Abstract
This is the first study carried out to describe the role of fetal microchimerism (FM) in the pathogenesis of uterine cancer. The prevalence and concentration of male fetal microchimeric cells (FMCs) were examined in endometrial tissues in relation to subtypes of uterine cancer, and the histological grade and stage of the tumor. FM occurrence was analyzed in relation to risk factors, including hypertension, obesity, type 2 diabetes, dyslipidemia, age at cancer diagnosis, and patient pregnancy history. The prevalence and concentration of FMCs were examined in endometrial tissues using real-time polymerase chain reaction, SRY and β-globin sequences as markers for male fetal FMCs and total DNA. The studied group involved 47 type 1 endometrial cancers, 28 type 2 endometrial cancers, and 41 benign uterine diseases. While the prevalence of FM was decreased only in type 1 endometrial cancer, compared with benign uterine disorders (38.3% vs.70.7%; odds ratio [OR]=0.257, 95% confidence interval [CI]: 0.105 to 0.628, p=0.003), FMC concentrations did not differ within examined groups. The lower FM prevalence was detected in low-grade (grade 1 and grade 2) endometrioid cancer (38.3% vs. 70.7%, OR=0.256, 95% CI: 0.105 to 0.627, p=0.003) and in FIGO 1 tumors (40.7% vs. 70.7%, OR=0.285, 95% CI: 0.120 to 0.675, p=0.004). No correlation between FM prevalence or FMC concentrations and risk factors was demonstrated. A lower prevalence of male FM seemed to be associated with better prognoses in uterine cancer based on tumor subtype, histological grade, and stage of the tumor.
Introduction
Fetal microchimerism (FM) is defined as the long-term persistence of small numbers of fetal-derived allogeneic cells in maternal organs and circulation. FM is a naturally occurring phenomenon accompanying each gestation (Artlett, 2005; Yan et al., 2005; Fleta et al., 2006; Lapaire et al., 2007). Fetal cells cross the placenta throughout gestation. While the majority of fetal cells (nucleated erythrocytes, leukocytes, and trophoblasts) are present in the maternal circulation throughout gestation, some fetal cells cross the placenta exclusively during certain stages of pregnancy. For instance, mesenchymal stem cells can be detected in maternal circulation only during the 7th and 14th weeks of gestation (Clayton et al., 1964; Walknowska et al., 1969; Mueller et al., 1990; Campagnoli et al., 2001). Stem and progenitor cells of fetal origin (hematopoietic and mesenchymal stem cells, endothelial progenitor cells) can engraft and proliferate in maternal bone marrow. Afterward, they settle in target maternal tissues, where, under appropriate micro-environmental stimuli, they can differentiate into cells expressing tissue-specific markers and carry out a variety of functions (Bianchi et al., 1996; Campagnoli et al., 2000; Guetta et al., 2003; O'Donoghue et al., 2003; Bayes-Genis et al., 2005; Khosrotehrani and Bianchi, 2005; Nguyen et al., 2006; O'Donoghue and Chan 2006; Buemi et al., 2007; Savvidou et al., 2008; Parant et al., 2009; Luppi et al., 2010). Fetal microchimeric cells (FMCs) have been recently shown to have diverse and controversial affects. FMCs can be involved in tissue repair or take part in inducement of chronic inflammation, leading to autoimmunity and cancer (Nassar et al., 2012). With regard to gynecologic malignancies, only a limited number of studies are available. A study by Cha et al. (2003) was the first to report the presence of male microchimeric cells in cervical tissues derived from patients with cervical cancer. Initial studies by Gadi and Nelson (2007) and Gadi et al. (2008) demonstrated that the presence of allogeneic FM in peripheral blood mononuclear cells in women, who had given birth to a son, contributed significantly to the reduction in the risk of breast cancer. They reported an FM prevalence of 56% in controls and 26% in patients with breast cancer. Their results suggested that the enigma of why some parous women are not afforded protection from breast cancer by pregnancy, might, in part, be explained by the absence of FM (Gadi et al., 2008). A contemporary study by Gilmore et al. (2008) also showed a higher prevalence of male cells (57% vs. 34%) in the maternal circulation of normal parous women compared with those with various malignant diseases, including, but not limited to, gynecologic malignancies, indicating that the absence of male microchimerism might be a risk factor for developing breast cancer. A study by Kamper-Jørgensen et al. (2012) highlighted the opposite effects of FM in blood samples on later development of breast and colon cancer. While the detection of male microchimerism was strongly associated with a reduced risk of developing breast cancer (70% vs. 40%), they found an increased risk of developing colon cancer (70% vs. 90%). Gadi (2010) found a protective association between FM in breast tissue and breast cancer. They reported a higher prevalence of FM in breast tissues from cancer-free women compared with unaffected breast tissues from patients with an invasive breast cancer diagnosis (63% vs. 26%, respectively). The latest study by Dhimolea et al. (2013) reported decreased FM prevalence in ductal invasive breast cancer compared with controls (21.0% vs. 56.0%, p<0.001).
Uterine cancer is defined as any invasive neoplasm of the uterine corpus. Invasive neoplasms of female pelvic organs account for almost 15% of all cancers in women. The most common of these malignancies, in the United States and Europe, is endometrial cancer (Schottenfeld, 1995; Olson et al., 2009). There are two forms of endometrial cancer. Type 1 endometrial cancer represented by low-grade endometrioid adenocarcinoma, which is thought to be primarily related to imbalances in reproductive hormones, usually constitutes more than 80% of all endometrial cancers (Milne et al., 2011) and has a favorable prognosis. Type 2 endometrial cancer, considered nonestrogen dependent, represented by serous carcinoma and cell clear carcinoma, is highly aggressive and is associated with a worse prognosis. Recently, it has been reported that grade 3 endometrioid carcinomas shared a molecular pathway with type 2 endometrial carcinomas (Alvarez et al., 2012). It has also been shown that grade 3 endometrioid carcinomas had a clinical behavior close to that reported in type 2 endometrial cancer (Alektiar et al., 2002; Soslow et al., 2007). That is why some authors start classifying high-grade endometrioid carcinomas (grade 3) as type 2 endometrial cancers (Amant et al., 2005; Bakkum-Gamez et al., 2008).
Since it is now universally accepted that carcinosarcomas are not uterine sarcomas but carcinomas with a sarcomatoid phenotype, they can also be grouped with high-grade (type 2) uterine carcinomas (Kurman et al., 2011).
Known risk factors for type 1 endometrial cancer include early menarche, late menopause, anovulation, infertility and/or nulliparity, obesity, diabetes, and higher lifetime estrogen exposure (Sherman, 2000; Purdie and Green, 2001; Kaaks et al., 2002; Akhmedkhanov et al., 2006; Trentham-Dietz et al., 2006; Wernli et al., 2006; Zagouri et al., 2009). The only known risk factor for type 2 endometrial cancer is age (>60 years), so most of these tumors occur after menopause.
To our knowledge, no study describing the role of FM in the pathogenesis of uterine cancer has been carried out. For that reason, we evaluated the prevalence of FM and FMC concentrations in tumor and control endometrial tissues in a population of Czech women. Further, we investigated the relationship between FM and the severity of the disease with regard to the uterine cancer subtype, the histological stage and grade of the tumor. The association part of the study focused on the risk factors, including patient age at diagnosis, patient pregnancy history, obesity, hypertension, dyslipidemia, and type 2 diabetes.
Materials and Methods
The study examined the presence of FM in frozen and/or fresh endometrial tissue specimens. The studied control group involved 41 patients (dysfunctional uterine bleeding, leiomyomas, endometrial polyps, benign ovarian cysts, prolapsed uterus, and benign endometrial hyperplasia) aged 36–75 years (mean 52.4 years). The patients in the control group suffered from type 2 diabetes (10.3%), hypertension (20.5%), and dyslipidemia (2.4%). Relative to BMI, the control group had the following distribution (38.9% normal range, 16.7% overweight, and 44.4% obese). The history of pregnancy in the control group was as follows: 5 - no pregnancies, 7 - one pregnancy, 8 - two pregnancies, 9 - three pregnancies, 7 - four pregnancies, and 5 - five or more pregnancies.
The cancer patient group enrolled 75 patients with uterine cancer. The group included 47 women with type 1 endometrial cancer (17 grade 1 endometrioid adenocarcinomas and 30 grade 2 endometrioid adenocarcinomas) and 28 women with type 2 endometrial cancer (19 grade 3 endometrioid adenocarcinomas, 2 clear cell endometrial carcinomas, 3 serous carcinomas, and 4 metaplastic carcinomas). The patients were diagnosed with uterine cancer at the mean age of 65.5 years (range 44–90 years). Tumor stages according to the FIGO 2009 classification (54 FIGO 1, 7 FIGO 2, 12 FIGO 3 and 2 FIGO 4) and tumor histological grades (17 G1, 30 G2 and 19 G3) were assessed. The prevalence of type 2 diabetes was 28.2%. The prevalence of hypertension and dyslipidemia in the uterine cancer group was 56.3% and 16.0%, respectively.
The cancer patients were subdivided according to BMI (11.3% BMI ≤25, 41.9% BMI >25, and 46.8% BMI >30). The pregnancy history of the uterine cancer group was as follows: 3 - no pregnancies, 11 - one pregnancy, 28 - two pregnancies, 19 - three pregnancies, 6 - four pregnancies, and 8 - five or more pregnancies. The study was performed in a retrospective manner using biological samples collected from January 2009 to December 2010. All patients who participated in this study provided written informed consent. The study was approved by the local ethics committee.
Processing of samples and real-time PCR analysis
DNA was extracted from 25 mg of endometrial tissue using a QIAamp DNA Mini kit (Qiagen, Hilden, Germany). In fresh tissue samples, DNA was eluted in 200 μL of AE buffer. To enrich DNA from frozen tissues, DNA was eluted using 60 μL of AE buffer. These two approaches were selected on the base of previous testing, yielding approximately the same quantity of fetal cells in identical tissues. The real-time PCR analysis was performed using a 7500 Real-Time PCR system (Applied Biosystems, Branchburg, NJ) as previously described. Two protocols produced the best results, identifying small numbers of male FMCs in uterine tissues. 15 μL and/or 47 μL were used as a template for the SRY-specific polymerase chain reaction. TaqMan amplification reactions were set up in a reaction volume of 50 μL and/or 100 μL using TaqMan Universal PCR Master Mix (Applied Biosystems). Each sample was analyzed in three replicate settings for the SRY gene. A patient's specimen was considered positive if the amplification signal occurred on a threshold cycle <50. The calibration curves for the SRY and β-globin (GLO) genes were run parallel to each analysis. The standard curves were prepared using two approaches. First, DNA from the peripheral blood of a healthy male donor was isolated, its concentration was measured using a spectrophotometer (NanoDrop-1000; Witec AG, Switzerland) and converted to the number of cells using a conversion factor (one diploid genome being equivalent to 6.6 pg of DNA). Second, DNA derived from a healthy male donor of known concentration (converted to the number of cells using a conversion factor) was spiked to the known concentration of female DNA. FM was expressed as the number of FMCs per 105 total cells. Five microliter of DNA was used as a template for the GLO PCR reaction.
Statistical analysis
The Chi-square test and univariate logistic regression model were used to compare the absence or the presence of FM across the groups. One-way analysis of variance (ANOVA) was used to test possible differences in mean concentrations of fetal-derived cells in endometrial tissues between groups. A series of multiple logistic regression analyses were performed to evaluate the effect of putative risk factors on the prevalence of FM in the groups of patients with uterine cancer and controls. Similarly, two-way ANOVA analyses were performed to compare the differences in concentrations of FMCs in endometrial tissues between the uterine cancer group and the control group in relation to particular risk factor.
In addition, the relationship between FM prevalence or FMC concentrations in endometrial tissue and the age of the patient at the time of diagnosis, patient pregnancy history (total number of pregnancies, including both completed and uncompleted pregnancies), and the body mass index was studied using linear regression models.
The significance level was established at a p-value of p<0.05. If there was statistical significance with the ANOVA test, then the Bonferroni's post-hoc analysis was applied.
Results
The prevalence of male FM in uterine cancer
Overall, a significantly decreased prevalence of male FM was observed in women who developed uterine cancer compared with uterine cancer-free controls (44.0% vs. 70.7%; odds ratio [OR]=3.076, 95% confidence interval [CI]: 1.365 to 6.933, p=0.007). However, the difference in concentrations of fetal-derived cells between uterine cancer and cancer free-groups did not achieve statistical significance (F=0.013, df=1,114, p=0.910). Fetal cells were detected at mean concentrations of 0.090 and 0.050 per 105 total cells in uterine cancer cases and controls, respectively.
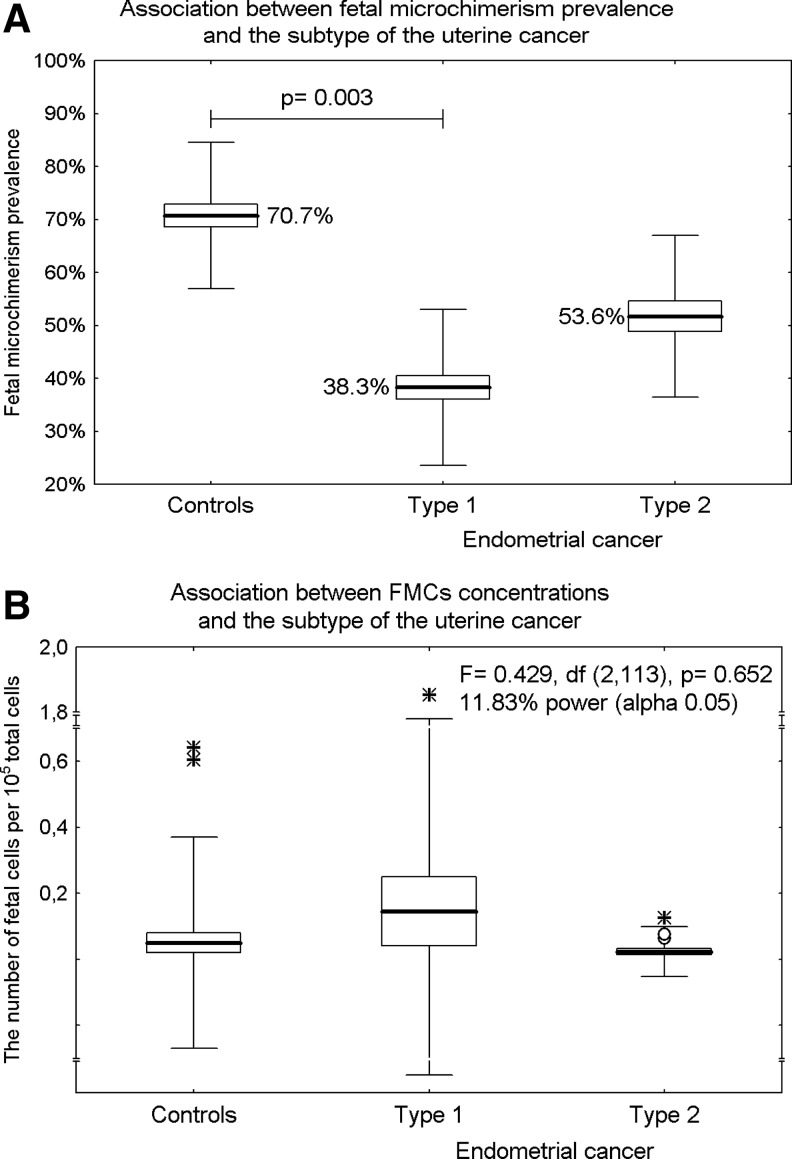
A significant difference in the prevalence of male FM was found between uterine cancer patients and the control group. While the prevalence of male FM between the control group and type 2 endometrial cancer (70.7% vs. 53.6%; OR=0.478, 95% CI: 0.175 to 1.301, p=0.148) did not differ, a lower prevalence was detected in patients with type 1 endometrial cancer (70.7% vs. 38.3%; OR=0.257, 95% CI: 0.105 to 0.628, p=0.003), (Fig. 1A). However, the number of chimeric cells did not differ within the examined groups (type 1 endometrial cancer: mean 0.146 per 105 total cells vs. type 2 endometrial cancer: mean 0.023 per 105 total cells vs. controls: mean 0.050 per 105 total cells), (F=0.429, df=2,113, p=0.652), (Fig. 1B).
FIG. 1.
The prevalence of male fetal microchimerism (FM) in uterine cancer. Results expressed as box plots; upper and lower limits of the boxes represent the mean±SE (standard error), upper and lower whiskers represent the mean±2SD (standard deviations), mean is indicated by the line in each box; outliers are indicated by circles and extremes by asterisks. The significance level was established at a p-value of p<0.05. (A) While the prevalence of male FM between the control group and type 2 endometrial cancer did not differ, a lower prevalence was detected in patients with type 1 endometrial cancer. (B) The number of chimeric cells did not differ within the examined groups (type 1 endometrial cancer vs. type 2 endometrial cancer vs. controls).
Association between male FM and the histological grade of endometrioid cancer
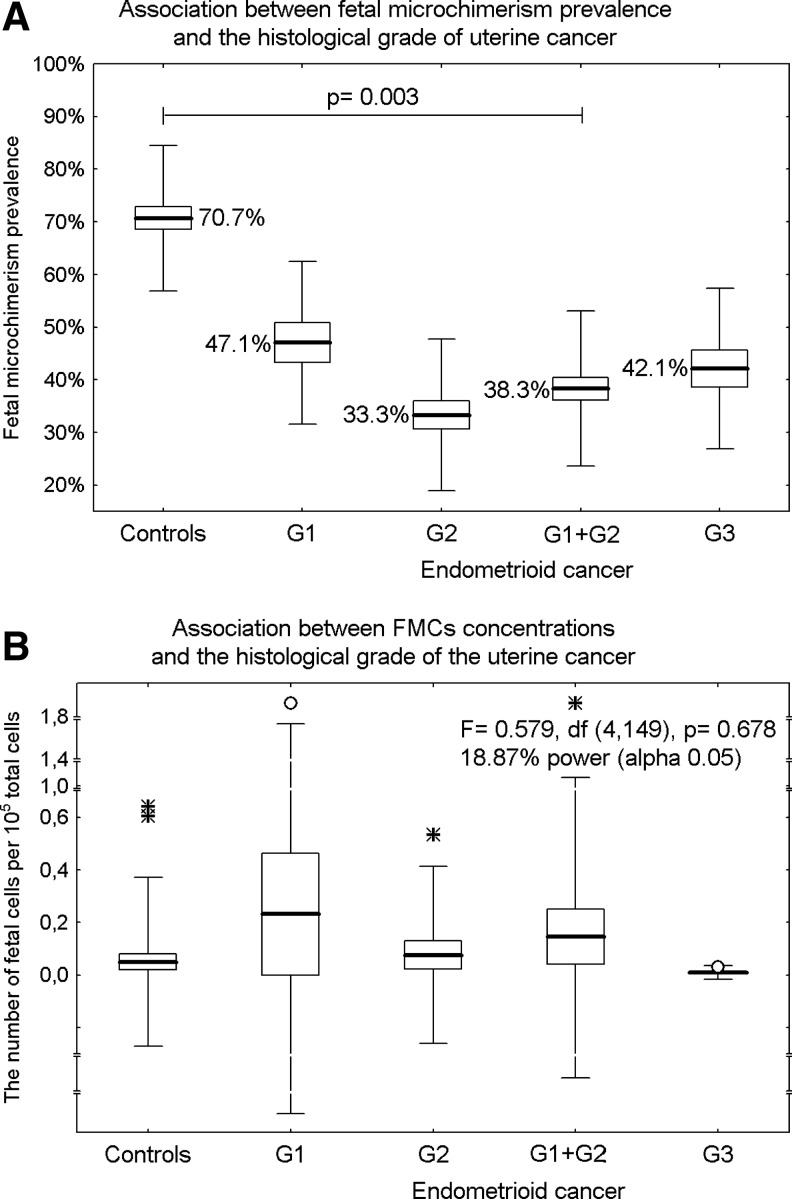
A significant effect of the histological grade of the tumor on the prevalence of male FM in examined endometrioid cancer tissues was revealed. A significantly lower prevalence of male FM was observed in low-grade (grade 1 and grade 2) endometrioid cancer (38.3% vs. 70.7%, OR=0.256, 95% CI: 0.105 to 0.627, p=0.003). The difference in prevalence of male FM between high-grade (grade 3) endometrioid tumors and the control group did not reach statistical significance (42.1% vs. 70.7%, OR=0.301, 95% CI: 0.097 to 0.934, p=0.138), (Fig. 2A).
FIG. 2.
Association between male FM and the histological grade of endometrioid cancer. Results expressed as box plots; upper and lower limits of the boxes represent the mean±SE, upper and lower whiskers represent the mean±2SD, mean is indicated by the line in each box; outliers are indicated by circles and extremes are indicated by asterisks. The significance level was established at a p-value of p<0.05. (A) While the difference in prevalence of male FM between high grade (grade 3) endometriod tumors and the control group did not reach statistical significance, a significantly lower prevalence of male FM was observed in low-grade (grade 1 and grade 2) endometrioid cancer. (B) No association between concentrations of male FMCs and the histological grade of the tumor within the endometrioid cancer group was found.
Mean FMC concentrations in endometrial tissues of patients with grade 1 endometrioid carcinomas (0.232 per 105 total cells), grade 2 endometrioid carcinomas (0.076 per 105 total cells), and grade 3 endometrioid carcinomas (0.010 per 105 total cells) were assessed. Statistical analyses showed no association between concentrations of male FMCs and the histological grade of the tumor within the endometrioid cancer group (F=0.579, df=4,149, p=0.678), (Fig. 2B).
Association between male FM and the stage of the uterine cancer according to the FIGO classification
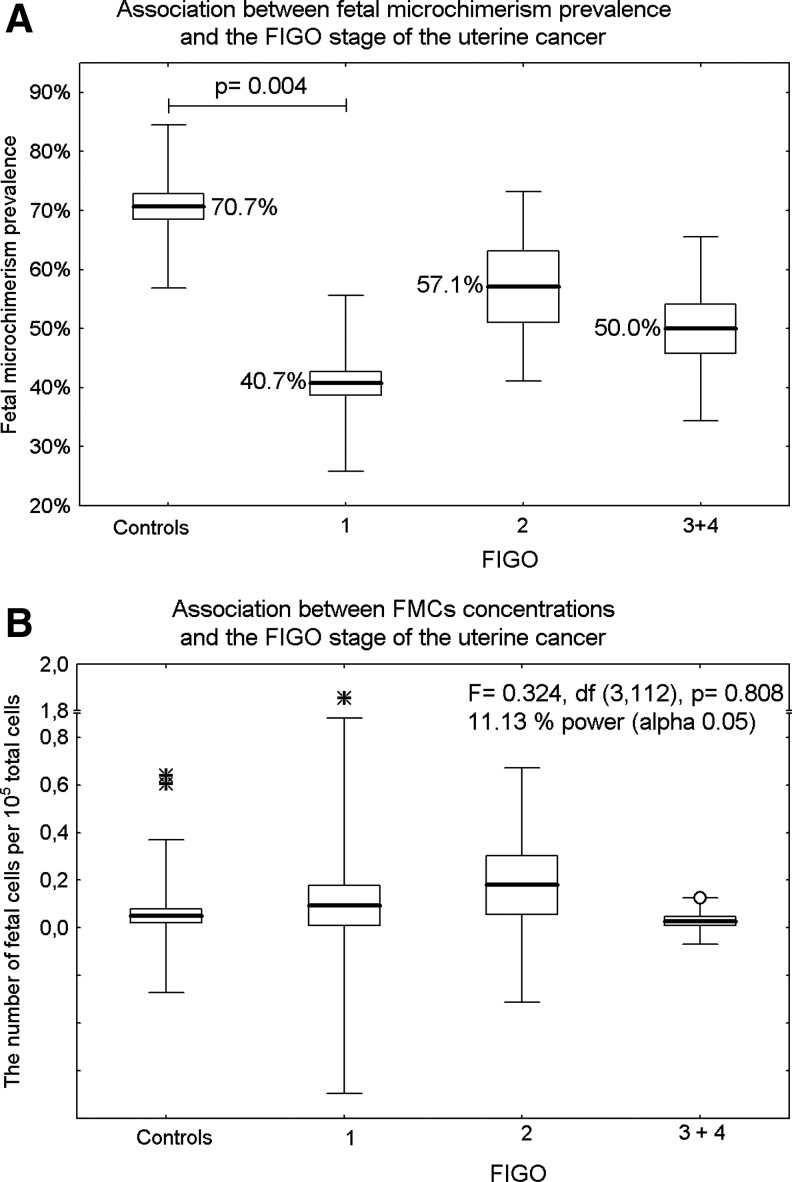
FM prevalence in endometrial tissues differed significantly depending on the stage of uterine cancer. While the lowest prevalence of FM was detected in FIGO 1 tumors compared with the control group (40.7% vs. 70.7%, OR=0.285, 95% CI: 0.120 to 0.675, p=0.004), no difference between uterine cancer-free tissue samples and FIGO 2 (70.7% vs. 57.1%, OR=0.552, 95% CI: 0.107 to 2.848, p=0.478) or FIGO 3 and FIGO 4 tumors (70.7% vs. 50.0%, OR=0.414, 95% CI: 0.119 to 1.437, p=0.165) was observed (Fig. 3A).
FIG. 3.
Association between male FM and the stage of the uterine cancer according to the FIGO classification. Results expressed as box plots; upper and lower limits of the boxes represent the mean±SE, upper and lower whiskers represent the mean±2SD, mean is indicated by the line in each box; outliers are indicated by circles and extremes are indicated by asterisks. The significance level was established at a p-value of p<0.05. (A) While the lowest prevalence of FM was detected in FIGO 1 tumors compared to the control group, no difference between uterine cancer-free tissue samples and FIGO 2 or FIGO 3 and FIGO 4 tumors was observed. (B) The concentrations of FMCs showed no difference between groups of patients with uterine cancer.
The concentrations of FMCs showed no difference between groups of patients with uterine cancer (F=0.324, df=3,112, p=0.808).The mean number of fetal cells per 105 total cells tested for microchimerism was 0.093 in FIGO 1, 0.180 in FIGO 2, and 0.028 in FIGO 3 and FIGO 4 (Fig. 3B).
The association study of FM and putative risk factors for uterine cancer
No relation between hypertension and FM prevalence was observed (OR=1.280, 95% CI: 0.561 to 2.918, p=0.558). Similarly, there was no difference in concentrations of FMCs (F=0.075, df=1,102, p=0.785) between hypertensive and normotensive subjects (F1=2.344, df=1,102, p=0.129) within the uterine cancer group and the control group (F2=0.033, df=1,102, p=0.785).
FM prevalence (OR=1.046, 95% CI: 0.405 to 2.702, p=0.925) and FMC concentrations (F=0.187, df=1,102, p=0.666; F1=0.013, df=1,102, p=0.908; F2=0.196, df=1,102, p=0.659) were approximately equal in the uterine cancer group and the control group, relative to type 2 diabetes.
The results indicate no association between the presence of dyslipidemia and the prevalence of FM (OR=2.465, 95% CI: 0.702 to 8.652, p=0.159) or FMC concentrations in endometrial tissues (F=0.003, df=1,112, p=0.959; F1=0.004, df=1,112, p=0.953; F2=0.158, df=1,112, p=0.692) in groups of patients with or without uterine cancer.
There was no significant effect of body mass index, when patients were subdivided into particular categories involving norm and overweight/obese patients, on FM prevalence (OR=0.473, 95% CI: 0.155 to 1.446, p=0.189) or FMC concentrations in endometrial tissues (F=1.384, df=1,90, p=0.243; F1=0.143, df=1,90, p=0.706; F2=0.085, df=1,90, p=0.771). In addition, no relationship between both FM prevalence and the concentration of FMCs in endometrial tissues and BMI was found in the uterine cancer group (R2=0.0194, p=0.426; R2=0.0092, p=0.385) or cancer-free controls (R2=0.0522, p=0.191; R2=0.0129, p=0.239).
Although uterine cancer is more common in patients older than 60 years of age, the association part of the study focused on the comparison of FM prevalence and FMC concentrations in endometrial tissues between age-matched groups. The control group and the uterine cancer group below the age of 60 also showed the difference in FM prevalence (73.3% vs. 41.6%; OR=3.385, 95% CI: 1.137 to 10.077, p=0.029); while no differences in FMC concentrations (F=1.148, df=1,57, p=0.289) were observed. Likewise, the benign uterine disorder group and the uterine cancer group older than 60 years of age differed in FM prevalence (63.5% vs. 45.1%; OR=2.275, 95% CI: 0.584 to 8.862, p=0.049) but did not differ in FMC concentrations (F=1.228, df=1,55, p=0.20). No difference in FM prevalence (controls: OR=1.571, 95% CI: 0.361 to 6.842, p=0.547; cancer patients: OR=1.056, 95% CI: 0.414 to 2.692, p=0.909) and in FMC concentrations (controls: F=1.352, df=1,39, p=0.259; cancer patients: F=0.935, df=1,73, p=0.337) was observed within individual groups with regard to age (below 60 yrs vs. over 60 yrs). Similarly, a linear regression analysis showed no association between testing positive for FM and concentrations of FMCs in endometrial tissues versus age at diagnosis in women who developed uterine cancer (R2=0.00047, p=0.853; R2=0.00053, p=0.844) or benign uterine disorders (R2=0.06941, p=0.126; R2=0.07944, p=0.074).
FM prevalence and FMC concentrations in endometrial tissues were also analyzed in relation to the patients' pregnancy history (i.e., number of childbirths, spontaneous abortions and miscarriages). No association between either FM prevalence or FMC concentrations and the number of pregnancies in the uterine cancer group (R2=0.0065, p=0.496; R2=0.000068, p=0.944) and the control group (R2=0.0013, p=0.838; R2=0.040, p=0.247) was revealed.
Discussion
We previously reported considerable inter-individual variation in the DYS-14 copy number in a group of healthy men; therefore, we believed that the DYS-14 sequence is not an optimal marker for male fetal DNA quantification (Hromadnikova et al., 2008, 2009). That is why our studies to assess the prevalence of FM were done preferentially using the SRY gene as the marker. Unfortunately, a recently discovered sex-independent fetal-specific marker, the hypermethylated RASSF1A sequence, cannot be used for fetal DNA quantification in cancer patients, because hypermethylation of the promoter, associated with the inactivation of the tumor suppressor gene, has been frequently observed in various tumors (van der Weyden and Adams, 2007; Pallarés et al., 2008; Banno et al., 2012).
Overall, FM prevalence is more common in patients with benign diseases of the uterus than in uterine cancer. With regard to the individual subtypes of uterine cancer, the prevalence of FM was different between cancer patients and controls. Patients with type 1 endometrial cancer were less likely to be FM positive than uterine cancer-free controls. On the other hand, the prevalence of FM in those patients with a more aggressive, faster-growing form of cancer that tends to have a poorer prognosis, such as type 2 endometrial cancer, was the same relative to the control group.
The analysis conducted to examine the association between the prevalence of FM and the histological tumor grade score revealed significantly decreased prevalence of FM in low-grade endometrioid adenocarcinomas compared with controls. Interestingly, no difference in FM prevalence between high-grade uterine tumors and uterine cancer-free controls was observed.
Similarly, we observed a lower prevalence of FM in patients in whom the cancer was restricted to the body of the uterus (FIGO 1) compared with the control group with benign diseases of the uterus. The results also suggested that there was no difference in FM prevalence in endometrial tissues within women in whom the cancer had spread from the body of the uterus to the supporting connective tissue of the cervix (FIGO 2) and/or outside the uterus (FIGO 3+4) and the control group with benign diseases of the uterus. Overall, the present study suggests that better prognoses of uterine cancer are usually associated with lower prevalence of FM in tumor tissues compared with control tissues derived from benign uterine disorders. Interestingly, the concentration of FMCs in endometrial tissues did not differ between the examined groups.
It is evident that most women with nonmalignant uterine diseases harbor FMCs in very low concentrations. This phenomenon may be associated with ongoing disorders that are accompanied by local inflammation for which control patients underwent surgery. Besides hormonal regulation, a number of other factors involving inflammatory processes have been reported to regulate uterine myoma (Miura et al., 2006; Khan et al., 2010). On the other hand, the occurrence of FM in endometrial tissue may be linked with protection against the development of uterine cancer. However, the occurrence of FM in women without these reproductive diseases, who could not be used as controls in the study, remains unclear. The endometrial biopsy is usually performed in women older than the age of 35 to determine the cause of abnormal menstrual periods, bleeding after menopause, bleeding associated with taking hormone replacement medications, or to screen for endometrial cancer. Normal endometrial tissue is described as proliferative or secretory endometrium that has the thickness of the uterine lining comparable to that of a healthy uterus and lacks the presence of precancerous and cancerous cell growth. However, even patients with abnormal vaginal bleeding have normal endometrial tissue on biopsy. Moreover, an endometrial biopsy does not provide a sufficient amount of biological material to study the occurrence of FM. The only option enabling study is utilization of biological material from surgery, indicated for uterine abnormalities identified with an endometrial biopsy.
The occurrence of male microchimeric cells in tumor tissues may be related to their active involvement in the complex process of tumorigenesis, involving tumor initiation and propagation inclusive of integration into the tumor stroma (Dubernard et al., 2008), neoangiogenesis, facilitation of metastasis (Nguyen et al., 2009), inducement of immune responses (Sawaya et al., 2004; Gadi, 2009) followed by reparation of inflammation damaged tissues, as previously suggested for certain autoimmune diseases and malignancies (Lee et al., 2010).
Since very low concentrations of FMCs are present in uterine cancer tissues and benign uterine disorders, the identification of their origin, phenotype, and role in maternal tissues, using the most sensitive currently available techniques, is relatively unfeasible. Nevertheless, an investigation of the biological consequences of pregnancy-associated FM is fundamental. Recently, reported developments of highly sensitive symptomatic qPCR assays have opened up the possibility of analyzing paraffin-embedded tissues that were previously unusable for chimerism studies (Dhimolea et al., 2013). This discovery represents the first step toward quantification of allogeneic cells in more accessible biological material for most research centers. The next steps should be directed toward the development of more precise techniques for visualization of rare fetal cells in paraffin-embedded tissues that can be dissected for consecutive single cell analysis. Incorporation of live cell imaging techniques, to obtain a better understanding of biological function, through the study of cellular dynamics, would be desirable as well.
With these types of advancements, the origin of allogeneic cells in maternal tissues might be definitely confirmed. Although pregnancy is the most common setting, in which FM is encountered, microchimerism can also occur after allogeneic blood transfusion (Vervoordeldonk et al., 1998; Lapierre et al., 2007). Microchimeric cells can also be transferred in utero from a twin and potentially from an unrecognized (vanished) twin, which is relatively common in healthy pregnancies (de Bellefon et al., 2010). Sexual transmission and needle sharing have also been suggested as possible mechanisms for microchimerism; however, to date, they have not been well documented (Bloch et al., 2013).
Some health-related lifestyle factors, such as type 2 diabetes or insulin resistance, obesity, hypertension, dyslipidemia, and increasing age, may be highly relevant to later uterine cancer development (Grossman et al., 2002; Amant et al., 2005; Rapp et al., 2005; Friberg et al., 2007; Lucenteforte et al., 2007; Pallarés et al., 2008; Schmandt et al., 2011; von Gruenigen et al., 2011; Seth et al., 2012; Chen et al., 2013; Edlinger et al., 2013). The lower prevalence of male FM (1.6 times) was also observed as affecting the development of uterine cancer.
We studied the association between FM prevalence and FMC concentrations in endometrial tissues and putative risk factors for uterine cancer. However, the association analyses pointed to no relationship between FM prevalence or FMC concentrations and appropriate risk factors. It is well known that multiparous women have a lower risk of developing hormone-dependent cancers. Several studies have provided evidence that multiparity might confer a protective effect on the risk of death from endometrial cancer (Chan et al., 2011; Cramer, 2012). We tested the possibility that the prevalence of FM and concentrations of FMCs increased with increasing numbers of pregnancies. However, the analysis indicated no trend of increasing FM prevalence and FMC concentrations associated with higher numbers of pregnancies. On the basis of these findings, we hypothesized that the protective effect of multiparity, relative to the onset of uterine cancer, is not associated with FM.
Conclusion
Low concentrations of FMCs are very common in endometrial tissues derived from patients treated for benign uterine disorders. In cases of uterine cancer, a lower prevalence of FM was demonstrated. A lower prevalence of FM seems to be associated with better prognoses in uterine cancer based on tumor subtype, histological grade, and stage of the tumor. A lower prevalence of FM was observed in low-grade type 1 endometrial cancer and pT1 tumors. No relationship between FM prevalence or FMC concentrations in endometrial tissues and the prevalence of hypertension, type 2 diabetes, dyslipidemia, overweight and obesity, age of patients, and total number of previous pregnancies was demonstrated.
Acknowledgment
This work was supported by grant no. 262704/SVV/2011 and PRVOUK P32.
Disclosure Statement
This work is original, unpublished, and not under consideration by another journal. No competing financial interests exist.
References
- Akhmedkhanov A., Zeleniuch-Jacquotte A., and Toniolo P. (2006). Role of exogenous and endogenous hormones in endometrial cancer. Ann N Y Acad Sci 943, 296–315 [DOI] [PubMed] [Google Scholar]
- Alektiar K.M., McKee A., Lin O., Venkatraman E., Zelefsky M.J., McKee B., Hoskins W.J., and Barakat R.R. (2002). Is there a difference in outcome between stage I-II endometrial cancer of papillary serous/clear cell and endometrioid FIGO grade 3 cancer? Int J Radiat Oncol Biol Phys 54, 79–85 [DOI] [PubMed] [Google Scholar]
- Alvarez T., Miller E., Duska L., and Oliva E. (2012). Molecular profile of grade 3 endometrioid endometrial carcinoma: Is it a type I or type II endometrial cancer? Am J Surg Pathol 36, 753–761 [DOI] [PubMed] [Google Scholar]
- Amant F., Moerman P., Neven P., Timmerman D., Van Limbergen E., and Vergote I. (2005). Endometrial cancer. Lancet 366, 491–505 [DOI] [PubMed] [Google Scholar]
- Artlett C.M. (2005). Pathophysiology of fetal microchimeric cells. Clin Chim Acta 360, 1–8 [DOI] [PubMed] [Google Scholar]
- Bakkum-Gamez J.N., Gonzalez-Bosquet J., Laack N.N., Mariani A., and Dowdy S.C. (2008). Current issues in the management of endometrial cancer. Mayo Clin Proc 83, 97–112 [DOI] [PubMed] [Google Scholar]
- Banno K., Kisu I., Yanokura M., Masuda K., Kobayashi Y., Ueki A., Tsuji K., Yamagami W., Nomura H., Susumu N., and Aoki D. (2012). Endometrial Cancer and Hypermethylation: Regulation of DNA and MicroRNA by Epigenetics. Biochem Res Int 2012, 738274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes-Genis A., Bellosillo B., de la Calle O., Salido M., Roura S., Ristol F.S., Soler C., Martinez M., Espinet B., Serrano S., Bayes de Luna A., and Cinca J. (2005). Identification of male cardiomyocytes of extracardiac origin in the hearts of women with male progeny: male fetal cell microchimerism of the heart. J Heart Lung Transplant 24, 2179–2183 [DOI] [PubMed] [Google Scholar]
- Bianchi D.W., Zickwolf G.K., Weil G.J., Sylvester S., and DeMaria M.A. (1996). Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA 93, 705–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E.M., Jackman R.P., Lee T.H., and Busch M.P. (2013). Transfusion-associated microchimerism: the hybrid within. Transfus Med Rev 1, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buemi M., Allegra A., D'Anna R., Coppolino G., Crascì E, Giordano D., Loddo S., Cucinotta M., Musolino C., and Teti D. (2007). Concentration of circulating endothelial progenitor cells (EPC) in normal pregnancy and in pregnant women with diabetes and hypertension. Am J Obstet Gynecol 196, 68.e1–e6 [DOI] [PubMed] [Google Scholar]
- Campagnoli C., Fisk N., Overton T., Bennett P., Watts T., and Roberts I. (2000). Circulating hematopoietic progenitor cells in first trimester fetal blood. Blood 95, 1967–1972 [PubMed] [Google Scholar]
- Campagnoli C., Roberts I.A., Kumar S., Bennett P.R., Bellantuono I., and Fisk N.M. (2001). Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98, 2396–2402 [DOI] [PubMed] [Google Scholar]
- Cha D., Khosrotehrani K., Kim Y., Stroh H., Bianchi D.W., and Johnson K.L. (2003). Cervical cancer and microchimeris. Obstet Gynecol 102, 774–781 [DOI] [PubMed] [Google Scholar]
- Chan T.F., Wu C.H., Changchien C.C., and Yang C.Y. (2011). Mortality from breast, endometrial and ovarian cancers among grand multiparous women in Taiwan. Aust N Z J Obstet Gynaecol 51, 548–552 [DOI] [PubMed] [Google Scholar]
- Chen Y.L., Wang K.L., Chen M.Y., Yu M.H., Wu C.H., Ke Y.M., Chen Y.J., Chang Y.Y., Hsu K.F., and Yen M.S. (2013). Risk factor analysis of coexisting endometrial carcinoma in patients with endometrial hyperplasia: a retrospective observational study of Taiwanese Gynecologic Oncology Group. J Gynecol Oncol 24, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E.M., Jr, Feldhaus W.D., and Whitacre F.E. (1964). Fetal erythrocytes in the maternal circulation of pregnant women. Obstet Gynecol 23, 915–919 [PubMed] [Google Scholar]
- Cramer D.W. (2012). The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am 26, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bellefon L.M., Heiman P., Kanaan S.B., Azzouz D.F., Rak J.M., Martin M., Roudier J., Roufosse F., and Lambert N.C. (2010). Cells from a vanished twin as a source of microchimerism 40 years later. Chimerism 2, 56–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhimolea E., Denes V., Lakk M., Al-Bazzaz S., Aziz-Zaman S., Pilichowska M., and Geck P. (2013). High male chimerism in the female breast shows quantitative links with cancer. Int J Cancer 133, 835–842 [DOI] [PubMed] [Google Scholar]
- Dubernard G., Aractingi S., Oster M., Rouzier R., Mathieu M.C., Uzan S., and Khosrotehrani K. (2008). Breast cancer stroma frequently recruits fetal derived cells during pregnancy. Breast Cancer Res 10:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlinger M., Concin N., Concin H., Nagel G., Ulmer H., and Göbel G. (2013). Lifestyle-related biomarkers and endometrial cancer survival: elevated gemma-glutamyltransferase as an important risk factor. Cancer Epidemiol 37, 156–161 [DOI] [PubMed] [Google Scholar]
- Fleta Asin B., Gonzalvo Liarte M.C., and Cia Gomez P. (2006). Chimerism: origin and medical implications. Rev Clin Esp 206, 340–342 [DOI] [PubMed] [Google Scholar]
- Friberg E., Orsini N., Mantzoros C.S., and Wolk A. (2007). Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 50, 1365–1374 [DOI] [PubMed] [Google Scholar]
- Gadi V.K., and Nelson J.L. (2007). Fetal microchimerism in women with breast cancer. Cancer Res 67, 9035–9038 [DOI] [PubMed] [Google Scholar]
- Gadi V.K., Malone K.E., Guthrie K.A., Porter P.L., and Nelson J.L. (2008). Case-control study of fetal microchimerism and breast cancer. PLoS One 3, e1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadi V.K. (2009). Fetal microchimerism and cancer. Cancer Lett 276, 8–13 [DOI] [PubMed] [Google Scholar]
- Gadi V.K. (2010). Fetal microchimerism in breast from women with and without breast cancer. Breast Cancer Res Treat 121, 241–244 [DOI] [PubMed] [Google Scholar]
- Gilmore G.L., Haq B., Shadduck R.K., Jasthy S.L., and Lister J. (2008). Fetal-maternal microchimerism in normal parous females and parous female cancer patients. Exp Hematol 36, 1073–1077 [DOI] [PubMed] [Google Scholar]
- Grossman E., Messerli F.H., Boyko V., and Goldbourt U. (2002). Is there an association between hypertension and cancer mortality? Am J Med 112, 479–486 [DOI] [PubMed] [Google Scholar]
- Guetta E., Gordon D., Simchen M.J., Goldman B., and Barkai G. (2003). Hematopoietic progenitor cells as targets for non-invasive prenatal diagnosis: detection of fetal CD34+ cells and assessment of post-delivery persistence in the maternal circulation. Blood Cells Mol Dis 30, 13–21 [DOI] [PubMed] [Google Scholar]
- Hromadnikova I., Benesova M., Zejskova L., Stehnova J., Doucha J., Sedlacek P., Dlouha K., and Krofta L. (2009). The effect of DYS-14 copy number variations on extracellular fetal DNA quantification in maternal circulation. DNA Cell Biol 28, 351–358 [DOI] [PubMed] [Google Scholar]
- Hromadnikova I., Zlacka D., Hien Nguyen T.T., Sedlackova L., Zejskova L., and Sosna A. (2008). Fetal cells of mesenchymal origin in cultures derived from synovial tissue and skin of patients with rheumatoid arthritis. Joint Bone Spine 75, 563–566 [DOI] [PubMed] [Google Scholar]
- Kaaks R., Lukanova A., and Kurzer M.S. (2002). Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 11, 1531–1543 [PubMed] [Google Scholar]
- Kamper-Jørgensen M., Biggar R.J., Tjønneland A., Hjalgrim H., Kroman N., Rostgaard K., Stamper C.L., Olsen A., Andersen A-MN, and Gadi V.K. (2012). Opposite effects of microchimerism on breast and colon cancer. Eur J Cancer 48, 2227–2235 [DOI] [PubMed] [Google Scholar]
- Khan K.N., Kitajima M., Hiraki K., Fujishita A., Sekine I., Ishimaru T., and Masuzaki H. (2010). Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod 25, 642–653 [DOI] [PubMed] [Google Scholar]
- Khosrotehrani K., and Bianchi D.W. (2005). Multi-lineage potential of fetal cells in maternal tissue: a legacy in reverse. J Cell Sci 118, 1559–1563 [DOI] [PubMed] [Google Scholar]
- Kurman R.J., Ellenson L.H., and Ronnett B.M. (2011). Blaustein's Pathology of the Female Genital Tract, 6th ed. (Springer; New York: ), pp. 460–476 [Google Scholar]
- Lapaire O., Hosli I., Zanetti-Daellenbach R., Huang D., Jaeggi C., Gatfield-Mergenthaler S., Hahn S., and Holzgreve W. (2007). Impact of fetal-maternal microchimerism on women's health—a review. J Matern Fetal Neonatal Med 20, 1–5 [DOI] [PubMed] [Google Scholar]
- Lapierre V., Aupérin A., Robinet E., Ferrand C., Oubouzar N., Tramalloni D., Saas P., Debaene B., Lasser P., and Tiberghien P. (2007). Immune modulation and microchimerism after unmodified versus leukoreduced allogeneic red blood cell transfusion in cancer patients: results of a randomized study. Transfusion 47, 1691–1699 [DOI] [PubMed] [Google Scholar]
- Lee E.S.M., Bou-Gharios G., Seppanen E., Khosrotehrani K., and Fisk N.M. (2010). Fetal stem cell microchimerism: natural-born healers or killers? Mol Hum Reprod 16, 869–878 [DOI] [PubMed] [Google Scholar]
- Lucenteforte E., Bosetti C., Talamini R., Montella M., Zucchetto A., Pelucchi C., Franceschi S., Negri E., Levi F., and La Vecchia C. (2007). Diabetes and endometrial cancer: effect modification by body weight, physical activity and hypertension. Br J Cancer 97, 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi P., Powers R.W., Verma V., Edmunds L., Plymire D., and Hubel C.A. (2010). Maternal circulating CD34+ VEGFR-2+ and CD133+ VEGFR-2+ progenitor cells increase during normal pregnancy but are reduced in women with preeclampsia. Reprod Sci 17, 643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne F.H., Judge D.S., Preen D.B., and Weinstein P. (2011). Early life environment, life history and risk of endometrial cancer. Med Hypotheses 77, 626–632 [DOI] [PubMed] [Google Scholar]
- Miura S., Khan K.N., Kitajima M., Hiraki M., Moriyama S., Masuzaki H., Samejima T., Fujishita A., and Ishimaru T. (2006). Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas. Hum Reprod 21, 2545–2554 [DOI] [PubMed] [Google Scholar]
- Mueller U.W., Hawes C.S., Wright A.E., Petropoulos A., DeBoni E., Firgaira F.A., Morley A.A., Turner D.R., and Jones W.R. (1990). Isolation of fetal trophoblast cells from peripheral blood of pregnant women. Lancet 336, 197–200 [DOI] [PubMed] [Google Scholar]
- Nassar D., Droitcourt C., Mathieu-d'Argent E., Kim M.J., Khosrotehrani K., and Aractingi S. (2012). Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J 26, 149–157 [DOI] [PubMed] [Google Scholar]
- Nguyen Huu S., Dubernard G., Aractingi S., and Khosrotehrani K. (2006). Feto-maternal cell trafficking: a transfer of pregnancy associated progenitor cells. Stem Cell Rev 2, 111–116 [DOI] [PubMed] [Google Scholar]
- Nguyen Huu S., Oster M., Avril M.F., Boitier F., Mortier L., Richard M.A., Kerob D., Maubec E., Souteyrand P., Moguelet P., Khosrotehrani K., and Aractingi S. (2009). Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy. Am J Pathol 174, 630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue K., and Chan J. (2006). Human fetal mesenchymal stem cells. Curr Stem Cell Res Ther 1, 371–386 [DOI] [PubMed] [Google Scholar]
- O'Donoghue K., Choolani M., Chan J., de la Fuente J., Kumar S., Campagnoli C., Bennett P.R., Roberts I.A., and Fisk N.M. (2003). Identification of fetal mesenchymal stem cells in maternal blood: implications for non-invasive prenatal diagnosis. Mol Hum Reprod 9, 497–502 [DOI] [PubMed] [Google Scholar]
- Olson S.H., Chen C., De Vivo I., Doherty J.A., Hartmuller V., Horn-Ross P.L., Lacey J.V., Lynch S.M., Sansbury L., and Setiawan V.W. (2009). Maximizing resources to study an uncommon cancer: E2C2-Epidemiology of Endometrial Cancer Consortium. Cancer Causes Control 20:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallarés J., Velasco A., Eritja N., Santacana M., Dolcet X., Cuatrecasas M., Palomar-Asenjo V., Catasús L., Prat J., and Matias-Guiu X. (2008). Promoter hypermethylation and reduced expression of RASSF1A are frequent molecular alterations of endometrial carcinoma. Mod Pathol 21, 691–699 [DOI] [PubMed] [Google Scholar]
- Parant O., Dubernard G., Challier J.C., Oster M., Uzan S., Aractingi S., and Khosrotehrani K. (2009). CD34+ cells in maternal placental blood are mainly fetal in origin and express endothelial markers. Lab Invest 89, 915–923 [DOI] [PubMed] [Google Scholar]
- Purdie D.M., and Green A.C. (2001). Epidemiology of endometrial cancer. Best Practise Res Clin Obstet Gynaecol 15, 341–354 [DOI] [PubMed] [Google Scholar]
- Rapp K., Schroeder J., Klenk J., Stoehr S., Ulmer H., Concin H., Diem G., Oberaigner W., and Weiland S.K. (2005). Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer 93, 1062–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvidou M.D., Xiao Q., Kaihura C., Anderson J.M., and Nicolaides K.H. (2008). Maternal circulating endothelial progenitor cells in normal singleton and twin pregnancy. Am J Obstet Gynecol 198, 414.e1–e5 [DOI] [PubMed] [Google Scholar]
- Sawaya H.H.B., Jimenez S.A., and Artlett C.M. (2004). Quantification of fetal microchimeric cells in clinically affected and unaffected skin of patients with systemic sclerosis. Rheumatology 43, 965–968 [DOI] [PubMed] [Google Scholar]
- Schmandt R.E., Iglesias D.A., Co N.N., and Lu K.H. (2011). Understanding obesity and endometrial cancer risk: opportunities for preventation. Am J Obstet Gynecol 205, 518–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld D.J. (1995). Epidemiology of endometrial neoplasia. Cell Biochem Suppl 23, 151–159 [DOI] [PubMed] [Google Scholar]
- Seth D., Garmo H., Wigertz A., Holmberg L., Hammar N., Jungner I., Lambe M., Walldius G., and van Hemelrijck M. (2012). Lipid profiles and the risk of endometrial cancer in the Swedish AMORIS study. Int J Mol Epidemiol Genet 3, 122–133 [PMC free article] [PubMed] [Google Scholar]
- Sherman M.E. (2000). Theories of endometrial carcinogenesis: a multidisciplinary approach. Med Pathol 13, 295–308 [DOI] [PubMed] [Google Scholar]
- Soslow R.A., Bissonnette J.P., Wilton A., Ferguson S.E., Alektiar K.M., Duska L.R., and Oliva E. (2007). Clinicopathologic analysis of 187 high-grade endometrial carcinomas of different histologic subtypes: similar outcomes belie distinctive biologic differences. Am J Surg Pathol 31, 979–987 [DOI] [PubMed] [Google Scholar]
- Trentham-Dietz A., Nichols H., Hampton J.M., and Newcomb P. (2006). Weight change and risk of endometrial cancer. Int J Epidemiol 35, 151–158 [DOI] [PubMed] [Google Scholar]
- van der Weyden L., and Adams D.J. (2007). The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta 1776, 58–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoordeldonk S.F., Doumaid K., Remmerswaal E.B., ten Berge I.J., Wilmink J.M., de Waal L.P., and Boog C.J. (1998). Long-term detection of microchimaerism in peripheral blood after pretransplantation blood transfusion. Br J Haematol 4, 1004–1009 [DOI] [PubMed] [Google Scholar]
- von Gruenigen V.E., Waggoner S.E., Frasure H.E., Kavanagh M.B., Janata J.W., Rose P.G., Courneya K.S., and Lerner E. (2011). Lifestyle challenges in endometrial cancer survivorship. Obstet Gynecol 117, 93–100 [DOI] [PubMed] [Google Scholar]
- Walknowska J., Conte F.A., and Grumbach M.M. (1969). Practical and theoretical implications of fetal-maternal lymphocyte transfer. Lancet 1, 1119–1122 [DOI] [PubMed] [Google Scholar]
- Wernli K.J., Ray R.M., Gao D.L., De Roos A.J., Checkoway H., and Thomas D.B. (2006). Menstrual and reproductive factors in relation to risk of endometrial cancer in Chinese women. Cancer Causes Control 17, 949–955 [DOI] [PubMed] [Google Scholar]
- Yan Z., Lambert N.C., Guthrie K.A., Porter A.J., Loubiere L.S., Madeleine M.M., Stevens A.M., Hermes H.M., and Nelson J.L. (2005). Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am J Med 118, 899–906 [DOI] [PubMed] [Google Scholar]
- Zagouri F., Dimopoulos A.M., Fotiou S., Kouloulias V., and Papadimitriou C.A. (2009). Treatment of early uterine sarcomas: disentangling adjuvant modalities. World J Surg Oncol 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]