Abstract
This article provides a brief overview of the work conducted by the Division of Reproductive Health at the Centers for Disease Control and Prevention on severe maternal morbidity and mortality in the United States. The article presents the latest data and trends in maternal mortality and severe maternal morbidity, as well as on maternal substance abuse and mental health disorders during pregnancy, two relatively recent topics of interest in the Division, and includes future directions of work in all these areas.
Introduction
Pregnant and postpartum women in the United States enjoy levels of health and healthcare that are considerably better than those of their counterparts in less developed and developing countries. Nonetheless, the perinatal and postpartum periods are a time of risk for women everywhere. At the Centers for Disease Control and Prevention (CDC), the Division of Reproductive Health (DRH) is the focal point for issues related to maternal health and to women's reproductive health more broadly. DRH houses the national Pregnancy Mortality Surveillance System and is involved in research and public health activities related to maternal mortality and morbidity, substance abuse, and mental health disorders during pregnancy.
Maternal Mortality in the United States
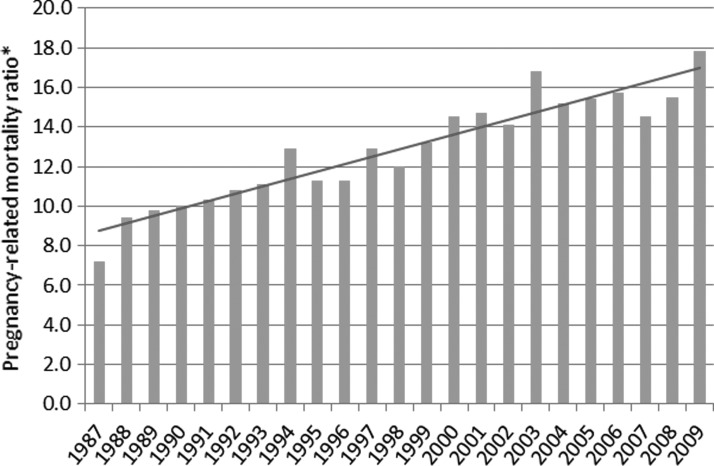
CDC's DRH has been conducting surveillance for pregnancy-related mortality since 1987.1 Fifty-two reporting areas (50 U.S. states, New York City, and District of Columbia) voluntarily submit de-identified copies of death certificates for all deaths occurring during or within 1 year of pregnancy regardless of the cause of death or the duration of pregnancy; matching birth or fetal death certificates are also sent if available. Clinically experienced medical epidemiologists review all the information available for each death and record information regarding cause of death, pregnancy outcomes, associated medical conditions, and demographic and obstetrical data. A pregnancy-related death is defined as the death of a woman during or within 1 year of pregnancy that was caused by a pregnancy complication, a chain of events initiated by pregnancy, or the aggravation of an unrelated condition by the physiologic effects of pregnancy. The temporal association between the pregnancy status and death is ascertained in one of the following ways: presence of a selected pregnancy checkbox on the death certificate indicating the woman was pregnant at the time of death or describing an interval between the end of a pregnancy and death; words or codes indicating a pregnancy on the death certificate; presence of a note on the death certificate indicating the duration of complications causing or events leading to death; or availability of a birth or fetal death certificate within 1 year of the woman's death. The causal association between the pregnancy status and death is based on the clinical cause of death, the interval between pregnancy termination and death, and the pathophysiology of pregnancy complications. Deaths attributable to conditions where the pregnant status likely did not have an impact on the fatal course are not considered pregnancy-related even if the temporal association with the pregnant status is met; these are defined as pregnancy-associated deaths. Trends in U.S. pregnancy-related mortality ratios, defined as the number of pregnancy-related deaths per 100,000 live births, are shown in Fig. 1 for the period between 1987 and 2009. The pregnancy-related mortality ratio increased steadily from 7.2 deaths per 100,000 live births in 1987 to 17.8 deaths per 100,000 live births in 2009. The reasons for this increase are unclear. The use of computerized data linkages by states, changes in the way causes of death are coded following implementation of the International Classification of Diseases version 10 (ICD-10) in 1999, or the addition of a pregnancy checkbox on the 2003 standard U.S. death certificate have likely improved the identification of pregnancy-related deaths over time.2 Thus, whether the actual risk of a woman dying from pregnancy-related causes has increased is unclear. Many studies show that an increasing number of pregnant women in the United States have chronic health conditions,3–6 and these conditions may put a pregnant woman at higher risk of adverse outcomes. The higher pregnancy-related mortality ratio in 2009 compared with previous years is caused by the 2009 H1N1 influenza pandemic, which disproportionally affected pregnant women.7–9 Surveillance data from 2010 are not yet available, but we expect to see additional impact on the mortality ratio from pregnant women affected by the 2009 H1N1 influenza pandemic. Comparisons of our data with data from other developed countries are difficult given the differences in mortality definitions and indicators used and in the type of methods employed for case identification. CDC's DRH identifies and reports all deaths occurring during pregnancy and within one year postpartum, while most other developed countries report only deaths occurring during pregnancy and within 42 days after the end of pregnancy (i.e., maternal deaths by the World Health Organization [WHO]/ICD-10 definition).10 Given the limited data available for each case, we cannot ascertain whether injury deaths such as drug overdoses, suicides or homicides, or cancer-related deaths during pregnancy or within 1 year postpartum are pregnancy related, and therefore, we consider such deaths pregnancy associated. In countries where more data on such cases are available, the causal relationship of each death to pregnancy can be established. On the other hand, our surveillance methods are superior to methods used in a majority of countries in Europe that rely exclusively on data recorded in routine vital statistics systems.10 Few countries use enhanced surveillance and review methods similar to ours (e.g., France, the Netherlands, United Kingdom), and differences between routine vital statistics and enhanced surveillance data have been shown to be significant.10 Despite these limitations in making data comparisons, it does appear that the risk of dying from pregnancy complications is higher in the United States than in many European countries.10
FIG. 1.
Trends in pregnancy-related mortality in the United States, 1987–2009. *Number of pregnancy-related deaths per 100,000 live births per year; test for trend p<0.001. Data from Centers for Disease Control and Prevention.2
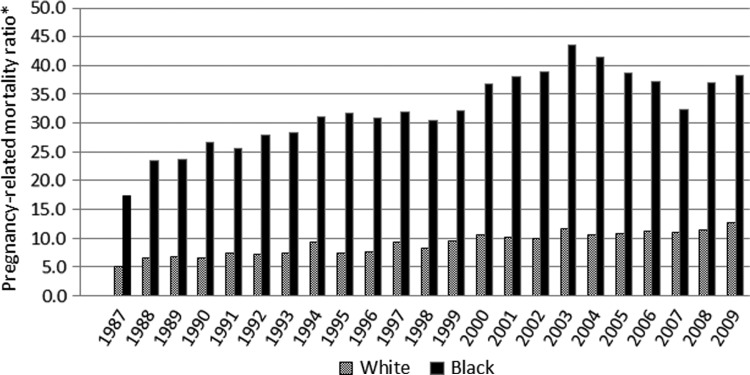
Some women in the United States are at a higher risk of dying from pregnancy-related causes than others.11 Most notably, pregnancy-related mortality ratios are 3–4 times higher among black than white women (Fig. 2), and for specific mortality causes (e.g., ectopic pregnancy), this gap appears to be even greater.11,12 A recent analysis of our pregnancy mortality surveillance data showed that except for foreign-born white women, all other race, ethnicity, and nativity groups were at higher risk of dying from pregnancy-related causes than U.S.-born white women after adjusting for age differences.11 There have been many attempts to explain these pronounced disparities, but due to data source limitations, most authors concluded that they are multifactorial and no single intervention is likely to reduce them.12 However, variability in the risk of death by race and other factors indicates that more can be done to understand and reduce pregnancy-related mortality. More research on this topic is needed, and this represents one of the top priorities of research in DRH.
FIG. 2.
Race differentials in pregnancy-related mortality in the United States, 1987–2009. *Number of pregnancy-related deaths per 100,000 live births per year.
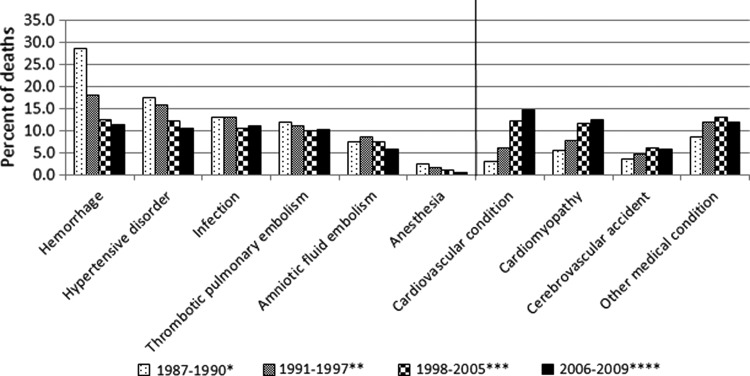
Causes of and risk factors for pregnancy-related deaths from 1987 to 2005 have already been published in the literature.1,13,14 Information on causes of pregnancy-related deaths occurring after 2005 have been released regularly on our website at www.cdc.gov/reproductivehealth/MaternalInfantHealth/PMSS.html as additional years of data became available; we will continue to provide such regular updates in the future. Changes in the causes of pregnancy-related deaths between 1987 and 2009 are shown in Fig. 3, and correspond to the data in our surveillance publications1,13,14 and the most current web update.2 We found a decline in the contribution of the traditional causes of pregnancy-related mortality (i.e., hemorrhage, sepsis, hypertensive disorders of pregnancy), and the emergence of cardiovascular and other medical conditions as important contributors to mortality in the United States. For the most recent surveillance period shown (2006–2009), cardiovascular conditions alone accounted for over a third of all pregnancy-related deaths, and together with other medical conditions, they accounted for half of all pregnancy-related deaths. Similar trends have been observed in other developed countries conducting enhanced identification and review of maternal deaths.15 More detailed analyses of our surveillance data are conducted and published on pooled 5-year data, and the next such analysis is due upon review of 2010 data.
FIG. 3.
Causes of pregnancy-related mortality in the United States, 1987–2009. *Data from Berg et al., 19961. **Data from Berg et al., 200313; ***Data from Berg et al., 201014; **** Data from Centers for Disease Control and Prevention.2
Severe Maternal Morbidity in the United States
Maternal morbidity encompasses physical and psychologic conditions that result from or are aggravated by pregnancy and have an adverse effect on a woman's health. Currently, there is no standard definition for maternal morbidity. The WHO has proposed a definition and a measurement approach for maternal near-miss (i.e., “a woman who nearly died but survived a complication that occurred during pregnancy, childbirth or within 42 days of termination of pregnancy”),16 and recently organized a working group to develop a definition and propose a measurement strategy for a larger group of severe maternal morbidities.17 The EURO-PERISTAT project uses a maternal morbidity indicator that includes any one of eclampsia, hysterectomy, embolization, blood transfusion, or a stay of more than 24 hours in an intensive care unit.10
By and large, there are three approaches for identifying severe morbidity using either disease-specific criteria to capture conditions like eclampsia or hemorrhage, management criteria to identify interventions like massive blood transfusion or hysterectomy, or organ-system dysfunction criteria to identify the dysfunction or failure of organs and systems.16 Of all, the organ-system dysfunction framework was identified as the most promising for establishing a standard set of criteria for measuring severe maternal morbidity (SMM). WHO used these criteria to define maternal near miss,16 and CDC's DRH employed such criteria to develop and refine an SMM measure to be used for population-based surveillance using administrative data in the United States.18 Specifically, for the latter, Callaghan et al. have explored lists of ICD-9 diagnosis and procedure codes from a national dataset including delivery and postpartum hospital discharge records, linked indicators of severe morbidity to those of in-hospital mortality, and identified 25 ICD-9 code-based categories that capture indicators of organ-system dysfunction that likely represent specific, well-defined severe events (these codes are available elsewhere18,19).
Currently, there is no systematic ongoing data collection for population-based maternal morbidity in the United States. CDC's DRH uses delivery hospitalization data and the above-mentioned SMM algorithm to identify potentially life-threatening maternal conditions or complications.18,19 Delivery hospitalizations are identified using another published ICD-9-based algorithm that incorporates diagnostic codes for an outcome of delivery, diagnosis-related group delivery codes, and procedure codes for selected delivery-related procedures.20 The source of data for CDC's national SMM estimates is the Nationwide Inpatient Sample (NIS), the largest all-payer hospital inpatient care database in the United States.21 This is one of a family of databases and software tools developed as part of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality.22 NIS is a stratified sample of approximately 20% of all community hospitals in the United States, with hospitals selected using five characteristics: rural or urban location, number of beds, region, teaching status, and ownership.21 The database includes all discharges from the selected hospitals and, for the most recent year (2011), provided information from 1,045 hospitals in 46 U.S. states.21 Each record is weighted to account for the complex sampling design, and nationwide estimates can be derived when analytic weights are applied during analysis.19
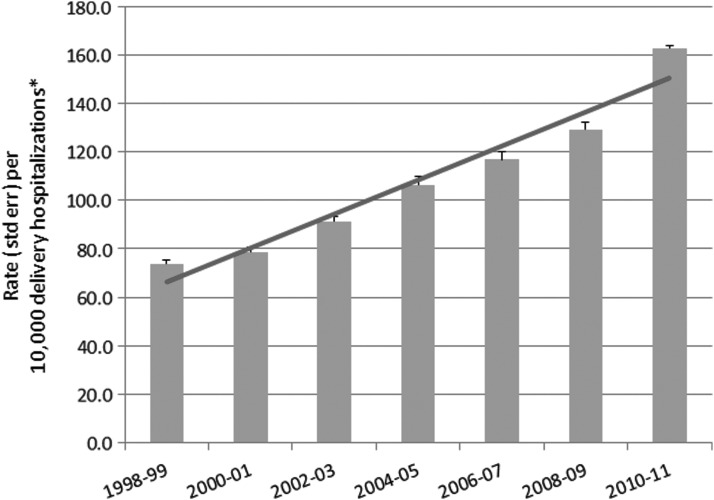
Trends in SMM in the United States between 1998 and 2011 are shown in 2-year increments in Fig. 4. We observed a clinically and statistically significant increase in overall SMM rates between 1998–1999 and 2010–2011 (Cuzick test for trend p=0.014). For every 10,000 delivery hospitalizations during the most recent 2-year period (2010–2011), there were 163 delivery hospitalizations with at least one SMM indicator. This represents a 26.1% rate increase from the previous 2-year period (2008–2009). Blood transfusion was the most common indicator of SMM during 1998–2011. For the most recent 2-year period (2010–2011), blood transfusion was a SMM indicator for 117 of 10,000 delivery hospitalizations. The second through fifth most frequent indicators of SMM during delivery hospitalizations were disseminated intravascular coagulation (32 per 10,000 delivery hospitalizations), heart failure during a procedure or surgery (18 per 10,000 delivery hospitalizations), hysterectomy (9 per 10,000 delivery hospitalizations), and operations on the heart or pericardium (7 per 10,000 delivery hospitalizations). While we cannot directly compare the overall SMM rate in the United States with the rate in other developed countries, such comparison is possible for eclampsia and hysterectomy, two of the five indicators included in the maternal morbidity measure of the EURO-PERISTAT project. In 2010, rates of eclampsia ranged between 1 and 9 per 10,000 deliveries among the 20 European countries that provided these data, while the 2010–2011 rate of eclampsia in the United States was 7 per 10,000 deliveries.10,19 Similarly, among the 17 European countries reporting hysterectomy data for 2010, the rate of hysterectomy ranged between 1 and 13 per 10,000 deliveries, while the corresponding 2010–2011 rate in the United States was 9.2 per 10,000 deliveries.10,19
FIG. 4.
Trends in severe maternal morbidity during delivery hospitalizations in the United States, 1998–2011. *The number of delivery hospitalizations with at least one severe maternal morbidity indicator per 10,000 delivery hospitalizations. Data from Centers for Disease Control and Prevention.19
At the current rate, SMM affects over 60,000 women in the United States every year, and this burden has been steadily increasing in recent years.19 Rises in SMM are likely driven by a combination of factors, including increases in maternal age,23 prepregnancy obesity,24–25 preexisting chronic medical conditions,3–6 and cesarean delivery.23,26 The consequences of the increasing SMM prevalence are wide ranging and include higher utilization of health services, higher direct medical costs, extended length of hospitalization, and need for long-term rehabilitation.18
Our analysis of SMM trends in the United States is not without limitations. To assess severe maternal morbidity, we used administrative data that are primarily collected for billing purposes, and in the absence of more nuanced clinical information, this may have led to misclassification. The SMM algorithm is based on ICD-9 diagnosis and procedure codes indicating untoward events during delivery hospitalizations, but likely incorporates a range of severity. Blood transfusion was found to be the most common indicator of SMM. However, information regarding the amount of blood transfused was not available, and evidence suggests wide variations in physicians' decisions to transfuse in diverse clinical settings.27
On the other hand, examination of maternal morbidity has many advantages. Most importantly, it provides a more comprehensive picture of disease patterns among pregnant and postpartum women, and hence, an opportunity to identify points of intervention for quality improvements in maternal care and a more relevant assessment of the range of resources needed to prevent and manage these conditions. Of note, CDC's measure of SMM can be used for population-level estimates of severe morbidity but cannot replace the in-depth review and analysis of risk factors for maternal morbidity—the latter should become the norm in all U.S. hospitals caring for pregnant women. Current and future work by DRH scientists aims to better understand current trends in SMM, and to explore ways to translate research findings into practice and improve pregnancy outcomes by advancing evidence-based clinical practices and processes.
Maternal Substance Abuse
Maternal substance abuse is a continuing public health problem in the United States. Smoking during pregnancy increases the risk of complications such as placenta previa, placental abruption, and premature rupture of the membranes.28 Based on the Pregnancy Risk Assessment Monitoring System (PRAMS) data from 29 states, an estimated 23% of women smoked in the three months before pregnancy and 13% during the last three months of pregnancy, with disparities in smoking prevalence by maternal age, race/ethnicity, socioeconomic status and state of residence.29 DRH aims to evaluate and promote effective clinical and policy interventions to prevent and reduce maternal smoking. A recent example of this work includes a free, interactive online training entitled “Smoking Cessation for Pregnancy and Beyond: A Virtual Clinic” which was supported by DRH and endorsed by the American College of Obstetricians and Gynecologists.30 This training is designed for healthcare professionals to hone their skills in best practice approaches for smoking cessation for pregnant women. In addition, DRH sponsored and participated in the evidence review and development of the first WHO recommendations for the prevention and management of tobacco use and secondhand smoke exposure in pregnancy.31 Future areas of research in this area include assessment of the cost-effectiveness of using financial incentives for prenatal smoking cessation, review of evidence for the effectiveness and reach of telephone-based quitlines for pregnant smokers and for the efficacy of interventions to prevent smoking during pregnancy and in the postpartum period.
Among pregnant women, both the prevalence of use and the quantity of illicit (i.e., street or prescription) drugs used are highest during the first trimester and lowest in the third trimester of pregnancy.32,33 However, some women continue to use illicit drugs throughout their pregnancies.32 The prevalence of illicit drug use by pregnant women aged 15–44 years has not changed significantly since the early 2000s, and the most recent estimate (2011) provided by the National Survey of Drug Use and Health (NSDUH) was 5.0%.34 Yet, evidence suggests that the types of drugs used during pregnancy did change during this period.35–37 Nationally, the maternal use of opiates increased from 1.2 (95% confidence interval [CI], 1.0–1.4) to 5.6 (95% CI, 4.4–6.7) per 1,000 births between 2000 and 2009,38 in line with significantly growing numbers of emergency visits and deaths related to misuse or abuse of opioid pain relievers among women of all ages.39
Neonates exposed to illicit substances or prescription medications, especially opiates, can develop neonatal abstinence syndrome (NAS).35 This syndrome represents a constellation of behavioral and physiological signs and symptoms including extreme irritability, inability to self-soothe, and respiratory and central and automatic nervous system dysfunctions.35 Between 2000 and 2009, the incidence of NAS increased from 1.2 (95% CI, 1.1–1.4) to 3.4 (95% CI, 3.1–3.7) per 1,000 births (p for trend<0.001).38 The abuse of illicit drugs during pregnancy has been linked with poor pregnancy and neonatal outcomes such as low birth weight, small for gestational age, and prematurity.35,40 In addition, as children, drug-exposed neonates experience developmental challenges and appear more likely to have psychomotor and cognitive deficits and behavioral disorders than unexposed neonates.41,42
CDC's DRH is currently engaged in a number of activities related to perinatal illicit drug use and NAS. In September of 2012, DRH organized and hosted an Expert Meeting on Perinatal Illicit Drug Abuse which convened clinicians and scientists from state and federal agencies, academia and other partners to discuss issues surrounding screening, brief interventions, and clinical treatment for pregnant and postpartum women abusing illicit drugs. Several manuscripts have been developed as a result of the expert meeting, and are currently under review at CDC and in peer-reviewed journals. Through collaborations with State Health Departments, we are now exploring the feasibility of using Prescription Drug Monitoring Program data, state-based electronic databases that collect information on medications dispensed in the state, to learn about the prescription histories of pregnant women and the subsequent development of NAS in their newborns.
Mental Health During Pregnancy and the Postpartum Period
Mental health disorders, especially depression and anxiety, are common and affect the health and well-being of women and their families. NSDUH data show that about 1 in every 10 women (8% of pregnant and 11% of nonpregnant women of reproductive age, 18–44 years) had at least one major depressive episode in the year before the survey interview.34 Women suffering from depression are at increased risk of substance abuse, developing chronic diseases, and having poorer health.43,44 For example, among U.S. women with major depression, most (89%) have one or more chronic medical conditions (e.g., diabetes, obesity) or medical risk factors (smoking, binge or heavy drinking, physical inactivity).43 Yet, only 50% of pregnant and 54% of nonpregnant women suffering from depression receive treatment for this condition.45 During pregnancy and the postpartum period, women's poor mental health may adversely impact pregnancy outcomes, maternal-infant bonding, maternal functioning, and infant health and development.46,47
Due to the burden and adverse impact of poor mental health on women and their families, CDC's DRH works to improve the mental health of women of reproductive age through research and technical assistance provided to other organizations working towards the same goals. Data from NSDUH, PRAMS, the Behavioral Risk Factor Surveillance System (BRFSS), and several other sources are used to provide national- and state-level information on the prevalence of and risk factors for depression, anxiety and other mental health conditions, as well as associated health conditions, obstetric outcomes, and diagnosis and treatment strategies. DRH assists states and nongovernmental organizations to conduct similar activities and to further efforts to develop and target appropriate public health strategies for addressing mental health among women of reproductive age.
Future priorities in this area include examining and evaluating ways to alleviate barriers to diagnosis and treatment of mental health disorders among women of reproductive age, determining effective ways to integrate mental health services into routine prenatal and well-woman care, and developing and testing interventions that simultaneously address risky health behaviors (i.e., smoking, drinking, and illicit drug abuse) and mental health disorders in this subpopulation.
Conclusion
Preventing maternal morbidity and mortality and eliminating health disparities in maternal health are national public health and research priorities. While maternal mortality has been the traditional sentinel event for monitoring maternal health, maternal morbidity occurs more frequently and much can be learned from reviewing maternal mortality and morbidity cases and their healthcare. CDC has been conducting surveillance for pregnancy-related mortality for 25 years, and data have been continuously reported on a voluntary basis by all U.S. states, District of Columbia, and New York City. CDC efforts to measure and ascertain SMM are currently coupled with work conducted on both the clinical and the public health sides by our long-term national partners, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American Academy of Pediatrics, the Maternal and Child Health Bureau, and the National Institutes of Health among many others, in a concerted effort to reduce maternal mortality and morbidity in the United States.
Disclosure Statement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. No competing financial interests exist.
References
- 1.Berg CJ, Atrash HK, Koonin LM, Tucker M. Pregnancy-related mortality in the United States, 1987–1990. Obstet Gynecol 1996;88:161–167 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Pregnancy-related mortality surveillance. 2013. Available at: www.cdc.gov/reproductivehealth/MaternalInfantHealth/PMSS.html
- 3.Campbell KH, Savitz D, Werner EF. Maternal morbidity and risk of death at delivery hospitalization. Obstet Gynecol 2013;122:627–633 [DOI] [PubMed] [Google Scholar]
- 4.Small MJ, James AH, Kershaw T, Thames B, Gunatilake R, Brown H. Near-miss maternal mortality: Cardiac dysfunction as the principal cause of obstetric intensive care unit admissions. Obstet Gynecol 2012;119:250–255 [DOI] [PubMed] [Google Scholar]
- 5.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol 2012;206:134..e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995–2006. BJOG 2011;118:345–352 [DOI] [PubMed] [Google Scholar]
- 7.Creanga AA, Johnson TF, Graitcer SB, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol 2010;115:717–726 [DOI] [PubMed] [Google Scholar]
- 8.Louie JK, Acosta M, Jamieson DJ, Honein MA; California Pandemic (H1N1) Working Group Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010;362:27–35 [DOI] [PubMed] [Google Scholar]
- 9.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic H1N1 Influenza in Pregnancy Working Group. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 21;303:1517–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EURO PERISTAT Report 2012. Available at: www.europeristat.com/reports/european-perinatal-health-report-2010.html
- 11.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce C, Callaghan WM. Race, ethnicity and nativity differentials in pregnancy-related mortality in the United States: 1993–2006. Obstet Gynecol 2012;120:261–268 [DOI] [PubMed] [Google Scholar]
- 12.Creanga AA, Shapiro-Mendoza CK, Bish CL, Zane S, Berg CJ, Callaghan WM. Trends in ectopic pregnancy mortality in the United States: 1980–2007. Obstet Gynecol 2011;117:837–843 [DOI] [PubMed] [Google Scholar]
- 13.Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991–1997. Obstet Gynecol 2003;101:289–296 [DOI] [PubMed] [Google Scholar]
- 14.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998–2005. Obstet Gynecol 2010;116:1302–1309 [DOI] [PubMed] [Google Scholar]
- 15.Centre for Maternal and Child Enquiries (CMACE) Saving mothers' lives: Reviewing maternal deaths to make motherhood safer: 2006–08. The eighth report on confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118:1–203 [DOI] [PubMed] [Google Scholar]
- 16.Say L, Souza JP, Pattinson RC; WHO Working Group on Maternal Mortality and Morbidity Classifications Maternal near miss—towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol 2009;23:287–296 [DOI] [PubMed] [Google Scholar]
- 17.Firoz T, Chou D, von Dadelszen P, et al. ; Maternal Morbidity Working Group Measuring maternal health: Focus on maternal morbidity. Bulletin of the World Health Organization. 2013; Bull World Health Organ 2013;91:794–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029–1036 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Severe maternal morbidity in the United States. 2013. Available at: www.cdc.gov/reproductivehealth/MaternalInfantHealth/Severe maternal morbidity.html
- 20.Kuklina E, Whiteman M, Hillis S, et al. An enhanced method for identifying obstetric deliveries: Implications for estimating maternal morbidity. Matern Child Health J 2008;12:9. [DOI] [PubMed] [Google Scholar]
- 21.Healthcare Cost and Utilization Project (HCUP) Overview of the National Inpatient Sample (NIS). 2013. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp
- 22.Healthcare Cost and Utilization Project (HCUP) Overview of the Healthcare Cost and Utilization Project. 2013. Available at: www.hcup-us.ahrq.gov
- 23.Martin JA, Hamilton BE, Ventura SJ, et al. Births: Final data for 2011. National vital statistics reports; vol 61 no 1. Hyattsville, MD: National Center for Health Statistics, 2013. Available at: www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_01.pdf [PubMed] [Google Scholar]
- 24.Hinkle SN, Sharma AJ, Kim SY, et al. Prepregnancy obesity trends among low-income women, United States, 1999–2008. Matern Child Health J 2012;16:1339–1348 [DOI] [PubMed] [Google Scholar]
- 25.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med 2013;56:372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber EL, Lundsberg LS, Belanger K, et al. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol 2011;118:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islam R, Tinmouth AT, Francis JJ, et al. A cross-country comparison of intensive care physicians' beliefs about their transfusion behavior: A qualitative study using the Theoretical Domains Framework. Implement Sci 2012;21:7.:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention PRAMS and smoking. 2013. Available at www.cdc.gov/prams/TobaccoandPrams.htm
- 29.Centers for Disease Control and Prevention How tobacco smoke causes disease: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, 2010 [Google Scholar]
- 30.Tong VT, Dietz PM, England LJ. “Smoking cessation for pregnancy and beyond: A virtual clinic,” an innovative web-based training for healthcare professionals. J Womens Health (Larchmt) 2012;21:1014–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization Guidelines for smoking during pregnancy. 2013. Available at: http://www.who.int/tobacco/publications/pregnancy/guidelinestobaccosmokeexposure/en/index.html
- 32.Della Grotta S, LaGasse LL, Arria AM, et al. Patterns of methamphetamine use during pregnancy: Results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J 2010;14:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muhuri PK, Gfroerer JC. Substance use among women: Associations with pregnancy, parenting, and race/ethnicity. Matern Child Health J 2009;13:376–385 [DOI] [PubMed] [Google Scholar]
- 34.Substance Abuse and Mental Health Services Administration, Results from the 2011 National Survey on Drug Use and Health: Mental Health Findings, NSDUH Series H-45, HHS Publication No. (SMA) 12-4725. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2012. Available at: www.samhsa.gov/data/nsduh/2k11results/nsduhresults2011.pdf [Google Scholar]
- 35.Creanga AA, Sabel JC, Ko JY, et al. Maternal drug use and its effect on neonates: A population-based study in Washington State. Obstet Gynecol 2012;119:924–933 [DOI] [PubMed] [Google Scholar]
- 36.Kellogg A, Rose CH, Harms RH, Watson WJ. Current trends in narcotic use in pregnancy and neonatal outcomes. Am J Obstet Gynecol 2011;204:259..e1–4. [DOI] [PubMed] [Google Scholar]
- 37.Substance Abuse and Mental Health Services Administration Trends in substances of abuse among pregnant women and women of childbearing age in treatment. 2013. Available at: www.samhsa.gov/data/spotlight/spot110-trends-pregnant-women-2013.pdf [PubMed]
- 38.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA 2012;307:1934–1940 [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention Vital signs: Overdoses of prescription opioid pain relievers and other drugs among women—United States, 1999–2010. Morb Mortal Wkly Rep 2013;62:537–542 [PMC free article] [PubMed] [Google Scholar]
- 40.Wendell AD. Overview and epidemiology of substance abuse in pregnancy. Clin Obstet Gynecol 2013;56:91–96 [DOI] [PubMed] [Google Scholar]
- 41.Breslau N, Chilcoat HD, Johnson EO, Andreski P, Lucia VC. Neurologic soft signs and low birthweight: Their association and neuropsychiatric implications. Biol Psychiatry 2000;47:71–79 [DOI] [PubMed] [Google Scholar]
- 42.Bada HS, Das A, Bauer CR, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics 2007;119:e348–359 [DOI] [PubMed] [Google Scholar]
- 43.Farr SL, Hayes DK, Bitsko RH, Bansil P, Dietz PM. Depression, diabetes, and chronic disease risk factors among U.S. women of reproductive age. Prev Chronic Dis 2011;8:A119. [PMC free article] [PubMed] [Google Scholar]
- 44.Farr SL, Bish CL. Preconception health among women with frequent mental distress: A population-based study. J Womens Health (Larachmt) 2013;22:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko JY, Farr SL, Dietz PM, Robbins CL. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005–2009. J Womens Health 2012;21:830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Hara MW. Postpartum depression: What we know. J Clin Psychol 2009;65:1258–1269 [DOI] [PubMed] [Google Scholar]
- 47.Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: A report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry 2009;31:403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]