective study of obese and normal weight women randomly assigned to use either 20/100 or 30/150 OC, no clinically or statistically significant changes in weight or body composition were observed in the overall group, in either BMI subgroup, or in either OC formulation subgroup, despite adequate power to identify small changes. No differences in weight or body composition change were observed in obese women compared to normal weight women or in women taking 30/150 OCs compared to those taking 20/100 OCs.
We observed only minor changes in weight and body composition in the overall sample and in the BMI and OC dose subgroups; these changes were not clinically or statistically significant. The standard deviations of these estimates were also relatively small, which indicates that it is unlikely that these null results are due to random error. While most women experienced no significant weight change, a few women gained or lost significant weight; among those women, weight loss was as common as weight gain.
Although average weight change was small in the overall group and in each subgroup, obese participants and participants assigned to the lower dose OCs lost a small amount of weight on average, while normal weight participants and participants assigned to the higher dose OCs gained a small amount of weight on average. The slight weight loss in the obese group and weight gain in the normal weight group is likely due to regression to the mean. It is possible that over a longer follow-up period these differences between groups could become clinically meaningful.
The present study compared two distinct BMI groups. To our knowledge, there are no published trials that compare weight and body composition change with OC use in obese and normal weight women, and only two published studies that compare weight change in obese (BMI≥30) and nonobese (BMI<30) OC users. The first study included adolescent girls who initiated OC use at a health clinic visit.19 Over 18 months of OC use, the nonobese participants gained significantly more weight than obese participants. The second study is a cohort study of women using EE/desogestrel OCs and nonhormonal methods over 36 months.20 Body composition change was measured with DXA. The authors found that women using OCs experienced a small increase in body fat compared to the control group, but that weight change did not differ between obese and nonobese women.
The findings of this study are limited by the duration of follow-up and the lack of a placebo group. It is possible that changes in weight or body composition could have been observed over a longer period of follow-up. Approximately 33% of participants either dropped out of the study prior to follow up examinations or were not consistent OC users and were excluded from this analysis. While BIA is not the gold standard measure of body composition, the present study was concerned with change rather than absolute values of body composition, and BIA has been shown to be a reliable measure of body composition.16,18 There are some limitations of using BIA to measure body composition. First, fat free mass and fat mass estimates are based on the estimate for total body water, so if total body water changes, fat free mass and fat mass estimates will also change. Since no changes in weight, total body water, fat free mass, or fat mass were observed, this did not limit our conclusions. Second, we did not standardize several factors known to affect BIA estimates: hydration status, time since last meal, time of day, recent physical activity, and phase of the menstrual cycle.16 This probably accounts for the four extreme outliers that were excluded from BIA analyses. Given the large number of participant study visits required for the parent study, it was not feasible to standardize time of day that BIA measurements were taken.
This study is innovative in that it included obese women, an understudied group that makes up over 30% of the population.11 Another strength is that participants were randomized to use one of two widely marketed OC formulations of the same estrogen and progestin types. Another strength of this study is that measurement procedures were standardized to minimize random variation and the study had sufficient statistical power to detect small changes in weight and body composition.
In conclusion, our findings support previous evidence that on average, OC use is not associated with weight change. Among the minority of women who did experience weight change, weight loss was as common as weight gain. Furthermore, our findings suggest that this association does not differ in obese women compared to normal weight women.
Acknowledgments
This study was supported by National Institutes of Health Grant R01 HD045786. Duramed Pharmaceuticals donated the oral contraceptives provided to study participants. Clinical Trial Registration URL: www.clinicaltrials.gov; Identifier: NCT00827632
Disclosure Statement
C.L.W. is an advisory board member of Agile and a consultant for Merck and Bayer.
The other authors have no potential conflicts of interest to report.
References
- 1.Gaudet L, Kives S, Hahn P, Reid R. What women believe about oral contraceptives and the effect of counseling. Contraception 2004;69:31–36 [DOI] [PubMed] [Google Scholar]
- 2.Gallo MF, Lopez LM, Grimes DA, Schulz KF, Helmerhorst FM. Combination contraceptives: Effects on weight. Cochrane Database Syst Rev 2011;9: CD003987DOI: 10.1002/14651858.CD003987.pub4 [DOI] [PubMed] [Google Scholar]
- 3.Picardo CM, Nichols M, Edelman A, Jensen JT. Women's knowledge and sources of information on the risks and benefits of oral contraception. J Am Med Womens Assoc 2003;58:112–116 [PubMed] [Google Scholar]
- 4.Daniels K, Mosher WD, Jones J. Contraceptive methods women have ever used: United States, 1982–2010. National health statistics reports; No. 62. Hyattsville, MD: National Center for Health Statistics, 2013 [PubMed] [Google Scholar]
- 5.Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception 2007;76:267–272 [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg MJ.Waugh MS, Meehan TE. Use and Misuse of Oral Contraceptives: Risk Indicators for Poor Pill Taking and Discontinuation. Contraception 1995;51:283–288 [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: A prospective evaluation of frequency and reasons. Am J Obstet Gynecol 1998; 179:577–582 [DOI] [PubMed] [Google Scholar]
- 8.Westhoff CL, Heartwell S, Edwards S, et al. . Oral contraceptive discontinuation: Do side effects matter? Am J Obstet Gynecol 2007;196:412e1–412e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med 1995; 40:355–360 [PubMed] [Google Scholar]
- 10.Mosher WD, Jones J, Abma JC. Intended and unintended births in the United States: 1982–2010. National health statistics reports; No. 55. Hyattsville, MD: National Center for Health Statistics, 2012 [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS data brief; No. 82. Hyattsville, MD: National Center for Health Statistics, 2012 [PubMed] [Google Scholar]
- 12.Oelkers W, Foidart JM, Dombrovicz N, Welter A, Heithecker R. Effects of a new oral contraceptive containing an antimineralocorticoid progestin, drospinerone, on the rennin-aldosterone system, body weight, blood pressure, gludcose tolerance, and lipid metabolism. J Clin Endocrinol Metab 1995;80:1816–1821 [DOI] [PubMed] [Google Scholar]
- 13.Nelson AL. Combined hormonal contraceptive methods. Oral contraceptives. In: Hatcher RA, Trussell J, Nelson A, Cates W, Stewart F, Kowal D. Contraceptive Technology, 19th ed. New York: Contraceptive Technologies, Inc., 2007:193–270 [Google Scholar]
- 14.Westhoff CL, Torgal AH, Mayeda ER, et al. . Ovarian Suppression in normal-weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol 2010; 116:275–283 [DOI] [PubMed] [Google Scholar]
- 15.Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care 2008;11:566–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bioelectrical impedance analysis in body composition measurement: NIH Technological Assessment Statement. Am J Clin Nutr 1996;64:524S–532S [DOI] [PubMed] [Google Scholar]
- 17.Guo SS, Chumlea WC, OCkram DB. Use of statistical methods to estimate body composition. Am J Clin Nutr 1996;64:428S–435S [DOI] [PubMed] [Google Scholar]
- 18.Kyle UG, Bosaeus I, De Lorenzo AD, et al. . Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin Nutr 2004;23:1226–1243 [DOI] [PubMed] [Google Scholar]
- 19.Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006;160:40–45 [DOI] [PubMed] [Google Scholar]
- 20.Berenson AB, Rahman M. Changes in weight, total fat, percent body fat and central-to-peripheral fat ratio associated with injectable oral contraceptive use. Am J Obstet Gynecol 2009;200:329e1–329e8 [DOI] [PMC free article] [PubMed] [Google Scholar]