Abstract
Significance: Renal oxidative stress can be a cause, a consequence, or more often a potentiating factor for hypertension. Increased reactive oxygen species (ROS) in the kidney have been reported in multiple models of hypertension and related to renal vasoconstriction and alterations of renal function. Nicotinamide adenine dinucleotide phosphate oxidase is the central source of ROS in the hypertensive kidney, but a defective antioxidant system also can contribute. Recent Advances: Superoxide has been identified as the principal ROS implicated for vascular and tubular dysfunction, but hydrogen peroxide (H2O2) has been implicated in diminishing preglomerular vascular reactivity, and promoting medullary blood flow and pressure natriuresis in hypertensive animals. Critical Issues and Future Directions: Increased renal ROS have been implicated in renal vasoconstriction, renin release, activation of renal afferent nerves, augmented contraction, and myogenic responses of afferent arterioles, enhanced tubuloglomerular feedback, dysfunction of glomerular cells, and proteinuria. Inhibition of ROS with antioxidants, superoxide dismutase mimetics, or blockers of the renin-angiotensin-aldosterone system or genetic deletion of one of the components of the signaling cascade often attenuates or delays the onset of hypertension and preserves the renal structure and function. Novel approaches are required to dampen the renal oxidative stress pathways to reduced O2−• rather than H2O2 selectivity and/or to enhance the endogenous antioxidant pathways to susceptible subjects to prevent the development and renal-damaging effects of hypertension. Antioxid. Redox Signal. 20, 74–101.
Introduction
Scope of the review
The involvement of oxidative stress and reactive oxygen species (ROS) in hypertension has been extensively studied. Comprehensive reviews on the generation and actions of ROS in vascular and cardiac systems have been published recently (88, 104, 192, 193, 199, 203, 204, 242, 255, 317, 319). These publications have emphasized the contribution of pro- and antioxidant enzymes, the signaling pathways involved, and the approaches for prevention and treatment of hypertension with the use of antioxidants drugs. This Forum will focus on the physiological and pathophysiological actions of ROS produced in the kidney and its blood vessels and their contribution to the development and maintenance of hypertension. Where data were not available in renal tissues, brief mention will be made of the important ROS pathways in systemic blood vessels.
Overview of renal ROS and hypertension
ROS-generating and metabolizing systems
ROS are generated as a normal product of cellular metabolism (204). ROS, such as superoxide anion (O2−•), hydrogen peroxide (H2O2), and hydroxyl anion (OH−•), are reactive byproducts of mitochondrial respiration or oxidases, including nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxireductase (XOR), and certain arachidonic acid oxygenases (316). ROS can be formed also by nitric oxide synthases (NOS) after depletion of the NOS substrate l-arginine or the cofactor tetrahydrobiopterin (BH4) or during partial inhibition of NOS by antagonists such as asymmetric dimethylarginine. While superoxide dismutase (SOD) is one of the major defense systems to remove O2−•, catalase, peroxiredoxins (Prxs), glutathione peroxidase (GPX), and thioredoxin (Trx) reductase, all are important to metabolize H2O2 (59, 76, 321). ROS also may be scavenged by antioxidant molecules such as tocopherols or ascorbate. The excessive production or decreased metabolism of ROS can lead to oxidative stress that alters the redox state in the tissue, resulting in redirection of redox-regulated signaling pathways and ultimately cellular dysfunction or damage (315, 316).
Nitric oxide, ROS, endothelial dysfunction, and hypertension
Oxidative stress can be a cause, a consequence, or a potentiating factor for hypertension. Increased production of O2−• in the vasculature impairs endothelium-derived relaxation factor/nitric oxide (EDRF/NO) and increases vascular smooth muscle cell (VSMC) contraction and proliferation, and attraction of inflammatory cells (75, 137, 161, 191, 294). Since the kidney contributes to the long-term control of blood pressure (BP) and this is, in part, dependent on NO (341) whose activity is regulated in the renal blood vessels and tubules by O2−• (317), it is important to understand the renal mechanisms of generation and metabolism of ROS, their interaction with NO, and their relationship to hypertension.
Renal oxidative stress
An increased production of ROS in the kidney can initiate hypertension. For example, mice with the SOD-3 isoform knockout (−/−) had higher basal BP compared with wild-type (+/+), which was associated with increased production of O2−• and inactivation of NO in the kidney (304). ROS also can accelerate the development of hypertension. For example, conscious SOD-3 (−/−) mice had an earlier and more rapid increase in BP with a slow-pressor infusion of angiotensin II (ANG II) than their wild-type (+/+) littermates, even though both achieved similar levels of BP after about 10 days (304). The lack of consistent findings of hypertension in SOD knockout models may relate to many factors. Some studies (38, 53, 89, 128, 243) reported tail-cuff measurements of BP, which lack precision. Others were limited to blood vessels or studied in animals under anesthesia (49, 53, 144). Those that used telemetry measurements of BP have reported no changes in SOD-1 (−/−) (28) or increased BP in SOD-3(−/−) (304) conscious mice. Importantly, these inconsistent conclusions cannot be explained simply by the different SOD isoforms, since, for example, SOD-3 (−/−) mice were reported to be hypertensive in one study (304), but normotensive in another (89). ROS promote or mediate hypertension initiated by many processes, such as activation of the renin-angiotensin-aldosterone system (RAAS) in rats with the two kidney, one clip (2K,1C) model of Goldblat renovascular hypertension (305). This was related to an activated neutrophil oxidase (NOX)-derived O2−• production and a reduced metabolism of O2−• by SOD (29, 304). Dietary salt loading increased renal and vascular ROS in many salt-sensitive models (24, 31, 136, 245, 315). This implies that oxidative stress contributes to both the renal and vascular mechanisms of both salt-independent and salt-sensitive hypertension (138, 179, 317).
Mechanisms of hypertension and renal ROS
Increased renal ROS production contribute to the development and progression of hypertension (2, 62, 159, 187, 245, 303, 304, 310) by increasing renal vasoconstriction (132), renin release (307), renal afferent nerve activity (31), contraction of afferent arterioles to increased renal perfusion pressure (myogenic response), or to ANG II (153), endothelin-1 (ET-1), and thromboxane prostanoid receptor (TP) activation (293). ROS also cause dysfunction of glomerular cells and proteinuria (73, 239, 246, 274, 330). Increased O2−• in the kidney leads to vascular dysfunction and disrupts water and sodium (Na+) homeostasis (104, 193, 267, 317). Vascular O2−• reacts with endothelium-derived NO and directly promotes vasoconstriction (167). The generation of O2−• in specific nephron segments in response to endogenous vasoconstrictors such as to ANG II increases (264) or decreases (224) tubular Na+ reabsorption, depending on the nephron site studied.
Intrarenal expression of pro- and antioxidant systems and hypertension
NADPH oxidase
NADPH oxidase is the predominant source of renal O2−•. NOX proteins are homologs of the phagocytic NADPH oxidase and are distributed widely in the renal vessels, glomeruli, and nephron segments, as summarized in Table 1 (27, 88, 170, 204, 254). NOX3 and NOX5 are expressed in the fetal, but not in the adult, kidneys (37). NOX1, NOX2, and NOX4 are expressed in the adult kidney. NOX4 is predominant in endothelial and tubular cells. All these NOX proteins require the chaperone protein p22phox and entail phosphorylation and/or upregulation of several cytoplasmic subunits (Rac1/2, p47phox, p67phox, p40phox, and Poldip2) for activity (88, 172). These subunits are demonstrated in most of the nephron segments, either by mRNA/protein expression or by physiologic experiments (Table 1).
Table 1.
Distribution of NADPH Oxidase Subunits Along the Nephron (mRNA and/or Protein)
| |
|
|
GLOM |
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| AA | EA | Podo | MC | PT | TAL | JGA/MD | DT | CCD | MCD | |
| NOX1 |
* |
N/A |
|
+ |
+ |
|
|
|
|
|
| NOX2 |
√ |
N/A |
+ |
|
+ |
+ |
+ |
|
+ |
± |
| NOX4 |
* |
N/A |
+ |
+ |
+ |
|
+ |
+ |
|
+ |
| P22phox |
* |
N/A |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
± |
| P40phox |
N/A |
N/A |
|
|
|
+ |
|
|
|
|
| P47phox |
√ |
N/A |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
± |
| P67phox | N/A | N/A | + | + | + | + | + | + | + | ± |
Presumed by physiologic experiments (28).
+, present; ±, weak; N/A, no data available.
mRNA detected in preglomerular vascular smooth muscle cells of WKY rats (170).
AA, afferent arteriole; EA, efferent arteriole; GLOM, glomerulus; Podo, podocytes; MC, mesangial cells; PT, proximal tubule; TAL, thick ascending limb; JGA/MD, juxtaglomerular apparatus/macula densa; DT, distal tubule; CCD, cortical collecting duct; MCD, medullary collecting duct; NADPH, nicotinamide adenine dinucleotide phosphate; NOX, neutrophil oxidase; WKY, Wistar Kyoto.
Specific NADPH oxidase subunits in the kidney are implicated in the development of hypertension in animal models. Thus, p67phox was required for salt-sensitive hypertension (62), p22phox and p47phox for ANG II-induced hypertension (103, 153, 191, 207) and for genetic hypertension in spontaneously hypertensive rats (SHR) (29, 265), and Poldip2 for hypertension in models of chronic kidney disease (CKD) (150).
Studies using gene-deleted (−/−) mice and pharmacological inhibitors implicate NADPH oxidase-dependent ROS generation in the kidney cortex in increased renal vascular resistance (RVR), enhanced mitochondrial respiration, and decreased efficiency of O2 usage for Na+ transport (TNa/QO2) in models of hypertension (301) and CKD (150). NADPH oxidase-dependent ROS generation in the renal medulla is limited by the availability of O2 (34). Medullary ROS enhance tubular Na+ reabsorption and diminish pressure natriuresis (209). Indeed, there are increased ROS and decreased NO, activities in the renal outer medulla in many hypertensive and salt-sensitive models (41, 209). An increased expression of p67phox in the renal outer medulla of the Dahl salt-sensitive (DS) rat mediates increased NADPH oxidase activity, salt-sensitive hypertension, and renal injury (62).
Activation of NADPH oxidase by p47phox in the renal afferent arteriole leads to endothelial dysfunction, the development of an endothelium-dependent constrictor factor (EDCF) response, enhanced contractility to ANG II, ET-1, and TP agonists, and enhanced myogenic responses to increased renal perfusion pressure (2, 129, 153, 193).
NADPH oxidase-derived O2−• has nephron-specific effects on tubular Na+ transport and fluid reabsorption (217, 224, 264). ROS decrease Na+ reabsorption in the proximal tubule (PT) (224), but increase Na+ reabsorption in the loop of Henle (LH) and in distal segments of the nephron (177, 264, 272). Activation of specific isoforms of NOS has the opposite effects at these nephron segments.
Enhanced production of O2−• by NADPH oxidase in the macula densa (MD) diminishes NO signaling and increases tubuloglomerular feedback (TGF) responses (312). These likely contribute to enhanced preglomerular vasoconstriction and reduced glomerular filtration rate (GFR) that would restrict the rapid elimination of a salt load and predispose to salt sensitivity (70, 165, 207, 312).
Superoxide dismutases
Increased ROS in the kidney results not only from overproduction by prooxidants enzymes but also from decreased metabolism by antioxidant enzymes (187, 245, 304). The ability of an intact antioxidant system to reduce or remove ROS is a key step in the limitation of tissue injury. Indeed, antioxidant enzymes are heavily expressed in the kidney where they restrict the levels of ROS and limit hypertensive tissue injury (29, 75, 304). The major scavenging system for removal of O2−• is the SOD family of enzymes that catalyzes the dismutation of O2−• to H2O2. H2O2, more stable than O2−•, induces a vasorelaxation that has been ascribed to the endothelium-derived hyperpolarizing factor (72, 166), upregulated eNOS (72), and to blunted myogenic responses (150). H2O2 contributes to the ANG II-dependent hypertrophic and remodeling responses in blood vessels (75).
Three SOD isoforms are detected in the normal kidney: the copper–zinc containing SOD (SOD-1), located predominately in the cytoplasm; the manganese-containing SOD (SOD-2) in the mitochondria; and the extracellular SOD (SOD-3) in the extracellular space, where it is anchored by a heparin-binding domain that is required for its full activity (67, 208, 210, 215, 278, 279, 342).
Studies have reported the distribution of the different SOD isoforms along the nephron using immunohistochemical or in situ hybridization techniques (67, 208, 210, 215, 278, 279, 342). These have yielded somewhat different conclusions depending on the species studied. (67, 208). A high level of SOD-3 immunoreactivity in the mouse is observed on the cortical and juxtamedullary PT and in VSMCs of renal blood vessels, whereas medullary areas are only weakly reactive, and glomeruli are not stained (66, 215). Although rats have a diminished tissue SOD-3 expression, it is demonstrated clearly in the rat kidney (29, 303). SOD-1 is expressed in the kidney of rats and dogs (278, 279). Its immunoreactivity in the dog kidney is prominent in the cortical thick ascending limb (cTAL) while, in the rat kidney, it is confined to the PT. SOD-1 represents ∼60% to 80% of total SOD activity in the kidneys of several species (181), but only about 30% of total SOD activity in the vasculature, where it preserves endothelial NO activity (28). Wild-type mice and transgenic mice expressing the human SOD-2 isoform have high levels of SOD-2 immunoreactivity in the mitochondria of all tubular cells. However, only transgenic mice have prominent SOD-2 immunoreactivity in endothelial cells (ECs), VSMCs, and interstitial and glomerular cells (210).
Generation and metabolism of O2−• in hypertension
A growing body of evidence supports the hypothesis that both overproduction of O2−•, notably by NADPH oxidase and mitochondria, and reduced O2−• metabolism by SOD and other antioxidant enzymes can initiate or potentiate the development of hypertension. Two weeks of salt loading or 2 weeks of prolonged ANG II infusion both increase renal oxidative stress by increasing O2−• generation via NADPH oxidase and decreasing O2−• metabolism via SOD (29, 138, 317). Since salt loading reduces renin release and ANG II, several hypotheses can be proposed to explain the mechanisms involved in O2−• production induced by ANG II or high salt. Some studies reported selective changes in the expression of NADPH oxidase components or SOD isoforms in the kidney after salt loading or ANG II infusion. Salt loading increases the expression of NOX2, NOX4, p22phox, p47phox, and p67phox and decreased SOD-1 and SOD-2 (15, 32, 71, 138, 187), whereas ANG II acting via AT-1 receptors (AT1R) increases the renal expression of NOX2, NOX4, p22phox, p47phox, p67phox, Rac1, and SOD-2 and decreased expression of SOD-3 (29, 30, 62, 70, 90, 93, 129, 138, 153, 187, 207, 312). Other studies reported increased mitochondrial generation of H2O2 by the medullary thick ascending limb (mTAL) cells in response to increased tubular flow that secondarily increased NADPH oxidase activity (213). A paradoxical activation of the intrarenal RAAS observed in DS rats fed high-salt diet (142) could represent another mechanism of NADPH oxidase activation in the kidney in salt-sensitive hypertension. Salt loading increased ET-1 production in the collecting duct (CD) of normal rats and mice (173). However, DS rats had lower medullary levels of ET-1 and endothelin receptor type-B (ETB) receptor in response to a salt load than Dahl salt-resistant (DR) rats (269). Since ET-1 negatively regulates Na+ reabsorption in the CD, the increased Na+ transport at this site provides another potential pathway for salt-dependent renal oxidative stress. The activation of parallel pathways may explain the very severe hypertension and renal damage caused by an infusion of ANG II into rats fed a high-salt diet.
As reviewed comprehensively (319), short- or long-term administration of polyethylene glycol covalently linked to SOD (PEG-SOD) to increase cellular uptake or SOD-mimetic compounds such as the redox-cycling nitroxide tempol decreases oxidative stress, improves vascular and renal function, and lowers the BP in conscious or anesthetized rats and/or mice in genetic, salt-sensitive or -resistant, ANG II-dependent or -independent models of hypertension (28, 134, 245, 251, 319).
Welch et al. (304) reported decreased SOD activity and decreased SOD-3 expression in the kidney cortex of mice after 12 days of ANG II infusion at a slow-pressor rate. In contrast, Fukai and Harrison (75) reported increased SOD-3 expression in blood vessels from mice infused with ANG II at pressor rates. ANG II infusion in the rat decreased the renal cortical mRNA for SOD-3, yet increased SOD-2 (29). Renal expression of SOD-1 and SOD-2 was unchanged in SHR, but SOD-3 was significantly decreased (2). The expression of SOD-1 and SOD-2 was reduced in the renal medulla of DS rats compared with DR rats (187). SOD-2 is inactivated rapidly by nanomolar concentrations of peroxynitrite (ONOO−). Guo et al. (95) reported that the nitrotyrosine content of SOD-2 in the kidneys of rats increased 13-fold after 2 weeks of infusion of ANG II at a pressor rate, and that renal SOD-2 activity was decreased by 50%, despite unchanged protein expression. This was ascribed to post-transcriptional modification by tyrosine nitration, which decreases the SOD-2 activity and increases oxidative stress in the kidney during ANG II-induced hypertension. Thus, there are site-specific effects of ANG II or salt loading on SOD isoform expression within the kidney and blood vessels.
Oxidative stress in the renal medulla has been assigned an important role in the development of hypertension (41). Increased medullary ROS induced by prolonged SOD inhibition are associated with reduced medullary blood flow (MBF), reduced Na+ excretion (UNaV), and hypertension (175, 176).
Collectively, these studies demonstrate that a defective renal SOD system contributes to increased renal oxidative stress and hypertension, but the patterns of altered SOD isoform expression vary with the hypertensive model.
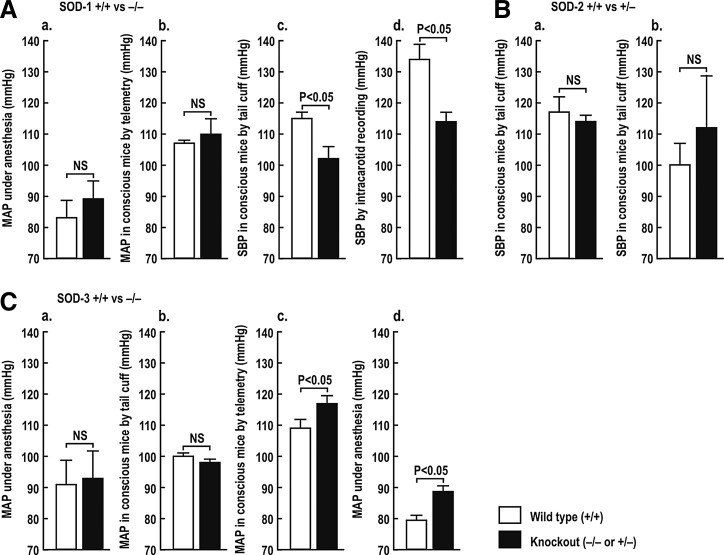
Genetic deletion of SOD isoforms has provided excellent models to study the effects of a prolonged compartmental increase in O2−• on BP and renal function. Mice with genetic deletion of SOD-1 or SOD-3 do not show obvious phenotype changes, but are more sensitive to tissue damage during oxidative stress despite some compensatory upregulation of other SOD isoforms (89, 304). In contrast, SOD-2 (−/−) mice have severe phenotypes, with embryonic or neonatal lethality. The absence of SOD-2 is not compensated by overexpression of SOD-1 (40). Thus, SOD-2 is essential for life (155, 163, 257). Viable SOD-2 (+/−) mice have ∼50% reduction in SOD-2 activity in the kidney (289) without compensatory upregulation of the other SOD isoforms (290).
Basal levels of BP are normal (28, 144) (Fig. 1A, panels a and b) or modestly reduced (53) (Fig. 1A, panels c and d) in SOD-1 (−/−) mice, but are not changed significantly in SOD-2 (+/−) mice (Fig. 1B, panels a and b) (35, 243). The BP is unchanged (49, 128) (Fig. 1C, panels a and b) or modestly elevated (Fig. 1C, panels c and d) (304) in SOD-3 (−/−) mice.
FIG. 1.
Effects of knockout of SOD isoforms on blood pressure in mice. Mean±SEM values of blood pressure in conscious or anesthetized mice. p<0.05 compared to wild-type mice. Redrawn from Refs.: A [a (144); b (28); c, d (53)]; B [a (38); b (243)], C [a (49); b (128); c, d (304)]. MAP, mean arterial pressure; SOD, superoxide dismutase; NS, not significant.
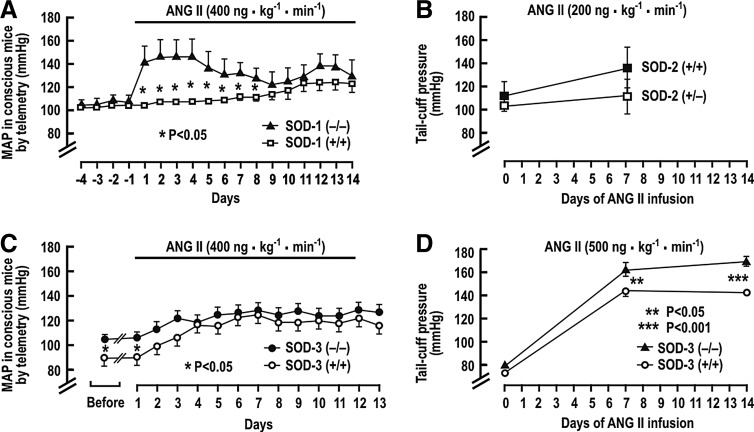
Several studies have reported the BP response to a prolonged infusion of ANG II in the SOD gene-deleted mice. Carlstrom et al. (28) reported an unchanged basal mean arterial pressure (MAP), but a much accelerated rise of MAP during a slow-pressor infusion of ANG II into SOD-1 (−/−) mice, although the final levels of BP after 2 weeks were not different from SOD-1 (+/+) mice (Fig. 2A). SOD-2 (+/−) mice had similar BP as wild-type mice after ANG II infusion (38, 243)(Fig. 2B). SOD-3 (−/−) mice had a higher basal level of MAP, but a similar response to ANG II in one study (304) (Fig. 2C), but an enhanced rise in BP to a rather high-rated ANG II infusion in another (89) (Fig. 2D). The RVR also was significantly higher in anesthetized SOD-3 (−/−) mice (304).
FIG. 2.
Blood pressure during prolonged infusion of ANG II in the SOD gene-deleted mice. Mean±SEM values of blood pressure in conscious mice. (A) SOD-1 (+/+) and (−/−) mice; (B) SOD-2 (+/+) and (+/−) mice; (C, D) SOD-3 (+/+) and (−/−) mice. *p<0.05, **p<0.001 compared to wild-type mice. Redrawn from Refs. [A (28); B (38), C (304), D (89)]. ANG II, angiotensin II.
Jung et al. (128) studied the effect of SOD-3 gene deletion on the development of ANG II-induced and in 2K,1C hypertension in mice. After renal artery clipping, both SOD-3 (−/−) and wild-type mice had impaired endothelium-dependent vascular relaxation, reduced vascular NO, and elevated O2−•. However, SOD-3 (−/−) mice had higher BP after 2 and 4 weeks of renal artery clipping, and after 1 week of ANG II infusion at a pressor rate. These effects were ameliorated by human recombinant SOD-3 and by the SOD mimetic tyron. This study demonstrated that the increased BP and limited NO bioavailability in the absence of SOD-3 were due to enhanced O2−•. These data demonstrate that a primary increase in tissue O2−• could enhance BP and RVR, and the rate of BP rises with ANG II, but the final degree of change is modest. Moreover, some studies did not detect a rise in basal BP in SOD-1-, SOD-2-, or SOD-3-gene deleted mice. Thus, these studies in aggregate demonstrate only modest and inconsistent effects of lifelong enhanced O2−• produced by gene deletion of SOD isoforms in mice. They indicate that prolonged increases in O2−• do not necessarily lead to hypertension, but may accelerate the development of hypertension, for example, with ANG II infusion.
Overexpression of specific SOD isoforms mitigates oxidative stress and hypertension in some (28, 63, 160, 198), but not all, studies (5, 63). Adenoviral gene transfer of human SOD-3 (AdECSOD) into SHR increased SOD-3 expression in the blood vessels and in the kidney, reduced the vascular O2−• (39), and improved the endothelial function and vascular reactivity. AdECSOD reduced the BP and Na+ balance, implying that both renal and vascular extracellular oxidative stress contributes to hypertension in the SHR. AdECSOD also improved endothelial function and increased vascular NO availability in the SHR stroke-prone (SHRSP) model (63). The injection into SHR of human recombinant SOD-1 containing a heparin-binding domain to mimic the vascular binding of SOD-3 decreased BP, whereas SOD-1 lacking this domain had a little effect (198). Overexpression of SOD-2 improved endothelial function in deoxycorticosterone acetate (DOCA) salt-hypertensive rats (160) and protected PT cells in vitro from mitochondrial injury and apoptosis induced by adenosine triphosphate (ATP) depletion (43). Perfused afferent arterioles isolated from transgenic mice overexpressing SOD-1 had a reduced sensitivity and contractile responsiveness to ANG II (28).
These studies demonstrate that maneuvers that increase SOD activity could prevent oxidative stress and mitigate the development of hypertension (319). The importance of each SOD isoform depends on its location, the origin of the oxidative insult, and the species studied, but most studies concur in identifying O2−•as the villain. Therefore, SOD mimetic agents might represent a rational strategy to prevent renal oxidative stress, high BP, and its consequences (319).
Other antioxidant enzymes
Catalase, GPX, Trx, and Prxs metabolize H2O2 to water and O2. Their dysregulation leads to the accumulation of H2O2, which interacts with transition elements to form the highly reactive OH−• by Fenton reaction. H2O2 is a mediator of cardiovascular and renal dysfunction and hypertension (36, 267, 268) and produces vasodilation (80), vasoconstriction (81), or a biphasic effect depending on the vascular bed (35, 44, 80).
Catalase is a 240-kDa homotetrameric heme-containing protein located predominantly in the peroxisome and expressed abundantly in the liver, lungs, and kidneys. Catalase deficiency results in overexpression of mitochondrial ROS and functional mitochondrial impairment (118). Catalase overexpression protects against H2O2 toxicity, whereas catalase deficiency exacerbates oxidative injury (110, 118, 140, 331).
Basal levels of H2O2 in the rat renal medulla are twofold higher than in the renal cortex (36). Exaggerated levels of H2O2 are reported in the renal medulla in several models of hypertension. They are implicated in the rise of BP, since intramedullary microperfusion of catalase reduced the H2O2 concentrations and moderated the hypertension, whereas intramedullary microperfusions of tempol or PEG-SOD were not fully effective (36, 267, 268).
Reduction of cellular glutathione (GSH) concentrations in rats by administration of buthionine sulfoximine caused progressive hypertension (292, 321). GPX not only metabolizes H2O2 but also converts toxic lipid peroxidation products into lipid alcohols in the presence of GSH. The reaction of O2−• with arachidonate (AA) forms lipid peroxides, termed isoprostanes (240). Isoprostanes, prostaglandin (PG) endoperoxides, cyclooxygenase (COX) metabolites of hydroxyeicosatetraenoic acid (HETE), the stable mimetic U-46,619, and perhaps even prostacyclin (61), in addition to thromboxane A2 (TxA2) all activate the TP. The TP is expressed in ECs and VSMCs and in the kidney (291). ANG II infusion in the mouse increases the generation of isoprostanes and TxA2. Remarkably, studies in TP (−/−) mice demonstrated that this receptor mediates not only the ANG II-induced increases in BP and RVR but also the oxidative stress (133). Increased production of renal eicosanoids activating TP receptors enhances Cl− reabsorption in the LH (311) and augments TGF responses (311). Thus, these multiple effects indicate that ligands for this receptor or oxidative stress increase TP activity (287) that may blunt the pressure natriuresis and contribute to salt sensitivity (321, 346).
The Trx system comprises Trx, thioredoxin reductase (TrxR), and Prx. These abundant proteins from different family members are widely distributed through the cytoplasm, mitochondria, and other cell compartments (59, 211). This system has been implicated in the regulation of the cellular redox state, DNA synthesis, cell proliferation, and apoptosis (74). Three Trxs have been identified in mammalian cells: Trx1, located in the cytoplasm and the nucleus; Trx2 in the mitochondria; and Trx3 in spermatozoa (211). The TrxR is a selenocysteine-containing flavoprotein with three isoforms that control the activity of Trxs and thereby the cellular redox state. TrxR1 is located in the nucleus and the cytoplasm, whereas TrxR2 and TrxR3 are located in the mitochondria (211). The proteins of the Trx system are expressed in both ECs and VSMCs (59). Trx reduces Prxs, which in turn reduce H2O2 to H2O (59). Prxs compose a large antioxidant gene family that uses conserved cysteine (Cys) residues at the sites of peroxidation. The Prx1 and Prx2 isoforms are expressed in the cytoplasm, Prx3 and Prx5 in the mitochondria, and Prx4 in the endoplasmic reticulum (281). Mitochondrial Trx systems (Trx2, TrxR2, and Prx3) protect cells from mitochondrion-dependent ROS and apoptosis (59, 74, 314). Trx proteins are abundant in the renal tubules (52), especially in PTs and distal tubules (DTs), while lower levels are reported in glomerular cells (54, 211).
There are only limited studies of Trx and TrxR in hypertension. The Trx system is upregulated (58) or downregulated (277) in hypertension, depending on the tissue, the stimulus, and the model. Decreased Trx expression was observed in the aorta, heart, and kidneys of SHR and SHRSP and was related to the hypertension (277). In contrast, Trx expression was increased in the hearts of mice infused with ANG II at a slow-pressor rate (58). Trx1 expression in human umbilical vein endothelial cells was enhanced by low concentrations (10–50 μM) of H2O2, but was degraded by higher concentrations (100–500 μM) (98, 99). Transgenic mice overexpressing EC-specific Trx2 (Trx2Tg) have reduced mitochondrial and total oxidative stress and increased NO activity and endothelium-dependent relaxations and lower basal BP. An infusion of Nω-Nitro-l-arginine methyl ester (L-NAME) increased the BP more in Trx2Tg mice than in wild-type mice, thereby implicating increased NO availability in the lower basal BP of these mice (347). Overexpression of human Trx2 in ANG II-infused mice limited the increased NOX2, p22phox, p47phox, and Rac-1 expression, diminished vascular and mitochondrial ROS, improved the endothelium-dependent vascular relaxation responses, and attenuated the hypertension (314). Since Trx2 is limited to the mitochondria, this implies that mitochondrial ROS contribute to decrease NO availability and increase NADPH oxidase activity and thereby sustains the oxidative stress (74, 314). Thus, inhibition of the renal Trx system could represent an additional mechanism to increase oxidative stress and lipid peroxidation and to impair endothelium-dependent relaxation in preglomerular vessels and perhaps exacerbate hypertension, but further studies are required.
In summary, the Trx system has been a focus of study in vascular biology where it has been shown to preserve NO bioavailability and endothelial function and to improve BP in ANG II-induced hypertension (74, 314, 347). Studies in the kidney are more limited, but generally concur in concluding that an impairment of the renal Trx system contributes to the impairment of NO activity associated with hypertension (277).
ROS and Renal Hemodynamics
Action of renal ROS on hemodynamics
Renal blood flow (RBF) determines GFR and tubular Na+ delivery and thereby is an important component of body salt and fluid homeostasis. The regulation of RBF by ROS has been studied extensively. A reduced RBF, or an altered intrarenal blood flow distribution, has been implicated in the development and maintenance of hypertension (236, 266, 317). Increased RVR attributable to ROS has been reported in genetic hypertension in the SHR, renovascular hypertension in the 2K,1C Goldblatt model and in the reduced renal mass (RRM) model of CKD, and in the model of ANG II-infused rats and/or mice (134, 251, 303, 305, 317). Infusion of ANG II at a slow-pressor rate increases the RVR in rats and mice before hypertension (134, 317) and is accompanied by increased renal and vascular ROS, increased renal excretion of lipid peroxidation products, and increased renal tyrosine nitration, implying ONOO− generation (29, 103, 133, 134, 216, 294, 312, 317). The coadministration of tempol prevents those events, reinforcing the evidence for involvement of O2−• in ANG II-dependent hypertension (134, 301–303).
ANG II acting on AT1Rs enhances the generation of O2−• by NADPH oxidase and the release of AA by phospholipase A2. AA is metabolized by COXs to PG endoperoxides (PGG2 and PGH2), which are further metabolized by enzymes, including thromboxane synthase to TxA2, or oxidized by O2−• to 8-isoprostane F2-alpha (8-iso). PGH2, TxA2, and 8-iso all activate TP (191). Welch and colleagues (33) reported that hypertension was maintained in rats at the ANG II-dependent phase of 2K,1C Goldblatt hypertension by AT1R and by COX-1 products that activated the TP (311). The TP also is implicated in the ANG II slow-pressor response, since rats given TP antagonists or TP (−/−) mice do not develop oxidative stress, renal vasoconstriction, or hypertension (133, 308). These prohypertensive effects of TPs were hypothesized to include increased pre- and postglomerular resistance and enhanced TGF responses (133).
Therefore, renal hemodynamics and GFR depend on the interaction of several elements, including ANG II, PGs, and TP activation, all of which can interact with ROS and NO to regulate the tone of the afferent and efferent arterioles.
Tubuloglomerular feedback
TGF regulates the glomerular arteriole resistance. There is a vasoconstrictive response of the renal afferent arteriole during increased NaCl delivery to, and reabsorption by, the MD segment. The proximal tubular fluid flow that yields 50% activation of TGF (set point) is close to the ambient rate measured by micropuncture. Thus, a reduction in tubular fluid flow will reduce TGF and vasodilate the afferent arteriole. TGF is a unique renal mechanism whereby vasoconstriction is regulated by tubular NaCl transport, and thereby the renal hemodynamics is adjusted to maintain the body fluid volume and BP. The MD cells at the junction of the thick ascending limb (TAL) of the LH are activated by reabsorption via the luminal Na+/K+/2Cl− transporter type-2 (NKCC2). This leads to the elaboration of positive (notably adenosine, ATP and vasoconstrictor PGs) and negative (notably NO and vasodilator PGs) signaling molecules, which adjust the tone of the renal afferent arteriole. Ren et al. (238) reported an efferent arteriole vasodilation in response to reabsorption of fluid by the MD that was mediated by adenosine receptors type-2. Thus, activation of TGF may reduce single-nephron glomerular filtration rate (SNGFR) both by increasing afferent arteriole vasoconstriction and by decreasing efferent arteriole vasoconstriction.
The myogenic response is a stretch-activated contraction of VSMC in the interlobular and afferent, but not efferent, arterioles. Myogenic and TGF responses together coordinate renal autoregulation whereby the RBF and the GFR remain stable despite changes in the renal perfusion pressure within a physiological range. ROS interact with and/or mediate both the myogenic and the TGF components of renal autoregulation and RVR, hypertension, and associated renal damage.
TGF- and MD-mediated renin release both have been implicated in the short- and long-term regulation of renal hemodynamics and in salt and water balance. Activation of TGF increases the afferent arteriolar resistance and reduces the SNGFR. Resetting of TGF by neuronal NOS (nNOS) in the MD during changes in BP or salt intake likely contributes to efficient excretion of excessive NaCl excretion (17, 282).
NaCl reabsorption in the MD stimulates nNOS-derived NO production, which puts a brake on TGF responses (217, 288, 322). Microperfusion of the tubular lumen of the MD of Sprague-Dawley or Wistar Kyoto (WKY) rats with nonspecific NOS inhibitors (NG-methyl-l-arginine acetate [L-NMMA] and L-NAME) or with a specific nNOS inhibitor, 7-nitroindazole (7-NI), enhances TGF responses (309, 310, 322), while perfusion with an NO donor blunts TGF (312). Blockade of nNOS reduces GFR and causes hypertension in rats (214, 310).
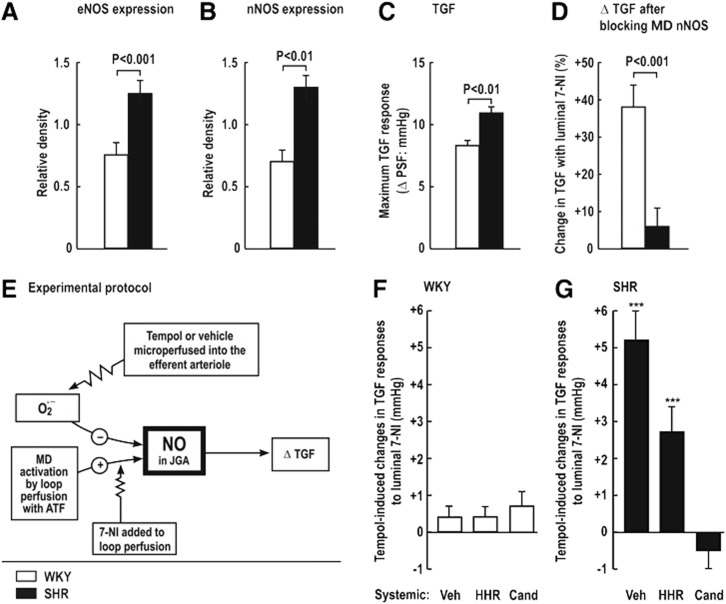
Increased MD levels of O2−• that enhance TGF responses contribute to renal vasoconstriction, salt retention, and hypertension, for example, during ANG II infusion or in the SHR. Thus, SHR, compared with WKY, have increased renal expression of eNOS and nNOS (Fig. 3A, B), yet paradoxically enhanced TGF responses (Fig. 3C) and diminished blocking of TGF by juxtaglomerular apparatus (JGA)-derived NO, as indexed from the increase in TGF during blockade of nNOS by luminal 7-NI added to the artificial tubular fluid (Fig. 3D). Tempol or vehicle was perfused into the star vessel (efferent arteriole) to reach the peritubular capillaries surrounding the test nephron to correct oxidative stress (Fig. 3E). The effects of tempol on NO signaling in the JGA again were assessed from the changes in TGF during luminal 7-NI. There were no significant tempol-induced changes in the response to luminal 7-NI in normotensive WKY rats, implying no interference in NO signaling by O2−• (Fig. 3F). In contrast, tempol microperfused into the efferent arteriole and peritubular capillaries of the SHR increased the TGF by circa 40%. This effect in SHR nephrons was prevented by 2 weeks of administration of the ANG II type-1 receptor blocker (ARB) candesartan, but not by equivalent lowering of BP with hydralazine, hydrochlorothiazide, and reserpine (Fig. 3G). Welch et al. (310, 311) conclude that AT1R-mediated increases in O2−• in the JGA of the SHR enhance TGF by preventing its buffering by MD-derived NO from nNOS. This reflects NO bioinactivation by O2−• in the JGA of the SHR. In other studies, systemic silencing of the p22phox gene during ANG II infusion reduced renal ROS, and prevented progressive hypertension (191) and moderated the TGF responses (207). Fu et al. (70) reported that activation of AT1R by ANG II mediated NOX2-dependent O2−• production in the MD cells, whereas basal O2−• generation was sustained by NOX4. Thus, increased NOX2-derived O2−• may inactivate nNOS-derived NO in the MD and eNOS-derived NO in the afferent arteriole in hypertensive models. This could account for the enhanced TGF responses that contributed to renal vasoconstriction and renal Na+ retention (70, 312, 316, 320, 321).
FIG. 3.
Interaction between macula densa derived nitric oxide and superoxide in the juxtaglomerular apparatus of the SHR. Comparison of protein expression for eNOS (A) and nNOS (B) and the change in tubuloglomerular feedback (TGF) with 7-nitroindazole (7-NI) in WKY rats (open bars, n=6) and SHR (solid bars, n=6) (C, D). (E) represents the experimental protocol for the subsequent study. (F, G) represent the effects of efferent arteriolar tempol on TGF responses to 7-NI compared to vehicle in WKY rats and SHR after 2 weeks of vehicle, candesartan (3 mg/kg/24 h), or hydralazine plus hydrochlorothiazide plus reserpine (30, 10, and 0.2 mg/kg/24 h). ***Significance of change: p<0.001. Redrawn from (310, 311). Veh, vehicle; HHR, hydrochlorothiazide, hydralazine and reserpine; Cand, candesartan; JGA, juxtaglomerular apparatus; NOS, nitric oxide synthase; nNOS, neuronal NOS; SHR, spontaneously hypertensive rats; WKY, Wistar Kyoto.
Arteriolar constriction and dilation
ROS regulate renal hemodynamics both directly, through vasoconstriction, and indirectly, through reducing NO activity (3, 175, 250). ROS are generated in microvessels and in genetic models such as the SHR and in the kidney in response to both short-term and prolonged infusions of ANG II, or other vasoconstrictors such as ET-1 or TP agonists (29, 226, 294). Thus, vasoconstriction produced by the TP activator U-46,619 on rabbit isolated, perfused afferent arteriole is moderated by coincubation with tempol, but enhanced by L-NAME (251).
ROS have been implicated in the acetylcholine-stimulated release of COX-1 and COX-2-derived EDCFs in renal afferent arterioles of rats or rabbits with ANG II or 2K,1C renovascular hypertension (250, 293, 294, 305). The EDCFs activates the TP on afferent arteriolar VSMCs to cause vasoconstriction. Tempol, COX-2 inhibitors, or TP antagonists, all improved endothelium-dependent relaxations and eliminated endothelium-dependent contractions in these models. Prolonged administration of a COX-2 antagonist or tempol also reduced the BP, increased the RBF, and restored the endothelial function of renal arteries of the 2K,2C renovascular hypertensive rats (283). These studies implicate intrarenal and afferent arteriolar ROS in the generation of an EDCF that caused TP-dependent renal vasoconstriction and contributed to hypertension.
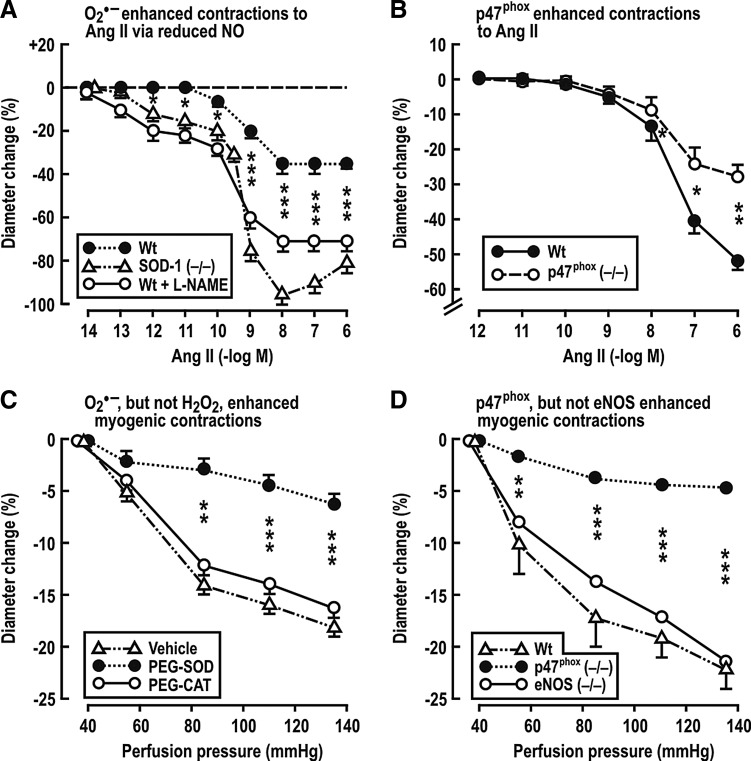
Adenosine receptors type-1 (A1AR) on afferent arterioles is required for TGF responses (21). Adenosine enhances the long-term constrictor effects of ANG II on preglomerular vessels (27, 69, 156). Lee et al. (156) reported that the infusion of ANG II at a slow-pressor rate in mice leads to hypertension and increases Na+ reabsorption that depends in part on A1ARs. A1AR (−/−) mice have blunted arteriolar and BP responses to L-NAME and ANG II (79). Gao et al. (79) propose that NO decreases, and ROS increases, adenosine production, and that adenosine mediates some of the renal effects of O2−• vasoconstriction. The p47phox component of NADPH oxidase is required for a full hypertensive response to ANG II (93). Likewise, Lai et al. demonstrated that p47phox also is required for increased BP and RVR in mice infused with ANG II (153). Studies by Carlstrom et al. (28) and Lai et al. (153) have investigated the roles of O2−•, H2O2, and NO on mouse isolated afferent arterioles. Both ANG II and increased perfusion pressure augmented afferent arteriolar ROS generation (153). Genetic deletion of SOD-1 increased O2−• and enhanced the response to ANG II by 89% and the sensitivity by >1000-fold (Fig. 4A). This was attributed to bioinactivation of NO, since, in the presence of L-NAME, ANG II contractions became similar in SOD-1 (+/+) and in (−/−) arterioles (28). The O2−• generated by ANG II requires the p47phox component of NADPH oxidase, since arterioles from p47phox (−/−) mice had diminished response to ANG II (Fig. 4B) and diminished ROS generation (153). Similarly, myogenic contractions of mouse afferent arterioles to increased perfusion pressure were diminished by PEG-SOD, but not by PEG-catalase (CAT) (Fig. 4C), and by maneuvers that blocked NADPH oxidase (154). However, unlike the response to ANG II, neither blockade of NO with L-NAME nor genetic deletion of eNOS affected the myogenic contractions (Fig. 4D) (153). These studies indicate that eNOS-derived endothelial NO potently inhibits the response to ANG II, but that this blunting is potently inhibited by O2−•. In contrast, myogenic responses do not depend on the endothelium and therefore are modulated by O2−• independent of NO (28). Carlstrom et al. (27) reported that NOX2 is required for contractile responses of the afferent arteriole to ANG II and/or adenosine and is implicated in prolonged ANG II-induced hypertension in mice. Ren et al. (236) reported that NOX2-derived O2−• contributes to the enhanced myogenic response, and hence to the high preglomerular resistance, of SHR, but these authors did not detect an effect of O2−• on myogenic responses of afferent arterioles from WKY rats.
FIG. 4.
Mean±SEM values for contractions of mouse isolated and perfused renal afferent arterioles. (A, B) depicts dose–response effects of bath addition of ANG II. In (A) are shown responses of afferent arterioles from normal wild-type mice, mice with deletion of SOD-1, or normal mice after the bath addition of 10−4M of l-nitroarginine methyl ester. In (B) are shown responses of p47phox knockout and wild-type mice. (C, D) depict the effects of graded increases in the afferent arteriolar perfusion pressure. In (C) are shown the responses of the afferent arteriole from normal mice after incubation with a vehicle, pegylated SOD, or pegylated catalase. In (D) are shown the responses of arterioles from wild-type mice or endothelial nitric oxide synthase or p47phox gene-deleted mice. *p<0.05, **p<0.01, and ***p<0.005 compared to wild-type or vehicle. Redrawn from Refs. [A (28); B, D (153); C (154)]. WT, wild-type mice; L-NAME, Nw-Nitro-l-arginine methyl ester; PEG-SOD, polyethylene glycol covalently linked to SOD; PEG-CAT, polyethylene glycol covalently linked to catalase.
In summary, inactivation of NO by increased renal O2−• production enhances afferent arteriole tone, Na+ reabsorption, and TGF responses, all of which contribute to hypertension (178–180, 310). ROS antagonizes the effects of eNOS-derived NO to increase renal plasma flow by dilating the glomerular arterioles. MD O2−• prevents the effects of NO formed via nNOS in the MD to blunt TGF and reduces afferent arteriole vasoconstriction. Therefore, an increase in the intrarenal ROS can have importance on renal hemodynamics that could lead to hypertension (284, 350).
Afferent arterioles from a rat model of type-1 diabetes mellitus (T1DM) are dilated (286). Pharmacological studies demonstrated that this could be ascribed to activation of ATP-sensitive K+ (KATP) channels and Kir1.1/Kir3.x inward-rectifier K+ channels in VSMCs. Activation of membrane K+ channels should hyperpolarize the VSMCs, inactivate the voltage-gated Ca2+ channels, and thereby lead to vasodilation. The activation of the K+ channels in T1DM rat arterioles is attributed to oxidative stress, which is prevented in rats given tempol (286). This is an important result, since it provides a mechanism for diabetes hyperfiltration. It is unusual in demonstrating that prolonged ROS can cause afferent arteriole vasodilation, and provides a novel mechanism to explain it.
Renal autoregulation
RBF and GFR are efficiently buffered against changes in the renal perfusion pressure by a coordinated response of the renal afferent arteriole and the interlobular artery. At least three mechanisms participate in autoregulation of RBF: a fast myogenic response, a slower TGF response, and a third, very slow component of uncertain causality (131, 256). Just and Arendhorst (130) demonstrated that the myogenic response in the mouse kidney contributed 60%, TGF 40%, and the third mechanism 5% to RBF autoregulation. Apocynin attenuated the myogenic responses, which implicates O2−•, and this was independent of NO. Similar conclusions were drawn by Lai et al. (154) (Fig. 4C, D).
Renal autoregulation is impaired in the RRM, DOCA/salt, and DS rat models of hypertension and in salt-supplemented SHRSP (109, 169, 194, 230, 247, 252). The glomerular capillary hydraulic pressure (PGC) and the blood flow in cortical glomeruli of SHR are maintained during elevation of the renal perfusion pressure by highly effective autoregulation (121, 233, 317). This suggests that SHR have increased preglomerular vascular resistance, which is consistent with the studies showing narrowed afferent arterioles (206, 317), with enhanced myogenic vasoconstriction (120) and augmented TGF responses (206, 310, 317). The connecting tubule tubuloglomerular feedback (CTGF) might modulate RBF autoregulation. The CTGF is activated by Na+ reabsorption in the connecting tubule (CNT) and leads to vasodilation the afferent arteriole (297). Thus, the CTGF counteracts the vasoconstrictive effects of macula densa TGF by decreasing the afferent arteriole resistance in responses to increased NaCl delivery (296). NaCl reabsorption and consequently activation of the CTGF are mediated by the thiazide-sensitive Na+/Cl− cotransporter and by the amiloride/benzamil-sensitive epithelial Na+ channel (ENaC) in the CNT (297). The role of the CTGF has not been investigated in hypertensive animals. However, Ren et al. reported that the intratubular perfusion of ANG II enhanced the CTGF through activation of AT1R, increased NaCl reabsorption mediated by the ENaC (235), which led to dilation of afferent arterioles mediated by protein kinase C (PKC)-dependent NOX2-derived O2−• production. Coperfusion of ANG II with tempol, apocynin, or a NOX2 inhibitor blocked the effect of intratubular ANG II (237). Thus, activation of the CTGF by NOX2-dependent generation of tubular O2−• could limit ANG II-induced vasoconstriction of afferent arterioles and preserve the GFR by activation of NADPH oxidase. This would constitute an unusual effect of renal ROS to limit renal vasoconstriction, autoregulation, and the development of hypertension.
Overall, these data imply that increased renal ROS in hypertensive animals enhance myogenic and macula densa TGF responses and the contraction of afferent arterioles to ANG II, ET-1, and TP activation. Oxidative stress leads to the elaboration of an EDCF that activates TP on VSMC whose activity also is enhanced by ROS. This is accompanied by blunted EDRF/NO (28, 79, 151, 293) and augmented TGF responses (207, 310, 312), all of which contribute to a renal vascular prohypertensive pathway activated during oxidative stress.
Intrarenal distribution of blood flow
Renal medullary ROS generation contributes to the development of hypertension in several models by direct effects of H2O2 and by limiting NO bioavailability. Medullary ROS restrict the medullary perfusion and Na+ excretion, and increase BP (3, 36, 52, 175, 176, 197, 209, 217, 222, 267, 284). Vascular H2O2 enhances eNOS expression and enhances the activity of NOS and NO generation by cultured ECs (23, 56). NO generation was markedly increased in the medulla of rats infused with ANG II at a subpressor rate (195, 351). However, this response was blunted in the medulla of DS rats, which have an impaired NOS expression and activity (275). The unchanged levels of O2−• found in the renal medulla of rats during ANG II infusion were ascribed to the reaction of O2−• with the high level of NO to form ONOO− (52, 222, 348). Hypertension in DS rats is accompanied by a reduced medullary expression of SOD-1 and SOD-2 that could contribute to enhance O2−• (187). ANG II infusion increases O2−•generation in the mTAL, which interacts with NO in the vasa recta to mediate a tubulovascular crosstalk that culminates in a reduced MBF and reduced UNaV (52).
Further studies implicate medullary H2O2 in salt-sensitive hypertension. Thus, the intramedullary infusion of SOD inhibitors, such as diethyl thiocarbamate, reduces the MBF and raises the BP (175), yet the intramedullary infusion of tempol did not reduce the BP unless coinfused with catalase to reduce H2O2. (36, 268). Although catalase expression is increased in the kidneys of SHR (265, 346), its activity is decreased (346). Catalase overexpression reduced the pressor response to ANG II in mice (331), and intramedullary catalase infusion prevented the hypertension accompanying an intramedullary infusion of H2O2 (175).
Sousa et al. (267) reported a selective increase in AT1R and NOX4 expression and in medullary H2O2 in rats infused with ANG II at slow-pressure rates. Intraperitoneal administration of PEG-CAT caused only a transient fall in BP that was attributed to a normalization of renal angiotensinogen (AGT) levels, which are rate limiting for ANG II generation in the kidney (141). Apparently, elevated levels of H2O2 generated during prolonged ANG II stimulate the intrarenal RAAS, but inhibit the systemic RAAS (175, 268, 331). Mice overexpressing AGT in the PT are hypertensive independent of the plasma renin concentration, reinforcing the concept that a selective activation of the renal RAAS causes hypertension probably by enhancing ROS, renal vasoconstriction, and Na+ retention (65).
An elevation of renal perfusion pressure during hypertension may directly increase ROS production in the renal medulla, MBF and NaCl delivery to, and reabsorption by, the TAL (125). Jin et al. (125) reported that a short-term increase of renal perfusion pressure enhanced the production of both NO and H2O2 in the outer medulla of rats. The increased NO was attributed to the pressure-induced increase in MBF that enhanced vascular shear stress and to the increased H2O2 with higher flow rates through the mTAL. These studies suggest that increased renal perfusion pressure creates oxidative stress within the outer medulla of the kidney that leads to generation of the medullary H2O2 that contributes to salt-sensitive hypertension, while increased levels of medullary NO counteract these effects and restrain the salt sensitivity and hypertension (125). Thus, H2O2 in the renal medulla rather than O2−• has been identified as the major ROS mediating hypertension.
ROS in the Glomerulus
The direct effects of ANG II on kidney cells have been studied extensively (16, 168, 329). Both podocytes and mesangial cells (MC) generate ANG II in response to overproduction of ROS, whereas ROS are produced by ANG II. Indeed, ANG II is implicated in glomerular hypertension, disruption of the glomerular filtration barrier (GFB), and proteinuria in several hypertensive models (73, 239, 246, 274, 330). This provides a potential for an ANG II-initiated, feed-forward production of glomerular ROS (249, 329). On the other hand, podocytes express angiotensin-converting enzyme 2 (ACE-2), which can metabolize ANG II to angiotensin-(1–7) (102). Thus, ACE-2 may provide a brake on this feed-forward process.
Podocyte injury or dysfunction has been implicated as an initial event in glomerulosclerosis and proteinuria (313). Although ANG II-induced oxidative stress contributes to podocyte injury, the mechanisms have not been elucidated completely. ANG II enhances ROS generation in podocytes, promotes podocyte autophagy through mitochondrial generation of ROS, decreases nephrin expression, and induces apoptosis (55, 124, 239). Prolonged infusion of ANG II into rats at a slow-pressor rate induced A1AR-dependent hypertension, proteinuria, and podocyte injury and decreased nephrin expression and caveolin-1 phosphorylation (239). Thus, ANG II-induced ROS generation could interrupt the crosstalk between caveolin-1 and nephrin that is required to maintain the integrity of podocytes and the GFB.
Aldosterone also has been implicated in ROS-dependent podocyte injury and MC proliferation (20) and also may be involved in a feed-forward signaling between ROS and the RAAS (20, 139, 202, 212). Tempol preserved podocyte function and attenuated glomerulosclerosis in rats with aldosterone plus salt-induced hypertension (202). Mice lacking the guanylyl cyclase-A (GC-A) receptor for natriuretic peptides and given aldosterone and a high-salt diet increased oxidative stress, podocyte injury, and albuminuria, which were ameliorated by tempol or an ARB (212). The authors attributed the renoprotective properties of the GC-A system to a local inhibition of the RAAS and oxidative stress in podocytes, independent of BP.
Although the major site of action of aldosterone is the CD, it also activates mineralocorticoid receptors (MR) on podocytes. Shibata et al. (259) reported proteinuria and decreased nephrin and podocin expression in uniphrectomized rats infused with aldosterone and fed a high-salt diet. The BP, renal ROS production, NOX2, p47phox, p67phox, Rac-1 and Sgk1 (an effector kinase of MR) all were elevated by aldosterone. This was related to ROS, since all these effects were normalized by tempol but not by nonspecific correction of hypertension.
ROS mediate the MC proliferation, migration and production of extracellular matrix (ECM) induced by ANG II or aldosterone (73, 90, 168, 189). ANG II increases NADPH oxidase-dependent O2−• generation in MC and increases production of ECM proteins such as fibronectin, which are implicated in the development of glomerulosclerosis (115). MR activation by aldosterone induces NADPH-dependent ROS generation in MC that leads to translocation of p47phox and p67phox. MR antagonists ameliorate glomerular injury and decrease proteinuria in several animal models of hypertension, independent of changes in BP (189, 202, 241). Thus, intrarenal ANG II and aldosterone signaling via the MR both elicit renal oxidative stress that is widely implicated in the pathogenesis of hypertension and associated renal damage in animal models.
ROS and Renal Tubular Transport
Proximal tubule
NADPH oxidase-derived ROS can reduce renal tubular Na+ transport in the PT either directly or by reducing NO activity. Although there is enhanced Na+ and fluid uptake in PT of young SHR (4, 280), Panico et al. (224) reported reduced reabsorption in the PT of adult SHR and relate this to increased intratubular NADPH oxidase-derived O2−•. An intratubular perfusion of tempol, apocynin or systemic silencing of p22phox with a siRNA restored PT reabsorption in adult SHR nephrons, thereby implicating NOX-derived O2−• production in the reduced PT fluid reabsorption (224). This was ascribed to increased expression of the Na+/H+ exchanger regulatory factor (NHERF2), which inhibited the Na+/H+ exchanger (NHE3) activity (Fig. 5). The authors suggest that downstream sites of Na+ reabsorption, increased by O2−•, would compensate for the dysfunction in the PT and thereby account for the similar Na+ and water excretion in both WKY and SHR. While NOX4 is the primary source of O2−• in the PT (87, 88), the increase in PT reabsorption in the SHR by luminal apocynin implicates other NOX isoforms that required p47phox, such as NOX1 or NOX2 (224). Inhibition of PT fluid reabsorption by O2−• may be secondary to limitation of NO, since fluid reabsorption is enhanced by NO and reduced by the intratubular perfusion of a nonspecific or by an nNOS- or an iNOS-specific inhibitor (10, 199). Moreover, iNOS (−/−) and nNOS (−/−) mice but not eNOS (−/−) mice had reduced PT reabsorption (298, 299).
FIG. 5.
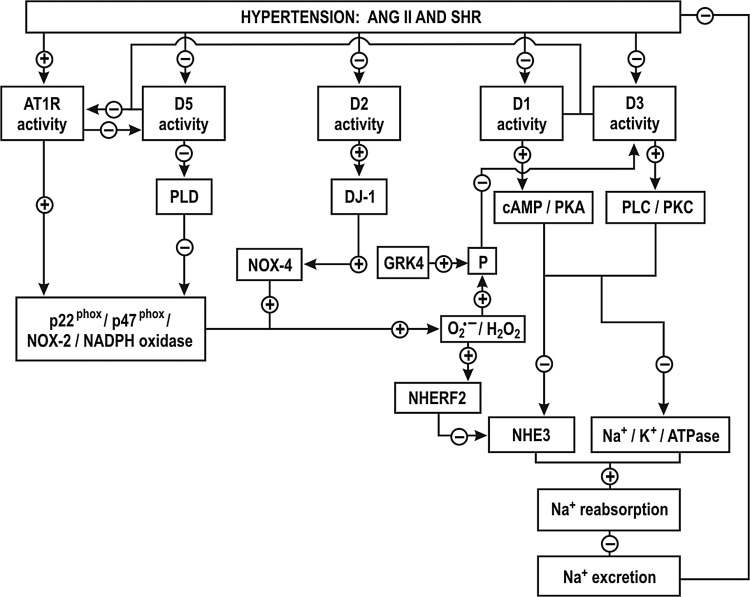
Hypothesis for proximal tubule signaling via reactive oxygen species after ANG II type-1 receptor or dopamine receptor activation or inhibition in hypertension. Activation of AT1R stimulates the NADPH-oxidase dependent O2−·production, which increases the expression of the NHE3 inhibitor factor (NHERF2) and decreases Na+ reabsorption in the proximal tubule by inhibition of NHE3. Superoxide (O2−·)- and G-coupled receptor kinase type 4 (GRK4)-dependent phosphorylation (P)/uncoupling of D1 and D3 leads to an impairment of these receptors, while AT1R impairs the D5 receptor (for explanation, see text). Drawn after Refs. (127, 224, 343–345). AT1R, angiotensin II receptor type-1; PLD, phospholipase D; GRK4, G-protein-coupled receptor kinase type 4; cAMP, 3′-5′-cyclic adenosine monophosphate; P, phosphate; PKA, phosphokinase A; PLC, phospholipase C; PKC, protein kinase C; NHE3, Na+/H+ exchanger; NHERF, Na+/H+ exchanger regulatory factor; NADPH, nicotinamide dinucleotide phosphate.
There are normal distributions of NHE3 and sodium-phosphate-cotransporter (NaPi2) proteins in the PT of young SHR, while in adult SHR, and in other some hypertensive rat models, the transporters are relocated from the luminal brush-border into the cytoplasm. This may contribute to decreased PT Na+ and fluid reabsorption (174, 185, 334, 338).
It is not yet clear whether O2−• and/or NO regulate luminal expression of sodium transporters in the PT. While hypertension causes a pressure-natriuresis, the infusion of ANG II causes an anti-natriuresis (157, 158, 185, 349). The infusion of a nonpressor concentration of ANG II enhanced renal ROS production (29) and translocated NHE3 and NaPi2 to the apical microvilli in the PT, which should increase Na+ and water reabsorption (157, 158). On the other hand, the infusion of pressor concentrations of ANG II had the opposite effect (185). A nonpressor infusion of ANG II was confirmed to simulate NHE3 activity in the PT brush border and in TAL cells (149, 328).
Blockade of A1ARs reduces Na+ transport in the PT and leads to natriuresis. Thus, the increased expression and function of A1ARs in the PT of rats during salt restriction may contribute to enhance PT reabsorption. (146, 323). Activation of A1AR augmented Na+ transport in human immortalized PT cells (HK-2) (276). H2O2 increased A1AR expression in smooth muscle cells of hamsters by activating NFκ-B (200). It will be important to determine the precise role of ROS and other related systems such as adenosine on tubular Na+ reabsorption.
A defect in the dopaminergic system of the PT has been implicated in the development of hypertension. Dopamine acting on D1-like and D2-like receptors decreases Na+ transport in volume-expanded states. Nevertheless, D2-like receptor activation can increase Na+ transport in volume-depleted states. The exact mechanisms whereby D2-like receptors regulate ion transport have not been established. There is reduced renal expression and/or impairment of dopamine receptors in several models of hypertension, such as the DS rats and the SHR (12, 270, 339, 343). D-1-like receptor function was impaired in the PT of volume expanded SHR and was implicated in a decreased natriuresis and the development of salt-sensitive hypertension (270, 343). This impairment was attributed to oxidative stress since it was corrected by tempol (12, 127). Indeed, D1, D3 and D5 were all impaired in the PT of SHR (Fig. 5) (127, 343–345). Increased renal O2−• and G protein-coupled receptor kinase type 4 (GRK4) in SHR induced the phosphorylation of D1 and D3 receptors in the PT, uncoupling them from their G protein effector protein and thereby preventing their activation (Fig. 5) (343). The activation of AT1R by ANG II impaired the D5 receptor (345). ANG II via AT1R stimulated the NADPH oxidase-dependent O2−• production in the PT of SHR and increased the expression of the NHE3 inhibitor factor (NHERF2), which decreased Na+ reabsorption in the PTs of SHR by inhibition of NHE3 (Fig. 5) (224). Since the D1, D3 and D5 receptors negatively regulate AT1R and oxidative stress (Fig. 5), their impairment in the in the PT of SHR would result in enhanced effects of ANG II that would account for the decreased Na+ reabsorption.
The interaction of D1-like with D2-like receptors regulates Na+ reabsorption in the PT and Na+ excretion (60, 335). The impairment of D1 receptor function in the PT and in the TAL of SHR (117, 201, 343) leads to increased luminal NHE3 and NaPi2, and basolateral Na/HCO3− co-transporters and Na+-K+-ATPase that together account for enhanced PT reabsorption (117, 127, 201, 343).
D2 (−/−) mice have hypertension that is in part dependent on oxidative stress. There is increased renal expression of NOX1, NOX2 and NOX4 and NADPH oxidase activity in D2 (−/−) mice (8). DJ-1, a multifunctional protein with antioxidant properties is required for the D2 receptor signaling (Fig. 5). Renal silencing of DJ-1 gene in mice increases NOX4 expression and NADPH oxidase activity and increases the BP (45). D5 (−/−) mice have hypertension that is dependent on oxidative stress (335). There is increased renal expression of NOX2, NOX4, p47phox and enhanced NADPH oxidase activity (335). D5 (−/−) mice also are salt sensitive and have higher expression of AT1R in the kidney (345). D3(−/−) mice also have increased renal AT1R expression but only a mild hypertension (344). This is attributed to a renal upregulation of D5 receptors, which prevents oxidative stress in D3 (−/−) mice (300). Thus, stimulation of D1-like and D2-like receptors activates antioxidant pathways while their impairment during hypertension exacerbates the oxidative stress induced by ANG II and its effects on Na+ reabsorption.
Loop of Henle
The apical Na+/K+/2Cl− cotransporter type-2 (NKCC2) in the TAL accounts for 70–80% of transcellular Na+ reabsorption whereas the NHE3 accounts for the remainder. The energy is provided by the basolateral Na+-K+-ATPase that maintains a low intracellular Na+ concentration. O2−• activates a signaling pathway in the mTAL that includes PKC-alpha and NKCC2 to enhance Na+ transport (217, 264). Although NADPH oxidase is the main source of O2−• in TAL, xanthine oxidase (XO), COX and CYP 450 enzymes also are expressed in this segment and might contribute to O2−• production (86). NOX2 and p47phox were identified as the main sources of O2−• generation in the TAL in response to ANG II or increased luminal flow (114, 254, 264). Feng et al. (62) reported that disruption of the gene for p67phox decreased medullary NADPH oxidase-dependent O2−• production and attenuated salt-sensitive hypertension in DS rats. However, Hong and Garvin (113) reported that flow-induced production of O2−• in the TAL was dependent on NOX4 but not on NOX2.
NO is a major regulator of NaCl transport in the TAL. Studies in isolated, perfused TAL demonstrated that NOS-3 derived NO inhibits NKCC2 and NHE3 at this site (85, 218, 219). The NKCC2 inhibition was mediated by a phophodiesterase-mediated degradation of 3′-5′-cyclic adenosine monophosphate (cAMP) whereas the inhibition of NHE3 was attributed to direct effect of NO (85). A high salt diet decreases NaCl reabsorption in the TAL. This is attributed both to an increased medullary osmolality and to release of ET-1, which through ETB receptors increased NOS-3 expression and NO-dependent inhibition of NKCC2 (108). Impairment of NO has been shown to increase NaCl reabsorption at this nephron segment in models of salt sensitive hypertension, which may thereby contribute to salt-dependent increases in BP (82).
T1DM is often associated with hypertension, salt sensitivity and oxidative stress (45, 171, 271). Studies report increased Na+ transport and oxygen consumption in diabetic rats (11, 231). Yang et al. (333) reported increased Na+ transport and O2 consumption in the mTAL of diabetic rats. This was ascribed to a NADPH oxidase- and PKC-dependent increase in O2−•, which entails NKCC2 and Na+ -K+-ATPase.
Renal production of 20-HETE is elevated in SHR, DOCA-salt or ANG II-infused rats and contributes to hypertension (111, 123, 196, 324). Roman and colleagues (196) reported that reduced 20-HETE production in the TAL of DS rats contributed to elevated Na+ reabsorption. The reduction of 20-HETE is dependent on ROS (111). Induction of 20-HETE synthesis improved pressure-natriuresis and attenuated the development of hypertension in DS rats (324). Thus, ROS generally enhance Na+ reabsorption in the TAL, in part via reduction in 20-HETE production and contributes to hypertension.
DT and CD
The ENaC in the distal nephron and cortical collecting duct (CCD) is responsible for much of the Na+ reabsorption in this segment. It provides the final renal tubular adjustments of Na+ reabsorption that are important for Na+ balance and thereby play a critical role in the regulation of BP (273). ANG II activation of AT1Rs in the CCD of rats increased ENaC activity by NOX-dependent ROS generation (272). Aldosterone increased ENaC activity in A6 distal nephron cells by O2−• generation, which reduced the inhibitory effects of NO on the ENaC (340). H2O2 generated in the CCD during a high NaCl intake stimulated the ENaC by diminishing its inhibition by arachidonic acid (272). These limited data imply that ROS-induced stimulation of the ENaC might be the result of diminishing the inhibitory factors acting on this Na+ channel. ET-1 inhibits Na+ transport in the CD via activation of ETB receptors and reduces ENaC expression and activity and Na+-K+-ATPase and thereby Na+ reabsorption (84). This entails the generation of NOS-1 dependent NO and accumulation of prostaglandin E2 (22, 143). ET-1 production in the CD was increased by elevated Na+ intake and associated with natriuresis (173). Paradoxically, medullary levels of ET-1 and ETB receptor expression were lower in DS rats than in DR rats on a high salt diet (269). However, renal expression of the ENaC was increased in DS rats (7) and this could represent a mechanism for the development of salt-sensitive hypertension.
Pressure-natriuresis
The kidney maintains the extracellular volume and BP by modulation of the pressure-natriuresis mechanism. An increase in pressure-natriuresis is accomplished by a vascular component, which has been related to an increased MBF leading to a decreased medullary solute gradient and an increased renal interstitial hydrostatic pressure both of which inhibit Na+ reabsorption and a primary tubular component, which has been with associated decreased Na+ reabsorption in the PT and TAL (83, 91, 244)
A shift of the pressure-natriuresis relationship to higher pressures occurs in many models of hypertension. The pressure-natriuresis was the outcome of opposing effects of ROS and NO, particularly in the renal medulla (41, 209). Inhibition of oxidative stress with tempol increases pressure-natriuresis and diminishes salt-sensitivity (94, 186, 312), whereas prolonged inhibition of NOS with L-NAME inhibits pressure-natriuresis and causes salt-sensitive hypertension (341). Thus, the balance between O2−• and NO determines the effectiveness of pressure-natriuresis and thereby the set point of BP.
ROS, tubular transport, and hypertension
Collectively, the studies linking ROS to tubular transport and hypertension suggest that O2−• decreases Na+ and fluid reabsorption in the PT largely by decreasing NO availability, while O2−• increases Na+ reabsorption in TAL and distal segments. NADPH oxidase was identified as the main source of O2−• in the tubules. However, increased Na+ delivery or ANG II both enhanced H2O2 production in medullary segments (213) and increased Na+ reabsorption in the mTAL (213) and CDs (272). NOX2 and NOX4 generate O2−• in the kidney, but their specific roles and sites of action are not yet well defined.
ROS, Renal Oxygenation, and Hypertension
PO2 and TNa/QO2 in the renal cortex and medulla
A reduction in renal tissue PO2 in the models of hypertension has been attributed to increase O2 usage for Na+ transport (TNa/QO2) (150, 303, 305, 312, 318). Welch et al. (301) reported a lower PO2 in the kidney cortex and the outer medulla of SHR that was dependent on renal AT1R and O2−•, since it was normalized by an ARB or tempol, whereas nonspecific correction of hypertension was less effective (57, 301, 302). The PO2 also was reduced in rats infused with ANG II at a slow-pressor rate (303) and in the clipped kidney of the 2K,1C rat (305). Tempol restored the PO2 levels and TNa/QO2 in both these models, whereas an ARB or an angiotensin-converting enzyme inhibitor (ACEi) had no effects in the clipped kidney of 2K,1C rats (223). Interestingly, baseline cortical PO2 was reduced in the clipped kidney in the early phase of 2K,1C, but it was restored in the chronic model, perhaps because a high BP and lack of autoregulation maintained the blood supply to the clipped kidney and the fall in the GFR limited Na+ filtration and reabsorption, thereby reducing renal work and O2 demands (223). Mice with 5/6 nephrectomy and a prolonged RRM also had reduced PO2 levels and TNa/QO2, especially in the renal medulla, which were corrected by tempol (150). The authors propose that tempol enhanced NO bioavailability in the renal medulla and thereby improved the mitochondrial efficiency and MBF (150). Prolonged inhibition of ANG II by an ACEi or an ARB in rats reduced age-related renal mitochondrial O2−• production and improved O2 usage (47). These studies suggest that an enhanced mitochondrial O2−• in several models of hypertension may have reduced NO sufficiently to disinhibit and uncouple mitochondrial respiration, increase QO2, but limit ATP production, and consequently to decrease TNa/QO2. Indeed, inactivation of NO signaling by O2−• is reported in the kidney of the SHR (310) in the clipped kidney of the 2K,1C rat (9, 19, 262, 263, 317) and the kidney of the ANG II-infused rat (9, 19, 50, 262, 263, 317). Since physiological levels of PO2 limit NADPH oxidase-dependent O2−• generation in the renal medulla (34, 301), the relative hypoxia may put a brake on severe medullary oxidative stress. Renal hypoxia also may contribute to the development of hypertension (184). Renal oxidative stress associated with upregulated Poldip2 and SOD accounted for the renal medullary hypoxia in a mouse model of RRM (150) and has been postulated to contribute to hypertension and progressive loss of function in patients with CKD (64).
Renal Nerves
Efferent and central effects
Activation of the sympathetic nervous system (SNS) raises the BP, enhances renal Na+ reabsorption, reduces RBF, and increases renin secretion (92). ROS increase SNS activity by decreasing NO bioavailability within the central nervous system (26, 31).
The efferent sympathetic renal nerves are distributed throughout the renal vasculature and tubular segments in the cortex and outer medulla, with high innervation along the afferent and efferent arterioles (145). β1-adrenergic receptors (β1AR) predominate on VSMCs of renal microvessels and MD epithelium, where they inhibit ROS generation and lead to vasodilation (18). Activation of β1AR by norepinephrine (NE) protected renal afferent arterioles of ANG II-infused rabbits from the enhanced contraction induced by increased ROS generation that was mediated by α-1 adrenergic receptors (295). Thus, α- and β-adrenergic receptor signaling can have opposite effects on renal microvascular ROS.
Afferent and renorenal reflex
The renal afferent nerves are mainly located in the renal pelvis, where they are activated by chemical stimuli, for example, high NaCl concentrations and physical stimuli, for example, increased renal interstitial pressure (145). The signals from the renal afferent nerves converge to brain centers involved in the control of arterial pressure (145). Unilateral renal denervation leads to ipsilateral natriuresis and contralateral increased renal sympathetic nerve activity (RSNA) and antinatriuresis. This has been termed the renorenal reflex.
Hypertension and renal nerves
Renal denervation prevents or attenuates hypertension in many models, including SHR, SHRSP, ANG II-induced, DOCA–salt, and 2K,1C hypertension (51). Renal denervation of rats early after renal injury prevented the enhanced NE secretion from the posterior hypothalamic nuclei (PH), the increased RSNA, and hypertension, thereby implicating the renal afferent nerves in centrally mediated neurogenic hypertension (31, 336). The central sympathetic activation after renal afferent nerve activation is dependent on ROS, since it is prevented by intracerebroventricular (ICV) infusion of tempol (31). A convenient model is produced by an intrarenal injection of phenol in rats. This enhances NE secretion from the PH and RSNA, and leads to hypertension that is prevented by denervation of the kidneys or by ICV injection of tempol or PEG-SOD (337). Several brain nuclei in this model have increased expression of NOX2, NOX4, p22phox, and p47phox, whereas interleukin-1β and nNOS are decreased (31, 42, 104, 225, 232, 337). A vitamin E-enriched diet attenuated activation of the SNS and hypertension initiated by intrarenal injection of phenol (25).
O2−• is elevated in the sympathetic ganglia of DOCA–salt-hypertensive rats, and this is attributed to ET-1 stimulation of the ETB receptors (46). The authors propose that O2−• could inactivate NO in the SNS and thereby increase the activity of sympathetic neurons and the release of vasoactive substances, resulting in vasoconstriction and hypertension. In other studies, an acute intravenous infusion of tempol caused a transient decrease in the BP, heart rate (HR), and RSNA in both DOCA–salt-hypertensive rats and SHR (26, 325–327). In DOCA–salt rats, the effect of tempol on BP, but not on HR and RSNA, was attenuated after NOS inhibition with NG-nitro-l-arginine (L-NNA). These studies suggest that the inactivation of NO by O2−• in peripheral sympathetic nerves may contribute directly to the increase in BP in hypertensive animals.
In contrast, ICV infusions of tempol do not decrease BP in WKY rats or SHR (261). These studies suggest that the ICV action of tempol on the SNS could involve NO-dependent and NO-independent pathways, while the systemic effects of tempol involved peripheral vasodilation and reflex activation of the SNS. Thus, ROS can activate the SNS both by increasing the central sympathetic drive and by facilitating peripheral SNS signaling. Microinjection of an AT1R antagonist into the rostral ventrolateral medulla decreased BP and peripheral sympathetic nerve activity in SHR, but not in WKY rats (6). These studies support the hypothesis that ROS may activate the SNS at the levels of renal afferents, central nuclei, and peripheral sympathetic nerves and thereby contribute to hypertension.
The Renin-Angiotensin-Aldosterone System
Renin and aldosterone release
Renin release is regulated by systemic and intrarenal signals. There is growing evidence that oxidative stress regulates renin expression and release (48, 77, 78, 119, 306). ANG II stimulates the production of cytokines, which are strong inhibitors of renin release (112). TNF-α inhibits renin expression and release through oxidative stress (48, 119).
The juxtaglomerular (JG) cells of the renal afferent arteriole are the primary site of storage for prorenin, activation of renin, and renin release. Renin release is stimulated by sympathetic activation of β1-adrenergic receptors on JG cells, decreased renal afferent arteriole stretch, and decreased NaCl delivery to the MD or by specific hormones and peptides (148, 229). The short-loop feedback inhibition of renin secretion by ANG II involves oxidative stress. Galle et al. (77) reported that JG cells isolated from mice had enhanced renin release when the tissue O2−• was increased. (77, 78, 119). Itani et al. (119) demonstrated that renin-expressing JG cells exposed to TNF-α and H2O2 had a time- and dose-dependent reduction in renin mRNA and renin promoter activity that was corrected by an antioxidant N-acetylcysteine (NAC). Interestingly, an effective antihypertensive dose of tempol increased the plasma renin activity in SHR, implying that inhibition of renin release did not contribute to the fall in MAP. Overall, these studies suggest that O2−• enhances, whereas H2O2 inhibits, renin expression and release, but further studies are needed due to the complexity of the factors involved in the JG cell function.
Aldosterone participates in the control of BP by central actions that lead to activation of the SNS, vascular actions that led to vasoconstriction, and renal actions that lead to enhanced Na+ reabsorption. Excessive aldosterone synthesis or release increases Na+ reabsorption by renal DTs and raises the BP (285). Aldosterone promoted renal and cardiovascular diseases associated with hypertension (20, 285). Aldosterone release from the zona glomerulosa of the adrenal cortex is driven by ANG II and [K+ ]. The pressor responses induced by prolonged aldosterone infusion and salt supplementation in rats were mediated by central AT1R and ROS, since they were inhibited by ICV administration of an ARB, apocynin, or tempol (285). Moreover, the pressor effects of a systemic infusion of low dose of ANG II also were dependent on central MR, since they were abolished by the ICV infusion of an MR blocker or an aldosterone synthase inhibitor (116). These studies disclose a crosstalk between ANG II and aldosterone in the brain to sustain hypertension that involved a central production of ROS. Fujita and Nagase (258) demonstrated that salt-dependent augmentation of ANG II effects in the kidney depended on renal MR/Rac1 activation. They proposed that a crosstalk between ANG II and aldosterone contributes to hypertensive kidney injury (135, 260). ANG II induced the synthesis and release of aldosterone in the adrenal cortex cells by activation of AT1Rs and increased CYP11B2 expression and activity (13, 205, 234) that were mediated by H2O2 derived from NOXs and mitochondria (234). Pretreatment with NAC, PEG-CAT, a NOX inhibitor, or an ARB prevented the ANG II stimulation of aldosterone release. These studies demonstrate that oxidative stress is an important regulator of the RAAS in the kidney, brain, and adrenal glomerulosa cells.
MD versus afferent arteriole mechanisms
The afferent arteriole is the effector site of TGF and renin secretion. ANG II enhances the afferent arteriolar constriction with TGF activation (188) and enhances the activity of the luminal NKCC2 in the MD (147), which generates the signal for TGF. Adenosine activity on AT1Rs enhances the afferent arteriolar response to ANG II (152). A1AR inhibition reverses the enhanced renal vasoconstriction in ANG II-infused rats (68). AT1AR (−/−) mice have an absent TGF response and attenuated effects of ANG II in reducing renal function and increasing RVR (79, 100, 253). Gao et al. (79) demonstrated that A1ARs increased afferent arteriolar contractility by increasing ROS generation. Therefore, an interaction between adenosine acting on A1AR and ANG II acting on AT1R modifies the degree of vasoconstriction induced by these agonists through increased arteriolar ROS. This would contribute to the development of hypertension by sensitizing TGF and the reactivity of preglomerular vessels.
Angiotensin type 1 and 2 receptor expression
Angiotensin II type 1 and 2 receptors (AT1Rs and AT2Rs) are demonstrated throughout the rat kidney (190, 221). AT1R protein is expressed on renal blood vessels, glomeruli, PTs, and DTs, especially in the outer medulla (106, 183). The highest expression of AT1R is found in the renal cortical vasculature and in the S3 segment of PT (190). Two AT1R subtypes are identified in rodents (122, 190, 248), AT1AR and AT1BR, but due to the high homology, their precise function in the kidney has not yet been fully defined. The mRNA for AT1AR and AT1BR is demonstrated in the glomeruli, tubules, and vessels from the renal medulla of rats (190). The AT1AR receptor has the highest homology with the human AT1R (42).
AT1AR (−/−) mice have a similar basal afferent arteriolar diameter to wild-type mice, but reduced constrictive responses to ANG II, whereas the diameter of efferent arterioles is higher in the AT1AR (−/−) mice, and they do not respond to Ang II (105). Kidney-specific AT1AR (−/−) mice infused with ANG II at a slow-pressor rate had no increases in BP. However, transplantation of a wild-type mouse kidney into systemic AT1AR (−/−) mice restored the responses to ANG II (42). AT1AR/AT1BR (−/−) mice have renal microvascular dysfunction, tubular injury, and interstitial inflammation despite a lower BP (220). These mice have enhanced renal expression of renin, AGT, NOX2, p40phox, p67phox, and XO, but decreased expression of NOX4. The authors attributed the increased NADPH oxidase components in the kidney to the infiltration of inflammatory cells. The cause for the paradoxical renal inflammation and dysfunction in mice lacking AT1Rs has not been determined.
AT2Rs are expressed on the renal vasculature, PTs, DTs, and CDs of rats, whereas glomeruli and mTAL are negative (190). AT2R regulates renal AT1R expression and function via the NO/cyclic guanine monophosphate (cGMP) pathway (332). Stimulation of AT2R reduced inflammation and increased renal production of NO and cGMP in the clipped kidney of 2K,1C hypertensive rats (182). Overexpression of AT2R in VSMC (126) or stimulation of AT2R in PT cells from rats (332) decreased AT1R expression. On the other hand, PT cells from AT2R (−/−) mice had increased AT1R expression. Inhibition of AT2R enhanced the upregulation of renal NADPH oxidase subunits by ANG II (29). Thus, there is extensive reciprocal regulation of AT1R and AT2R in the kidney that determines the expression of the NADPH oxidase isoforms.
AT2R (−/−) mice have high BP, decreased renal function, impaired water and Na+ handling, augmented vasopressor response to ANG II, and increased expression of AT1R in the kidney. AT2R activation in the TAL decreased Na+ reabsorption by an NO-mediated reduction of the NKCC2 cotransporter activity (107). NKCC2-dependent transport is impaired in the TAL of DS rats with oxidative stress, which may contribute to Na+ retention and salt-sensitive hypertension (113).
Recent studies have emphasized the importance of ANG II receptors in the nuclei and mitochondria of renal cells in modulating intracellular oxidative stress (1, 96, 162, 164, 227). Activation of AT1R in the isolated nuclei from the rat renal cortex was linked to increased ROS generation (228), whereas the activation of the AT2R and ANG II (1–7) receptors increased NO generation (97). Studies employing immunoelectron microscopy demonstrated AT2R bound to ANG II in the mitochondria of mouse kidney cells (1). An AT2R agonist stimulated the production of NO in the renal mitochondria and inhibited respiration in the heart mitochondria (1). These findings reinforce the role of AT2R in buffering the intracellular actions of AT1R on ROS production and extended this to the mitochondria and nuclei. These recent studies identify an intracellular RAAS system in the kidney that modulates nuclear and mitochondrial oxidative stress in hypertension.
ROS, hypertension, and the RAAS
Activation of the RAAS causes a widespread increase in ROS in the kidney via NADPH oxidase, mitochondrial dysfunction, decreased NO availability, and decreased antioxidant enzymes. This is implicated in Na+ retention, vasoconstriction, and hypertension. Moreover, ACE inhibitors, AT1R blockers, and MR antagonists decreased oxidative stress and ameliorated hypertension and protected the kidney in several hypertensive models (14, 15, 29, 57, 73, 157, 223, 302, 305, 308, 312, 334).
Increased ANG II generation of ROS is implicated in the development of hypertension in several mouse or rat models, including ANG II infusion at a slow-pressor rate (134, 216), prolonged NOS inhibition (214), salt sensitivity (101, 187), SHR (301, 306), 2K,1C rats (305), and salt-dependent hypertension in DOCA-salt rats (328).
The renal AT1R is a key element for the development of hypertension. (177, 224, 294, 305). Activation of this receptor engages renal afferent and efferent arteriolar vasoconstriction and decreased relaxation, limits the GFR and RBF, increases Na+ and fluid reabsorption, decreases pressure natriuresis, and augments TGF (177, 224, 294, 305). Many of the AT1R signaling pathways are antagonized by AT2R. AT1R activation in the kidney enhances the expression and stimulated the assembly of NADPH oxidase subunits and decreases antioxidant enzymes, resulting in increased ROS (29, 191, 304), whereas AT2R activation stimulates NO production and counteracts the effect of AT1R activation (29, 274). Aldosterone causes glomerulosclerosis and proteinuria by activation of the MR linked to ROS. Salt intake potentiates the deleterious effects of ANG II and MR activation via increasing ROS in the kidney, even with a suppressed systemic RAAS (135). Thus, a crosstalk between ANG II and MR in the kidney that both engage ROS could contribute to the renal hemodynamic alterations and injury involved in hypertension.
Innovation
This Forum reviews the most recent publications in basic research focusing on the contribution of renal oxidative stress to hypertension. Studies have shown that, on one hand, NADPH oxidase/O2−• is an essential requirement for hypertension during slow-pressor infusion of ANG II. On the other hand, prolonged O2−• tissue excess from knockout of SOD-1 or SOD-3 either does not change BP or increase it modestly, but does enhance BP with ANG II infusion. These and other studies suggest a critical modulating, but not necessarily mediating, role for ROS in hypertension development.
Conclusions
The kidney plays a crucial role in the development of hypertension. Current treatments utilizing ACE inhibitors, ARBs, and renin inhibitors have been effective in reducing BP, but do not fully prevent the progressive loss of kidney function in CKD. The therapeutic role of effective antioxidant strategies in human hypertension and CKD remains to be explored.
Studies with isolated preglomerular microvessels or renal cells have distinguished between the direct vascular actions of ANG II and those exerted by the high BP. Studies of microperfusion of vascular and tubular segments in vitro or in vivo have defined a clear role for ROS in mediating many of the effects of the renal RAAS on renal Na+ transport, TGF, RBF, and GFR. The development of knockout mice with global or site-specific deletion of a target gene has demonstrated that a primary increase in ROS, independent of ANG II, can be a provocative stimulus to increase BP. Finally, studies of the renal signaling pathways and transcription factors mediated by ROS have expanded the knowledge on the role of oxidative stress in the kidney and hypertension.
Abbreviations Used
- 7-NI
7-nitroindazole
- 8-iso
8-isoprostane F2-alpha
- A1AR
adenosine receptors type-1
- AA
arachidonate
- ACE
angiotensin-converting enzyme
- AGT
angiotensinogen
- ANG II
angiotensin II
- ARB
angiotensin II type-1 receptor blocker
- AT1R
angiotensin II receptors type-1
- AT2R
angiotensin II receptor type-2
- ATP
adenosine triphosphate
- BH4
tetrahydrobiopterin
- BP
blood pressure
- cAMP
3′-5′-cyclic adenosine monophosphate
- Cand
candesartan
- CCD
cortical collecting duct
- CD
collecting duct
- CKD
chronic kidney disease
- CNT
connecting tubule
- COX
cyclooxygenase
- cTAL
cortical thick ascending limb
- CTGF
connecting tubule tubuloglomerular feedback
- DOCA
deoxycorticosterone acetate
- DR
Dahl salt-resistant
- DS
Dahl salt-sensitive
- DT
distal tubule
- EA
efferent arteriole
- ECs
endothelial cells
- EDCF
endothelium-dependent constrictor factor
- EDRF/NO
endothelium-derived relaxation factor/nitric oxide
- ET-1
endothelin-1
- ETB
endothelin receptor type-B
- GFR
glomerular filtration rate
- GLOM
glomerulus
- GPX
glutathione peroxidase
- GRK4
G protein-coupled receptor kinase type 4
- GSH
glutathione
- H2O2
hydrogen peroxide
- HETE
hydroxyeicosatetraenoic acid
- HHR
hydrochlorothiazide, hydralazine, and reserpine
- HR
heart rate
- ICV
intracerebroventricular
- JGA
juxtaglomerular apparatus
- LH
loop of Henle
- L-NAME
Nω-Nitro-l-arginine methyl ester
- L-NMMA
NG-Methyl-l-arginine acetate
- L-NNA
NG-nitro-l-arginine
- MAP
mean arterial pressure
- MBF
medullary blood flow
- MCD
medullary collecting duct
- MC
mesangial cells
- MD
macula densa
- MR
mineralocorticoid receptors
- mTAL
medullary thick ascending limb
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NaPi2
Na+–phosphate cotransporter
- NE
norepinephrine
- NHE3
Na+/H+ exchanger
- NHERF2
Na+/H+ exchanger regulatory factor
- NKCC2
Na+/K+/2Cl− transporter type-2
- NOS
nitric oxide synthase
- NOX
neutrophil oxidase
- O2−•
superoxide
- OH−•
hydroxyl anion
- ONOO−
peroxynitrite
- PEG-CAT
polyethylene glycol covalently linked to catalase
- PEG-SOD
polyethylene glycol covalently linked to superoxide dismutase
- PGG2/PGH2
prostaglandins/endoperoxides
- PGs
prostaglandins
- PH
posterior hypothalamic nuclei
- PKA
phosphokinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PLD
phospholipase D
- Podo
podocytes
- P
phosphate
- Prx
peroxiredoxin
- PT
proximal tubule
- RAAS
renin-angiotensin-aldosterone system
- RBF
renal blood flow
- ROS
reactive oxygen species
- RRM
reduced renal mass
- RSNA
renal sympathetic nerve activity
- RVR
renal vascular resistance
- SHR
spontaneously hypertensive rats
- SHRSP
spontaneously hypertensive rats stroke-prone
- SNGFR
single-nephron glomerular filtration rate
- SNS
sympathetic nervous system
- SOD
superoxide dismutase
- T1DM
type 1 diabetes mellitus
- TGF
tubuloglomerular feedback
- TP
thromboxane prostanoid receptor
- TrxR
thioredoxin reductase
- Trx
thioredoxin
- TxA2
thromboxane A2
- Veh
vehicle
- VSMC
vascular smooth muscle cell
- WKY
Wistar Kyoto
- WT
wild-type mice
- XOR
xanthine oxireductase
- XO
xanthine oxidase
Acknowledgments
This was supported by National Institutes of Health grants from the NIDDK (DK-049870 and DK-036079) and by the NHLBI (PO1 HL068686) and by funds from the George E. Schreiner Chair of Nephrology.
References
- 1.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, and Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A 108: 14849–14854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler S. and Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 287: F907–F913, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ahmeda AF. and Johns EJ. The regulation of blood perfusion in the renal cortex and medulla by reactive oxygen species and nitric oxide in the anaesthetised rat. Acta Physiol (Oxf) 204: 443–450, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Aldred KL, Harris PJ, and Eitle E. Increased proximal tubule NHE-3 and H+-ATPase activities in spontaneously hypertensive rats. J Hypertens 18: 623–628, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Alexander MY, Brosnan MJ, Hamilton CA, Fennell JP, Beattie EC, Jardine E, Heistad DD, and Dominiczak AF. Gene transfer of endothelial nitric oxide synthase but not Cu/Zn superoxide dismutase restores nitric oxide availability in the SHRSP. Cardiovasc Res 47: 609–617, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Allen AM. Blockade of angiotensin AT1-receptors in the rostral ventrolateral medulla of spontaneously hypertensive rats reduces blood pressure and sympathetic nerve discharge. J Renin Angiotensin Aldosterone Syst 2: S120–S124, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Aoi W, Niisato N, Sawabe Y, Miyazaki H, Tokuda S, Nishio K, Yoshikawa T, and Marunaka Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertensive rats. Cell Biol Int 31: 1288–1291, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, and Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension 49: 672–678, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bachmann S. and Mundel P. Nitric oxide in the kidney: synthesis, localization, and function. Am J Kidney Dis 24: 112–129, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Bagnall NM, Dent PC, Walkowska A, Sadowski J, and Johns EJ. Nitric oxide inhibition and the impact on renal nerve-mediated antinatriuresis and antidiuresis in the anaesthetized rat. J Physiol 569: 849–856, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baines A. and Ho P. Glucose stimulates O2 consumption, NOS, and Na/H exchange in diabetic rat proximal tubules. Am J Physiol Renal Physiol 283: F286–F293, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Banday AA. and Lokhandwala MF. Dopamine receptors and hypertension. Curr Hypertens Rep 10: 268–275, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bassett MH, Suzuki T, Sasano H, White PC, and Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol 18: 279–290, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Bayorh MA, Ganafa AA, Eatman D, Walton M, and Feuerstein GZ. Simvastatin and losartan enhance nitric oxide and reduce oxidative stress in salt-induced hypertension. Am J Hypertens 18: 1496–1502, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Bayorh MA, Rollins-Hairston A, Adiyiah J, Lyn D, and Eatman D. Eplerenone suppresses aldosterone/salt-induced expression of NOX-4. J Renin Angiotensin Aldosterone Syst 12: 195–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhaskaran M, Reddy K, Radhakrishanan N, Franki N, Ding G, and Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol 284: F955–F965, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Blantz RC, Deng A, Lortie M, Munger K, Vallon V, Gabbai FB, and Thomson SC. The complex role of nitric oxide in the regulation of glomerular ultrafiltration. Kidney Int 61: 782–785, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Boivin V, Jahns R, Gambaryan S, Ness W, Boege F, and Lohse MJ. Immunofluorescent imaging of beta 1- and beta 2-adrenergic receptors in rat kidney. Kidney Int 59: 515–531, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Bosse HM. and Bachmann S. Immunohistochemically detected protein nitration indicates sites of renal nitric oxide release in Goldblatt hypertension. Hypertension 30: 948–952, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Briet M. and Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol 6: 261–273, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, and Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, and Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai H, Davis ME, Drummond GR, and Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol 21: 1571–1576, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Campese VM, Shaohua Y, and Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 46: 533–539, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Campese VM. and Ye S. A vitamin-E-fortified diet reduces oxidative stress, sympathetic nerve activity, and hypertension in the phenol-renal injury model in rats. J Am Soc Hypertens 1: 242–250, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, and Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 287: H695–H703, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Carlstrom M, Lai EY, Ma Z, Patzak A, Brown RD, and Persson AE. Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol 296: R72–R79, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Carlstrom M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, and Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension 56: 907–913, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, and Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, and Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Chan SH, Tai MH, Li CY, and Chan JY. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic Biol Med 40: 2028–2039, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, and Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol 28: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, and Wilcox CS. Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 293: H3246–H3253, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Gill PS, and Welch WJ. Oxygen availability limits renal NADPH-dependent superoxide production. Am J Physiol Renal Physiol 289: F749–F753, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Pearlman A, Luo Z, and Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol 293: H2085–H2092, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Chen YF, Cowley AW, Jr., and Zou AP. Increased H(2)O(2) counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol Regul Integr Comp Physiol 285: R827–R833, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Chrissobolis S. and Faraci FM. Sex differences in protection against angiotensin II-induced endothelial dysfunction by manganese superoxide dismutase in the cerebral circulation. Hypertension 55: 905–910, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu Y, Iida S, Lund DD, Weiss RM, DiBona GF, Watanabe Y, Faraci FM, and Heistad DD. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res 92: 461–468, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Copin JC, Gasche Y, and Chan PH. Overexpression of copper/zinc superoxide dismutase does not prevent neonatal lethality in mutant mice that lack manganese superoxide dismutase. Free Radic Biol Med 28: 1571–1576, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Cowley AW, Jr., Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, and Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruthirds DL, Saba H, and MacMillan-Crow LA. Overexpression of manganese superoxide dismutase protects against ATP depletion-mediated cell death of proximal tubule cells. Arch Biochem Biophys 437: 96–105, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Cseko C, Bagi Z, and Koller A. Biphasic effect of hydrogen peroxide on skeletal muscle arteriolar tone via activation of endothelial and smooth muscle signaling pathways. J Appl Physiol 97: 1130–1137, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Cuevas S, Zhang Y, Yang Y, Escano C, Asico L, Jones JE, Armando I, and Jose PA. Role of renal DJ-1 in the pathogenesis of hypertension associated with increased reactive oxygen species production. Hypertension 59: 446–452, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai X, Galligan JJ, Watts SW, Fink GD, and Kreulen DL. Increased O2*- production and upregulation of ETB receptors by sympathetic neurons in DOCA-salt hypertensive rats. Hypertension 43: 1048–1054, 2004 [DOI] [PubMed] [Google Scholar]
- 47.de Cavanagh EM, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, and Fraga CG. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J 17: 1096–1098, 2003 [DOI] [PubMed] [Google Scholar]
- 48.De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, and Griendling KK. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J 329 (Pt 3): 653–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demchenko IT, Oury TD, Crapo JD, and Piantadosi CA. Regulation of the brain's vascular responses to oxygen. Circ Res 91: 1031–1037, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Deng X, Welch WJ, and Wilcox CS. Role of nitric oxide in short-term and prolonged effects of angiotensin II on renal hemodynamics. Hypertension 27: 1173–1179, 1996 [DOI] [PubMed] [Google Scholar]
- 51.DiBona GF. and Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol 298: R245–R253, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Dickhout JG, Mori T, and Cowley AW, Jr., Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487–493, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, and Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res 91: 938–944, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Dikalov SI. and Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal 19: 1085–1094, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, and Singhal PC. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol 283: F173–F180, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Drummond GR, Cai H, Davis ME, Ramasamy S, and Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 86: 347–354, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Dworkin LD, Grosser M, Feiner HD, Ullian M, and Parker M. Renal vascular effects of antihypertensive therapy in uninephrectomized SHR. Kidney Int 35: 790–798, 1989 [DOI] [PubMed] [Google Scholar]
- 58.Ebrahimian T, He Y, Schiffrin EL, and Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens 25: 1263–1271, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Ebrahimian T. and Touyz RM. Thioredoxin in vascular biology: role in hypertension. Antioxid Redox Signal 10: 1127–1136, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Eklof AC. The natriuretic response to a dopamine DA1 agonist requires endogenous activation of dopamine DA2 receptors. Acta Physiol Scand 160: 311–314, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Feletou M, Verbeuren TJ, and Vanhoutte PM. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. Br J Pharmacol 156: 563–574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, and Cowley AW, Jr., Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fennell JP, Brosnan MJ, Frater AJ, Hamilton CA, Alexander MY, Nicklin SA, Heistad DD, Baker AH, and Dominiczak AF. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther 9: 110–117, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Fine LG, Bandyopadhay D, and Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl 75: S22–S26, 2000 [PubMed] [Google Scholar]
- 65.Fischer LR, Li Y, Asress SA, Jones DP, and Glass JD. Absence of SOD1 leads to oxidative stress in peripheral nerve and causes a progressive distal motor axonopathy. Exp Neurol 233: 163–171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Folz RJ. and Crapo JD. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics 22: 162–171, 1994 [DOI] [PubMed] [Google Scholar]
- 67.Folz RJ, Guan J, Seldin MF, Oury TD, Enghild JJ, and Crapo JD. Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am J Respir Cell Mol Biol 17: 393–403, 1997 [DOI] [PubMed] [Google Scholar]
- 68.Franco M, Bautista R, Perez-Mendez O, Gonzalez L, Pacheco U, Sanchez-Lozada LG, Santamaria J, Tapia E, Monreal R, and Martinez F. Renal interstitial adenosine is increased in angiotensin II-induced hypertensive rats. Am J Physiol Renal Physiol 294: F84–F92, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Franco M, Perez-Mendez O, and Martinez F. Interaction of intrarenal adenosine and angiotensin II in kidney vascular resistance. Curr Opin Nephrol Hypertens 18: 63–67, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Fu Y, Zhang R, Lu D, Liu H, Chandrashekar K, Juncos LA, and Liu R. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol 298: R707–R712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujii S, Zhang L, Igarashi J, and Kosaka H. L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension 42: 1014–1020, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Fujiki T, Shimokawa H, Morikawa K, Kubota H, Hatanaka M, Talukder MA, Matoba T, Takeshita A, and Sunagawa K. Endothelium-derived hydrogen peroxide accounts for the enhancing effect of an angiotensin-converting enzyme inhibitor on endothelium-derived hyperpolarizing factor-mediated responses in mice. Arterioscler Thromb Vasc Biol 25: 766–771, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Fujimoto S, Satoh M, Horike H, Hatta H, Haruna Y, Kobayashi S, Namikoshi T, Arakawa S, Tomita N, and Kashihara N. Olmesartan ameliorates progressive glomerular injury in subtotal nephrectomized rats through suppression of superoxide production. Hypertens Res 31: 305–313, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Fukai T. Mitochondrial thioredoxin: novel regulator for NADPH oxidase and angiotensin II-induced hypertension. Hypertension 54: 224–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukai T, Siegfried MR, Ushio-Fukai M, Griendling KK, and Harrison DG. Modulation of extracellular superoxide dismutase expression by angiotensin II and hypertension. Circ Res 85: 23–28, 1999 [DOI] [PubMed] [Google Scholar]
- 76.Fukai T. and Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galle J, Herzog C, Schollmeyer P, and Wanner C. Oxygen-derived radicals stimulate renin release of isolated juxtaglomerular cells. FEBS Lett 351: 314–316, 1994 [DOI] [PubMed] [Google Scholar]
- 78.Galle J, Stunz P, Schollmeyer P, and Wanner C. Oxidized LDL and lipoprotein(a) stimulate renin release of juxtaglomerular cells. Kidney Int 47: 45–52, 1995 [DOI] [PubMed] [Google Scholar]
- 79.Gao X, Patzak A, Sendeski M, Scheffer PG, Teerlink T, Sallstrom J, Fredholm BB, Persson AE, and Carlstrom M. Adenosine A(1)-receptor deficiency diminishes afferent arteriolar and blood pressure responses during nitric oxide inhibition and angiotensin II treatment. Am J Physiol Regul Integr Comp Physiol 301: R1669–R1681, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Gao YJ, Hirota S, Zhang DW, Janssen LJ, and Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol 138: 1085–1092, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao YJ. and Lee RM. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A(2) production. Br J Pharmacol 134: 1639–1646, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia NH, Plato CF, Stoos BA, and Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension 34: 508–513, 1999 [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Estan J. and Roman RJ. Role of renal interstitial hydrostatic pressure in the pressure diuresis response. Am J Physiol 256: F63–F70, 1989 [DOI] [PubMed] [Google Scholar]
- 84.Garvin JL, Herrera M, and Ortiz PA. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: clinical implications. Annu Rev Physiol 73: 359–376, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Garvin JL. and Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. Am J Physiol 277: F377–F382, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Garvin JL. and Ortiz PA. The role of reactive oxygen species in the regulation of tubular function. Acta Physiol Scand 179: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Geiszt M, Kopp JB, Varnai P, and Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gill PS. and Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, and Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension 48: 473–481, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, and Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285: F219– F229, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Granger JP, Alexander BT, and Llinas M. Mechanisms of pressure natriuresis. Curr Hypertens Rep 4: 152–159, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Grassi G, Seravalle G, Brambilla G, and Mancia G. The sympathetic nervous system and new nonpharmacologic approaches to treating hypertension: a focus on renal denervation. Can J Cardiol 28: 311–317, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Grote K, Ortmann M, Salguero G, Doerries C, Landmesser U, Luchtefeld M, Brandes RP, Gwinner W, Tschernig T, Brabant EG, Klos A, Schaefer A, Drexler H, and Schieffer B. Critical role for p47phox in renin-angiotensin system activation and blood pressure regulation. Cardiovasc Res 71: 596–605, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Guo P, Nishiyama A, Rahman M, Nagai Y, Noma T, Namba T, Ishizawa M, Murakami K, Miyatake A, Kimura S, Mizushige K, Abe Y, Ohmori K, and Kohno M. Contribution of reactive oxygen species to the pathogenesis of left ventricular failure in Dahl salt-sensitive hypertensive rats: effects of angiotensin II blockade. J Hypertens 24: 1097–1104, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, and Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol 285: H1396–H1403, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Gwathmey TM, Alzayadneh EM, Pendergrass KD, and Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R518–R530, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, and Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol 296: F1484–F1493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haendeler J, Popp R, Goy C, Tischler V, Zeiher AM, and Dimmeler S. Cathepsin D and H2O2 stimulate degradation of thioredoxin-1: implication for endothelial cell apoptosis. J Biol Chem 280: 42945–42951, 2005 [DOI] [PubMed] [Google Scholar]
- 99.Haendeler J, Tischler V, Hoffmann J, Zeiher AM, and Dimmeler S. Low doses of reactive oxygen species protect endothelial cells from apoptosis by increasing thioredoxin-1 expression. FEBS Lett 577: 427–433, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Hansen PB, Hashimoto S, Briggs J, and Schnermann J. Attenuated renovascular constrictor responses to angiotensin II in adenosine 1 receptor knockout mice. Am J Physiol Regul Integr Comp Physiol 285: R44–R49, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Haque MZ. and Majid DS. High salt intake delayed angiotensin II-induced hypertension in mice with a genetic variant of NADPH oxidase. Am J Hypertens 24: 114–118, 2011 [DOI] [PubMed] [Google Scholar]
- 102.Harris RC. Podocyte ACE2 protects against diabetic nephropathy. Kidney Int 82: 255–256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrison DG, Cai H, Landmesser U, and Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst 4: 51–61, 2003 [DOI] [PubMed] [Google Scholar]
- 104.Harrison DG, Gongora MC, Guzik TJ, and Widder J. Oxidative stress and hypertension. J Am Soc Hypertens 1: 30–44, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Harrison-Bernard LM, Cook AK, Oliverio MI, and Coffman TM. Renal segmental microvascular responses to ANG II in AT1A receptor null mice. Am J Physiol Renal Physiol 284: F538–F545, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, and el-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol 273: F170–F177, 1997 [DOI] [PubMed] [Google Scholar]
- 107.Herrera M. and Garvin JL. Angiotensin II stimulates thick ascending limb NO production via AT(2) receptors and Akt1-dependent nitric-oxide synthase 3 (NOS3) activation. J Biol Chem 285: 14932–14940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herrera M, Ortiz PA, and Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am J Physiol Renal Physiol 290: F1279–F1284, 2006 [DOI] [PubMed] [Google Scholar]
- 109.Hill GS. and Heptinstall RH. Steroid-induced hypertension in the rat. A microangiographic and histologic study on the pathogenesis of hypertensive vascular and glomerular lesions. Am J Pathol 52: 1–40, 1968 [PMC free article] [PubMed] [Google Scholar]
- 110.Ho YS, Xiong Y, Ma W, Spector A, and Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 279: 32804–32812, 2004 [DOI] [PubMed] [Google Scholar]
- 111.Hoagland KM, Maier KG, and Roman RJ. Contributions of 20-HETE to the antihypertensive effects of Tempol in Dahl salt-sensitive rats. Hypertension 41: 697–702, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, and Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296: R208–R216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hong NJ. and Garvin JL. NADPH oxidase 4 mediates flow-induced superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 303: F1151–F1156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hong NJ, Silva GB, and Garvin JL. PKC-alpha mediates flow-stimulated superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 298: F885–F891, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hua P, Feng W, Rezonzew G, Chumley P, and Jaimes EA. The transcription factor ETS-1 regulates angiotensin II-stimulated fibronectin production in mesangial cells. Am J Physiol Renal Physiol 302: F1418–F1429, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Huang BS, Ahmadi S, Ahmad M, White RA, and Leenen FH. Central neuronal activation and pressor responses induced by circulating ANG II: role of the brain aldosterone-“ouabain” pathway. Am J Physiol Heart Circ Physiol 299: H422–H430, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Hussain T. and Lokhandwala MF. Altered arachidonic acid metabolism contributes to the failure of dopamine to inhibit Na+,K(+)-ATPase in kidney of spontaneously hypertensive rats. Clin Exp Hypertens 18: 963–974, 1996 [DOI] [PubMed] [Google Scholar]
- 118.Hwang I, Lee J, Huh JY, Park J, Lee HB, Ho YS, and Ha H. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes 61: 728–738, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Itani H, Liu X, Sarsour EH, Goswami PC, Born E, Keen HL, and Sigmund CD. Regulation of renin gene expression by oxidative stress. Hypertension 53: 1070–1076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ito S, Juncos LA, and Carretero OA. Pressure-induced constriction of the afferent arteriole of spontaneously hypertensive rats. Hypertension 19: II164–II167, 1992 [DOI] [PubMed] [Google Scholar]
- 121.Iversen BM, Amann K, Kvam FI, Wang X, and Ofstad J. Increased glomerular capillary pressure and size mediate glomerulosclerosis in SHR juxtamedullary cortex. Am J Physiol 274: F365–F373, 1998 [DOI] [PubMed] [Google Scholar]
- 122.Iwai N. and Inagami T. Identification of two subtypes in the rat type I angiotensin II receptor. FEBS Lett 298: 257–260, 1992 [DOI] [PubMed] [Google Scholar]
- 123.Jennings BL, Anderson LJ, Estes AM, Yaghini FA, Fang XR, Porter J, Gonzalez FJ, Campbell WB, and Malik KU. Cytochrome P450 1B1 contributes to renal dysfunction and damage caused by angiotensin II in mice. Hypertension 59: 348–354, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, and Singhal PC. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol 28: 500–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin C, Hu C, Polichnowski A, Mori T, Skelton M, Ito S, and Cowley AW, Jr., Effects of renal perfusion pressure on renal medullary hydrogen peroxide and nitric oxide production. Hypertension 53: 1048–1053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin XQ, Fukuda N, Su JZ, Lai YM, Suzuki R, Tahira Y, Takagi H, Ikeda Y, Kanmatsuse K, and Miyazaki H. Angiotensin II type 2 receptor gene transfer downregulates angiotensin II type 1a receptor in vascular smooth muscle cells. Hypertension 39: 1021–1027, 2002 [DOI] [PubMed] [Google Scholar]
- 127.Jose PA, Eisner GM, and Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens 11: 87–92, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, and Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res 93: 622–629, 2003 [DOI] [PubMed] [Google Scholar]
- 129.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, and Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Just A. and Arendhorst WJ. The role of reactive oxygen species in renal blood flow autoregulation. FASEB J 22: 761.19, 2008 [Google Scholar]
- 131.Just A. and Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol 293: F1489–F1500, 2007 [DOI] [PubMed] [Google Scholar]
- 132.Just A, Whitten CL, and Arendshorst WJ. Reactive oxygen species participate in acute renal vasoconstrictor responses induced by ETA and ETB receptors. Am J Physiol Renal Physiol 294: F719–F728, 2008 [DOI] [PubMed] [Google Scholar]
- 133.Kawada N, Dennehy K, Solis G, Modlinger P, Hamel R, Kawada JT, Aslam S, Moriyama T, Imai E, Welch WJ, and Wilcox CS. TP receptors regulate renal hemodynamics during angiotensin II slow pressor response. Am J Physiol Renal Physiol 287: F753–F759, 2004 [DOI] [PubMed] [Google Scholar]
- 134.Kawada N, Imai E, Karber A, Welch WJ, and Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol 13: 2860–2868, 2002 [DOI] [PubMed] [Google Scholar]
- 135.Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, Ueda K, and Fujita T. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol 23: 997–1007, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, and Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 109: 2357–2362, 2004 [DOI] [PubMed] [Google Scholar]
- 137.Kitayama J, Yi C, Faraci FM, and Heistad DD. Modulation of dilator responses of cerebral arterioles by extracellular superoxide dismutase. Stroke 37: 2802–2806, 2006 [DOI] [PubMed] [Google Scholar]
- 138.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, and Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782, 2003 [DOI] [PubMed] [Google Scholar]
- 139.Klar J, Vitzthum H, and Kurtz A. Aldosterone enhances renin gene expression in juxtaglomerular cells. Am J Physiol Renal Physiol 286: F349–F355, 2004 [DOI] [PubMed] [Google Scholar]
- 140.Kobayashi M, Sugiyama H, Wang DH, Toda N, Maeshima Y, Yamasaki Y, Masuoka N, Yamada M, Kira S, and Makino H. Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int 68: 1018–1031, 2005 [DOI] [PubMed] [Google Scholar]
- 141.Kobori H, Nangaku M, Navar LG, and Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 142.Kobori H, Nishiyama A, Abe Y, and Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kohan DE, Padilla E, and Hughes AK. Endothelin B receptor mediates ET-1 effects on cAMP and PGE2 accumulation in rat IMCD. Am J Physiol 265: F670–F676, 1993 [DOI] [PubMed] [Google Scholar]
- 144.Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, and Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci 17: 4180–4189, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kopp UC. Neural Control of Renal Function. San Rafael, CA: Morgan Claypool Life Sciences, 2011 [PubMed] [Google Scholar]
- 146.Kost CK, Jr., Herzer WA, Rominski BR, Mi Z, and Jackson EK. Diuretic response to adenosine A(1) receptor blockade in normotensive and spontaneously hypertensive rats: role of pertussis toxin-sensitive G-proteins. J Pharmacol Exp Ther 292: 752–760, 2000 [PubMed] [Google Scholar]
- 147.Kovacs G, Peti-Peterdi J, Rosivall L, and Bell PD. Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT(1) receptors. Am J Physiol Renal Physiol 282: F301–F306, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Kurtz A. Control of renin synthesis and secretion. Am J Hypertens 25: 839–847, 2012 [DOI] [PubMed] [Google Scholar]
- 149.Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, and Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol 285: F152–F165, 2003 [DOI] [PubMed] [Google Scholar]
- 150.Lai EY, Luo Z, Onozato ML, Rudolph EH, Solis G, Jose PA, Wellstein A, Aslam S, Quinn MT, Griendling K, Le T, Li P, Palm F, Welch WJ, and Wilcox CS. Effects of the antioxidant drug tempol on renal oxygenation in mice with reduced renal mass. Am J Physiol Renal Physiol 303: F64–F74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ, and Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertension 55: 983–989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, and Persson AE. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int 70: 690–698, 2006 [DOI] [PubMed] [Google Scholar]
- 153.Lai EY, Solis G, Luo Z, Carlstrom M, Sandberg K, Holland S, Wellstein A, Welch WJ, and Wilcox CS. p47(phox) is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension 59: 415–420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lai EY, Wellstein A, Welch WJ, and Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension 58: 650–656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr., Dionne L, Lu N, Huang S, and Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A 93: 9782–9787, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee DL, Bell TD, Bhupatkar J, Solis G, and Welch WJ. Adenosine A1-receptor knockout mice have a decreased blood pressure response to low-dose ANG II infusion. Am J Physiol Regul Integr Comp Physiol 303: R683–R688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leong PK, Devillez A, Sandberg MB, Yang LE, Yip DK, Klein JB, and McDonough AA. Effects of ACE inhibition on proximal tubule sodium transport. Am J Physiol Renal Physiol 290: F854–F863, 2006 [DOI] [PubMed] [Google Scholar]
- 158.Leong PK, Zhang Y, Yang LE, Holstein-Rathlou NH, and McDonough AA. Diuretic response to acute hypertension is blunted during angiotensin II clamp. Am J Physiol Regul Integr Comp Physiol 283: R837–R842, 2002 [DOI] [PubMed] [Google Scholar]
- 159.Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, and Romero JC. Increased oxidative stress in experimental renovascular hypertension. Hypertension 37: 541–546, 2001 [DOI] [PubMed] [Google Scholar]
- 160.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, and Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation 107: 1053–1058, 2003 [DOI] [PubMed] [Google Scholar]
- 161.Li L. and Frei B. Iron chelation inhibits NF-kappaB-mediated adhesion molecule expression by inhibiting p22(phox) protein expression and NADPH oxidase activity. Arterioscler Thromb Vasc Biol 26: 2638–2643, 2006 [DOI] [PubMed] [Google Scholar]
- 162.Li XC. and Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-beta1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol 294: C1034–C1045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, and Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11: 376–381, 1995 [DOI] [PubMed] [Google Scholar]
- 164.Licea H, Walters MR, and Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung 89: 427–438, 2002 [DOI] [PubMed] [Google Scholar]
- 165.Liu R, Ren Y, Garvin JL, and Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 166.Liu Y, Bubolz AH, Mendoza S, Zhang DX, and Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 108: 566–573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lob HE, Vinh A, Li L, Blinder Y, Offermanns S, and Harrison DG. Role of vascular extracellular superoxide dismutase in hypertension. Hypertension 58: 232–239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lodha S, Dani D, Mehta R, Bhaskaran M, Reddy K, Ding G, and Singhal PC. Angiotensin II-induced mesangial cell apoptosis: role of oxidative stress. Mol Med 8: 830–840, 2002 [PMC free article] [PubMed] [Google Scholar]
- 169.Loutzenhiser R, Griffin K, Williamson G, and Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Luo Z, Chen Y, Chen S, Welch WJ, Andresen BT, Jose PA, and Wilcox CS. Comparison of inhibitors of superoxide generation in vascular smooth muscle cells. Br J Pharmacol 157: 935–943, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, and Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 347: 797–805, 2002 [DOI] [PubMed] [Google Scholar]
- 172.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, and Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Magyar CE, Zhang Y, Holstein-Rathlou NH, and McDonough AA. Proximal tubule Na transporter responses are the same during acute and chronic hypertension. Am J Physiol Renal Physiol 279: F358–F369, 2000 [DOI] [PubMed] [Google Scholar]
- 175.Makino A, Skelton MM, Zou AP, and Cowley AW, Jr., Increased renal medullary H2O2 leads to hypertension. Hypertension 42: 25–30, 2003 [DOI] [PubMed] [Google Scholar]
- 176.Makino A, Skelton MM, Zou AP, Roman RJ, and Cowley AW, Jr., Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667–672, 2002 [DOI] [PubMed] [Google Scholar]
- 177.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, and Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Manning RD, Jr., Hu L, and Reckelhoff JF. Role of nitric oxide in the arterial pressure and renal adaptations to long-term changes in sodium intake. Am J Physiol 272: R1162–R1169, 1997 [DOI] [PubMed] [Google Scholar]
- 179.Manning RD, Jr., Meng S, and Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand 179: 243–250, 2003 [DOI] [PubMed] [Google Scholar]
- 180.Manning RD, Jr., Tian N, and Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol 25: 311–317, 2005 [DOI] [PubMed] [Google Scholar]
- 181.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J 222: 649–655, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Matavelli LC, Huang J, and Siragy HM. Angiotensin AT(2) receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension 57: 308–313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matsubara H, Sugaya T, Murasawa S, Nozawa Y, Mori Y, Masaki H, Maruyama K, Tsutumi Y, Shibasaki Y, Moriguchi Y, Tanaka Y, Iwasaka T, and Inada M. Tissue-specific expression of human angiotensin II AT1 and AT2 receptors and cellular localization of subtype mRNAs in adult human renal cortex using in situ hybridization. Nephron 80: 25–34, 1998 [DOI] [PubMed] [Google Scholar]
- 184.Mazzali M, Jefferson JA, Ni Z, Vaziri ND, and Johnson RJ. Microvascular and tubulointerstitial injury associated with chronic hypoxia-induced hypertension. Kidney Int 63: 2088–2093, 2003 [DOI] [PubMed] [Google Scholar]
- 185.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298: R851–R861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Meng S, Cason GW, Gannon AW, Racusen LC, and Manning RD, Jr., Oxidative stress in Dahl salt-sensitive hypertension. Hypertension 41: 1346–1352, 2003 [DOI] [PubMed] [Google Scholar]
- 187.Meng S, Roberts LJ, 2nd, Cason GW, Curry TS, and Manning RD, Jr., Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regul Integr Comp Physiol 283: R732–R738, 2002 [DOI] [PubMed] [Google Scholar]
- 188.Mitchell KD. and Navar LG. Enhanced tubuloglomerular feedback during peritubular infusions of angiotensins I and II. Am J Physiol 255: F383–F390, 1988 [DOI] [PubMed] [Google Scholar]
- 189.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, and Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 16: 2906–2912, 2005 [DOI] [PubMed] [Google Scholar]
- 190.Miyata N, Park F, Li XF, and Cowley AW, Jr., Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol 277: F437–F446, 1999 [DOI] [PubMed] [Google Scholar]
- 191.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, and Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47: 238–244, 2006 [DOI] [PubMed] [Google Scholar]
- 192.Montezano AC. and Touyz RM. Molecular mechanisms of hypertension—reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol 28: 288–295, 2012 [DOI] [PubMed] [Google Scholar]
- 193.Montezano AC. and Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med 44Suppl 1: S2–S16, 2012 [DOI] [PubMed] [Google Scholar]
- 194.Moore LC, Schnermann J, and Yarimizu S. Feedback mediation of SNGFR autoregulation in hydropenic and DOCA- and salt-loaded rats. Am J Physiol 237: F63–F74, 1979 [DOI] [PubMed] [Google Scholar]
- 195.Moreno C, Lopez A, Llinas MT, Rodriguez F, Lopez-Farre A, Nava E, and Salazar FJ. Changes in NOS activity and protein expression during acute and prolonged ANG II administration. Am J Physiol Regul Integr Comp Physiol 282: R31–R37, 2002 [DOI] [PubMed] [Google Scholar]
- 196.Moreno C, Maier KG, Hoagland KM, Yu M, and Roman RJ. Abnormal pressure-natriuresis in hypertension: role of cytochrome P450 metabolites of arachidonic acid. Am J Hypertens 14: 90S–97S, 2001 [DOI] [PubMed] [Google Scholar]
- 197.Mori T, Ogawa S, Cowely AW, Jr., and Ito S. Role of renal medullary oxidative and/or carbonyl stress in salt-sensitive hypertension and diabetes. Clin Exp Pharmacol Physiol 39: 125–131, 2012 [DOI] [PubMed] [Google Scholar]
- 198.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, and Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A 88: 10045–10048, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nie J LQ, Chen S, Wilcox CS, and Welch WJ. Regulation of fluid reabsorption in the proximal tubule: role of nitric oxide, ADMA and DDAH. J Am Soc Nephrol 19: 23A, 2008 [Google Scholar]
- 200.Nie Z, Mei Y, Ford M, Rybak L, Marcuzzi A, Ren H, Stiles GL, and Ramkumar V. Oxidative stress increases A1 adenosine receptor expression by activating nuclear factor kappa B. Mol Pharmacol 53: 663–669, 1998 [DOI] [PubMed] [Google Scholar]
- 201.Nishi A, Eklof AC, Bertorello AM, and Aperia A. Dopamine regulation of renal Na+,K(+)-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension 21: 767–771, 1993 [DOI] [PubMed] [Google Scholar]
- 202.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, and Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 43: 841–848, 2004 [DOI] [PubMed] [Google Scholar]
- 203.Nistala R, Wei Y, Sowers JR, and Whaley-Connell A. Renin-angiotensin-aldosterone system-mediated redox effects in chronic kidney disease. Transl Res 153: 102–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nistala R, Whaley-Connell A, and Sowers JR. Redox control of renal function and hypertension. Antioxid Redox Signal 10: 2047–2089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nogueira EF, Xing Y, Morris CA, and Rainey WE. Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. J Mol Endocrinol 42: 319–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Norrelund H, Christensen KL, Samani NJ, Kimber P, Mulvany MJ, and Korsgaard N. Early narrowed afferent arteriole is a contributor to the development of hypertension. Hypertension 24: 301–308, 1994 [DOI] [PubMed] [Google Scholar]
- 207.Nouri P, Gill P, Li M, Wilcox CS, and Welch WJ. p22phox in the macula densa regulates single nephron GFR during angiotensin II infusion in rats. Am J Physiol Heart Circ Physiol 292: H1685–H1689, 2007 [DOI] [PubMed] [Google Scholar]
- 208.Nozik-Grayck E, Suliman HB, and Piantadosi CA. Extracellular superoxide dismutase. Int J Biochem Cell Biol 37: 2466–2471, 2005 [DOI] [PubMed] [Google Scholar]
- 209.O'Connor PM. and Cowley AW, Jr., Modulation of pressure-natriuresis by renal medullary reactive oxygen species and nitric oxide. Curr Hypertens Rep 12: 86–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Oberley TD, Coursin DB, Cihla HP, Oberley LW, el-Sayyad N, and Ho YS. Immunolocalization of manganese superoxide dismutase in normal and transgenic mice expressing the human enzyme. Histochem J 25: 267–279, 1993 [DOI] [PubMed] [Google Scholar]
- 211.Oberley TD, Verwiebe E, Zhong W, Kang SW, and Rhee SG. Localization of the thioredoxin system in normal rat kidney. Free Radic Biol Med 30: 412–424, 2001 [DOI] [PubMed] [Google Scholar]
- 212.Ogawa Y, Mukoyama M, Yokoi H, Kasahara M, Mori K, Kato Y, Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Tokudome T, Kishimoto I, Sugawara A, and Nakao K. Natriuretic Peptide receptor guanylyl cyclase-a protects podocytes from aldosterone-induced glomerular injury. J Am Soc Nephrol 23: 1198–1209, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ohsaki Y, O'Connor P, Mori T, Ryan RP, Dickinson BC, Chang CJ, Lu Y, Ito S, and Cowley AW, Jr., Increase of sodium delivery stimulates the mitochondrial respiratory chain H2O2 production in rat renal medullary thick ascending limb. Am J Physiol Renal Physiol 302: F95–F102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ollerstam A, Pittner J, Persson AE, and Thorup C. Increased blood pressure in rats after long-term inhibition of the neuronal isoform of nitric oxide synthase. J Clin Invest 99: 2212–2218, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ookawara T, Imazeki N, Matsubara O, Kizaki T, Oh-Ishi S, Nakao C, Sato Y, and Ohno H. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am J Physiol 275: C840– C847, 1998 [DOI] [PubMed] [Google Scholar]
- 216.Ortiz MC, Manriquez MC, Romero JC, and Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension 38: 655–659, 2001 [DOI] [PubMed] [Google Scholar]
- 217.Ortiz PA. and Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol 282: F777–F784, 2002 [DOI] [PubMed] [Google Scholar]
- 218.Ortiz PA, Hong NJ, and Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na(+)-K(+)-2Cl(-) cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 219.Ortiz PA, Hong NJ, Wang D, and Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of L-arginine on NaCl absorption. Hypertension 42: 674–679, 2003 [DOI] [PubMed] [Google Scholar]
- 220.Ouyang X, Le TH, Roncal C, Gersch C, Herrera-Acosta J, Rodriguez-Iturbe B, Coffman TM, Johnson RJ, and Mu W. Th1 inflammatory response with altered expression of profibrotic and vasoactive mediators in AT1A and AT1B double-knockout mice. Am J Physiol Renal Physiol 289: F902–F910, 2005 [DOI] [PubMed] [Google Scholar]
- 221.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, and Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension 30: 1238–1246, 1997 [DOI] [PubMed] [Google Scholar]
- 222.Pallone TL. and Mattson DL. Role of nitric oxide in regulation of the renal medulla in normal and hypertensive kidneys. Curr Opin Nephrol Hypertens 11: 93–98, 2002 [DOI] [PubMed] [Google Scholar]
- 223.Palm F, Onozato M, Welch WJ, and Wilcox CS. Blood pressure, blood flow, and oxygenation in the clipped kidney of chronic 2-kidney, 1-clip rats: effects of tempol and Angiotensin blockade. Hypertension 55: 298–304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Panico C, Luo Z, Damiano S, Artigiano F, Gill P, and Welch WJ. Renal proximal tubular reabsorption is reduced in adult spontaneously hypertensive rats: roles of superoxide and Na+/H+ exchanger 3. Hypertension 54: 1291–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Patel K, Chen Y, Dennehy K, Blau J, Connors S, Mendonca M, Tarpey M, Krishna M, Mitchell JB, Welch WJ, and Wilcox CS. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol 290: R37–R43, 2006 [DOI] [PubMed] [Google Scholar]
- 226.Patzak A, Lai E, Persson PB, and Persson AE. Angiotensin II-nitric oxide interaction in glomerular arterioles. Clin Exp Pharmacol Physiol 32: 410–414, 2005 [DOI] [PubMed] [Google Scholar]
- 227.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, and Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 [DOI] [PubMed] [Google Scholar]
- 228.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, and Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun 384: 149–154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Peti-Peterdi J. and Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol 21: 1093–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ploth DW, Schnermann J, Dahlheim H, Hermle M, and Schmidmeier E. Autoregulation and tubuloglomerular feedback in normotensive and hypertensive rats. Kidney Int 12: 253–267, 1977 [DOI] [PubMed] [Google Scholar]
- 231.Pollock CA, Lawrence JR, and Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol 260: F946–F952, 1991 [DOI] [PubMed] [Google Scholar]
- 232.Qi Z, Cai H, Morrow JD, and Breyer MD. Differentiation of cyclooxygenase 1- and 2-derived prostanoids in mouse kidney and aorta. Hypertension 48: 323–328, 2006 [DOI] [PubMed] [Google Scholar]
- 233.Racasan S, Joles JA, Boer P, Koomans HA, and Braam B. NO dependency of RBF and autoregulation in the spontaneously hypertensive rat. Am J Physiol Renal Physiol 285: F105–F112, 2003 [DOI] [PubMed] [Google Scholar]
- 234.Rajamohan SB, Raghuraman G, Prabhakar NR, and Kumar GK. NADPH oxidase-derived H(2)O(2) contributes to angiotensin II-induced aldosterone synthesis in human and rat adrenal cortical cells. Antioxid Redox Signal 17: 445–459, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ren Y, D'Ambrosio MA, Garvin JL, and Carretero OA. Angiotensin II enhances connecting tubule glomerular feedback. Hypertension 56: 636–642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, and Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 298: H1769–H1775, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ren Y, D'Ambrosio MA, Wang H, Peterson EL, Garvin JL, and Carretero OA. Mechanisms of angiotensin II-enhanced connecting tubule glomerular feedback. Am J Physiol Renal Physiol 303: F259–F265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ren Y, Garvin JL, and Carretero OA. Efferent arteriole tubuloglomerular feedback in the renal nephron. Kidney Int 59: 222–229, 2001 [DOI] [PubMed] [Google Scholar]
- 239.Ren Z, Liang W, Chen C, Yang H, Singhal PC, and Ding G. Angiotensin II induces nephrin dephosphorylation and podocyte injury: role of caveolin-1. Cell Signal 24: 443–450, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Roberts LJ, 2nd, and Milne GL. Isoprostanes. J Lipid Res 50Suppl: S219–S223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rocha R, Chander PN, Zuckerman A, and Stier CT, Jr., Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 33: 232–237, 1999 [DOI] [PubMed] [Google Scholar]
- 242.Rodrigo R, Gonzalez J, and Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 34: 431–440, 2011 [DOI] [PubMed] [Google Scholar]
- 243.Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, and Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol 102: 255–260, 2007 [DOI] [PubMed] [Google Scholar]
- 244.Roman RJ. Pressure-diuresis in volume-expanded rats. Tubular reabsorption in superficial and deep nephrons. Hypertension 12: 177–183, 1988 [DOI] [PubMed] [Google Scholar]
- 245.Sainz J, Wangensteen R, Rodriguez Gomez I, Moreno JM, Chamorro V, Osuna A, Bueno P, and Vargas F. Antioxidant enzymes and effects of tempol on the development of hypertension induced by nitric oxide inhibition. Am J Hypertens 18: 871–877, 2005 [DOI] [PubMed] [Google Scholar]
- 246.Sakamoto A, Hongo M, Saito K, Nagai R, and Ishizaka N. Reduction of renal lipid content and proteinuria by a PPAR-gamma agonist in a rat model of angiotensin II-induced hypertension. Eur J Pharmacol 682: 131–136, 2012 [DOI] [PubMed] [Google Scholar]
- 247.Salmond R. and Seney FD, Jr., Reset tubuloglomerular feedback permits and sustains glomerular hyperfunction after extensive renal ablation. Am J Physiol 260: F395–F401, 1991 [DOI] [PubMed] [Google Scholar]
- 248.Sasamura H, Hein L, Krieger JE, Pratt RE, Kobilka BK, and Dzau VJ. Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem Biophys Res Commun 185: 253–259, 1992 [DOI] [PubMed] [Google Scholar]
- 249.Schlondorff D. and Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20: 1179–1187, 2009 [DOI] [PubMed] [Google Scholar]
- 250.Schnackenberg CG. Physiological and pathophysiological roles of oxygen radicals in the renal microvasculature. Am J Physiol Regul Integr Comp Physiol 282: R335–R342, 2002 [DOI] [PubMed] [Google Scholar]
- 251.Schnackenberg CG. and Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 33: 424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 252.Schnermann J, Gokel M, Weber PC, Schubert G, and Briggs JP. Tubuloglomerular feedback and glomerular morphology in Goldblatt hypertensive rats on varying protein diets. Kidney Int 29: 520–529, 1986 [DOI] [PubMed] [Google Scholar]
- 253.Schnermann JB, Traynor T, Yang T, Huang YG, Oliverio MI, Coffman T, and Briggs JP. Absence of tubuloglomerular feedback responses in AT1A receptor-deficient mice. Am J Physiol 273: F315–F320, 1997 [DOI] [PubMed] [Google Scholar]
- 254.Schreck C and O'Connor PM. NAD(P)H oxidase and renal epithelial ion transport. Am J Physiol Regul Integr Comp Physiol 300: R1023–R1029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sedeek M, Hebert RL, Kennedy CR, Burns KD, and Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens 18: 122–127, 2009 [DOI] [PubMed] [Google Scholar]
- 256.Seeliger E, Wronski T, Ladwig M, Dobrowolski L, Vogel T, Godes M, Persson PB, and Flemming B. The renin-angiotensin system and the third mechanism of renal blood flow autoregulation. Am J Physiol Renal Physiol 296: F1334–F1345, 2009 [DOI] [PubMed] [Google Scholar]
- 257.Sentman ML, Granstrom M, Jakobson H, Reaume A, Basu S, and Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem 281: 6904–6909, 2006 [DOI] [PubMed] [Google Scholar]
- 258.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, and Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 121: 3233–3243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shibata S, Nagase M, Yoshida S, Kawachi H, and Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 49: 355–364, 2007 [DOI] [PubMed] [Google Scholar]
- 260.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, and Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 261.Shokoji T, Nishiyama A, Fujisawa Y, Hitomi H, Kiyomoto H, Takahashi N, Kimura S, Kohno M, and Abe Y. Renal sympathetic nerve responses to tempol in spontaneously hypertensive rats. Hypertension 41: 266–273, 2003 [DOI] [PubMed] [Google Scholar]
- 262.Sigmon DH. and Beierwaltes WH. Degree of renal artery stenosis alters nitric oxide regulation of renal hemodynamics. J Am Soc Nephrol 5: 1369–1377, 1994 [DOI] [PubMed] [Google Scholar]
- 263.Sigmon DH. and Beierwaltes WH. Influence of nitric oxide in the chronic phase of two-kidney, one clip renovascular hypertension. Hypertension 31: 649–656, 1998 [DOI] [PubMed] [Google Scholar]
- 264.Silva GB, Ortiz PA, Hong NJ, and Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 265.Simao S, Gomes P, Pinto V, Silva E, Amaral JS, Igreja B, Afonso J, Serrao MP, Pinho MJ, and Soares-da-Silva P. Age-related changes in renal expression of oxidant and antioxidant enzymes and oxidative stress markers in male SHR and WKY rats. Exp Gerontol 46: 468–474, 2011 [DOI] [PubMed] [Google Scholar]
- 266.Skov K. and Mulvany MJ. Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta Physiol Scand 181: 397–405, 2004 [DOI] [PubMed] [Google Scholar]
- 267.Sousa T, Oliveira S, Afonso J, Morato M, Patinha D, Fraga S, Carvalho F, and Albino-Teixeira A. Role of H(2) O(2) in hypertension, renin-angiotensin system activation and renal medullary disfunction caused by angiotensin II. Br J Pharmacol 166: 2386–2401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sousa T, Pinho D, Morato M, Marques-Lopes J, Fernandes E, Afonso J, Oliveira S, Carvalho F, and Albino-Teixeira A. Role of superoxide and hydrogen peroxide in hypertension induced by an antagonist of adenosine receptors. Eur J Pharmacol 588: 267–276, 2008 [DOI] [PubMed] [Google Scholar]
- 269.Speed JS, LaMarca B, Berry H, Cockrell K, George EM, and Granger JP. Renal medullary endothelin-1 is decreased in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 301: R519–R523, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Stier CT, Jr., Itskovitz HD, and Chen YH. Urinary dopamine and sodium excretion in spontaneously hypertensive rats. Clin Exp Hypertens 15: 105–123, 1993 [DOI] [PubMed] [Google Scholar]
- 271.Strojek K, Grzeszczak W, Lacka B, Gorska J, Keller CK, and Ritz E. Increased prevalence of salt sensitivity of blood pressure in IDDM with and without microalbuminuria. Diabetologia 38: 1443–1448, 1995 [DOI] [PubMed] [Google Scholar]
- 272.Sun P, Yue P, and Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sun Y, Zhang JN, Zhao D, Wang QS, Gu YC, Ma HP, and Zhang ZR. Role of the epithelial sodium channel in salt-sensitive hypertension. Acta Pharmacol Sin 32: 789–797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, Koike H, Shimizu F, and Kawachi H. Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol 170: 1841–1853, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Szentivanyi M, Jr., Zou AP, Mattson DL, Soares P, Moreno C, Roman RJ, and Cowley AW, Jr., Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 283: R266–R272, 2002 [DOI] [PubMed] [Google Scholar]
- 276.Tang Y. and Zhou L. Characterization of adenosine A1 receptors in human proximal tubule epithelial (HK-2) cells. Receptors Channels 9: 67–75, 2003 [PubMed] [Google Scholar]
- 277.Tanito M, Nakamura H, Kwon YW, Teratani A, Masutani H, Shioji K, Kishimoto C, Ohira A, Horie R, and Yodoi J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid Redox Signal 6: 89–97, 2004 [DOI] [PubMed] [Google Scholar]
- 278.Thaete LG, Crouch RK, Schulte BA, and Spicer SS. The immunolocalization of copper-zinc superoxide dismutase in canine tissues. J Histochem Cytochem 31: 1399–1406, 1983 [DOI] [PubMed] [Google Scholar]
- 279.Thaete LG, Crouch RK, and Spicer SS. Immunolocalization of copper-zinc superoxide dismutase. II. Rat. J Histochem Cytochem 33: 803–808, 1985 [DOI] [PubMed] [Google Scholar]
- 280.Thomas D, Harris PJ, and Morgan TO. Age-related changes in angiotensin II-stimulated proximal tubule fluid reabsorption in the spontaneously hypertensive rat. J Hypertens Suppl 6: S449–S451, 1988 [DOI] [PubMed] [Google Scholar]
- 281.Thomas SR, Witting PK, and Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 10: 1713–1765, 2008 [DOI] [PubMed] [Google Scholar]
- 282.Thomson SC, Vallon V, and Blantz RC. Resetting protects efficiency of tubuloglomerular feedback. Kidney Int Suppl 67: S65–S70, 1998 [DOI] [PubMed] [Google Scholar]
- 283.Tian XY, Wong WT, Leung FP, Zhang Y, Wang YX, Lee HK, Ng CF, Chen ZY, Yao X, Au CL, Lau CW, Vanhoutte PM, Cooke JP, and Huang Y. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin f(2alpha) impairs endothelial function in renovascular hypertensive rats. Antioxid Redox Signal 16: 363–373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Toda N. and Okamura T. Modulation of renal blood flow and vascular tone by neuronal nitric oxide synthase-derived nitric oxide. J Vasc Res 48: 1–10, 2011 [DOI] [PubMed] [Google Scholar]
- 285.Tomaschitz A, Pilz S, Ritz E, Obermayer-Pietsch B, and Pieber TR. Aldosterone and arterial hypertension. Nat Rev Endocrinol 6: 83–93, 2010 [DOI] [PubMed] [Google Scholar]
- 286.Troncoso Brindeiro CM, Lane PH, and Carmines PK. Tempol prevents altered K(+) channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension 59: 657–664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Valentin F, Field MC, and Tippins JR. The mechanism of oxidative stress stabilization of the thromboxane receptor in COS-7 cells. J Biol Chem 279: 8316–8324, 2004 [DOI] [PubMed] [Google Scholar]
- 288.Vallon V, Traynor T, Barajas L, Huang YG, Briggs JP, and Schnermann J. Feedback control of glomerular vascular tone in neuronal nitric oxide synthase knockout mice. J Am Soc Nephrol 12: 1599–1606, 2001 [DOI] [PubMed] [Google Scholar]
- 289.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, and Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics 16: 29–37, 2003 [DOI] [PubMed] [Google Scholar]
- 290.Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, and Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys 363: 91–97, 1999 [DOI] [PubMed] [Google Scholar]
- 291.Vanhoutte PM, Feletou M, and Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol 144: 449–458, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Vaziri ND, Wang XQ, Oveisi F, and Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 36: 142–146, 2000 [DOI] [PubMed] [Google Scholar]
- 293.Wang D, Chabrashvili T, and Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ Res 94: 1436–1442, 2004 [DOI] [PubMed] [Google Scholar]
- 294.Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego Conde LJ, Umans JG, and Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. J Am Soc Nephrol 14: 2783–2789, 2003 [DOI] [PubMed] [Google Scholar]
- 295.Wang D, Jose P, and Wilcox CS. beta(1) Receptors protect the renal afferent arteriole of angiotensin-infused rabbits from norepinephrine-induced oxidative stress. J Am Soc Nephrol 17: 3347–3354, 2006 [DOI] [PubMed] [Google Scholar]
- 296.Wang H, D'Ambrosio MA, Garvin JL, Ren Y, and Carretero OA. Connecting tubule glomerular feedback mediates acute tubuloglomerular feedback resetting. Am J Physiol Renal Physiol 302: F1300–F1304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wang H, Garvin JL, D'Ambrosio MA, Ren Y, and Carretero OA. Connecting tubule glomerular feedback antagonizes tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 299: F1374–F1378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wang T. Role of iNOS and eNOS in modulating proximal tubule transport and acid-base balance. Am J Physiol Renal Physiol 283: F658–F662, 2002 [DOI] [PubMed] [Google Scholar]
- 299.Wang T, Inglis FM, and Kalb RG. Defective fluid and HCO(3)(−) absorption in proximal tubule of neuronal nitric oxide synthase-knockout mice. Am J Physiol Renal Physiol 279: F518–F524, 2000 [DOI] [PubMed] [Google Scholar]
- 300.Wang X, Escano CS, Asico L, Jones JE, Barte A, and Jose PA. Upregulation of renal D5 dopamine receptor ameliorates hypertension in D3-deficient mice. High Blood Pressure Council Research 2012 Scientific Sessions 133, 2012 [Google Scholar]
- 301.Welch WJ, Baumgartl H, Lubbers D, and Wilcox CS. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int 59: 230–237, 2001 [DOI] [PubMed] [Google Scholar]
- 302.Welch WJ, Baumgartl H, Lubbers D, and Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat: role of AT1 receptors. Kidney Int 63: 202–208, 2003 [DOI] [PubMed] [Google Scholar]
- 303.Welch WJ, Blau J, Xie H, Chabrashvili T, and Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22–H28, 2005 [DOI] [PubMed] [Google Scholar]
- 304.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, and Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48: 934–941, 2006 [DOI] [PubMed] [Google Scholar]
- 305.Welch WJ, Mendonca M, Aslam S, and Wilcox CS. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K,1C kidney. Hypertension 41: 692–696, 2003 [DOI] [PubMed] [Google Scholar]
- 306.Welch WJ, Mendonca M, Blau J, Karber A, Dennehy K, Patel K, Lao YS, Jose PA, and Wilcox CS. Antihypertensive response to prolonged tempol in the spontaneously hypertensive rat. Kidney Int 68: 179–187, 2005 [DOI] [PubMed] [Google Scholar]
- 307.Welch WJ, Ott CE, Guthrie GP, Jr., and Kotchen TA. Mechanism of increased renin release in the adrenalectomized rat. Adrenal insufficiency and renin. Hypertension 5: I47–I52, 1983 [DOI] [PubMed] [Google Scholar]
- 308.Welch WJ, Patel K, Modlinger P, Mendonca M, Kawada N, Dennehy K, Aslam S, and Wilcox CS. Roles of vasoconstrictor prostaglandins, COX-1 and −2, and AT1, AT2, and TP receptors in a rat model of early 2K,1C hypertension. Am J Physiol Heart Circ Physiol 293: H2644–H2649, 2007 [DOI] [PubMed] [Google Scholar]
- 309.Welch WJ, Tojo A, Lee JU, Kang DG, Schnackenberg CG, and Wilcox CS. Nitric oxide synthase in the JGA of the SHR: expression and role in tubuloglomerular feedback. Am J Physiol 277: F130–F138, 1999 [DOI] [PubMed] [Google Scholar]
- 310.Welch WJ, Tojo A, and Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000 [DOI] [PubMed] [Google Scholar]
- 311.Welch WJ. and Wilcox CS. Potentiation of tubuloglomerular feedback in the rat by thromboxane mimetic. Role of macula densa. J Clin Invest 89: 1857–1865, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Welch WJ. and Wilcox CS. AT1 receptor antagonist combats oxidative stress and restores nitric oxide signaling in the SHR. Kidney Int 59: 1257–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 313.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, and Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 314.Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, and Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension 54: 338–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep 4: 160–166, 2002 [DOI] [PubMed] [Google Scholar]
- 316.Wilcox CS. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol Scand 179: 217–223, 2003 [DOI] [PubMed] [Google Scholar]
- 317.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 318.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126: 119–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wilcox CS. and Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60: 418–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wilcox CS. and Welch WJ. Macula densa nitric oxide synthase: expression, regulation, and function. Kidney Int Suppl 67: S53–S57, 1998 [DOI] [PubMed] [Google Scholar]
- 321.Wilcox CS. and Welch WJ. Oxidative stress: cause or consequence of hypertension. Exp Biol Med (Maywood) 226: 619–620, 2001 [DOI] [PubMed] [Google Scholar]
- 322.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, and Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci U S A 89: 11993–11997, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wilcox CS, Welch WJ, Schreiner GF, and Belardinelli L. Natriuretic and diuretic actions of a highly selective adenosine A1 receptor antagonist. J Am Soc Nephrol 10: 714–720, 1999 [DOI] [PubMed] [Google Scholar]
- 324.Williams JM, Sarkis A, Hoagland KM, Fredrich K, Ryan RP, Moreno C, Lopez B, Lazar J, Fenoy FJ, Sharma M, Garrett MR, Jacob HJ, and Roman RJ. Transfer of the CYP4A region of chromosome 5 from Lewis to Dahl S rats attenuates renal injury. Am J Physiol Renal Physiol 295: F1764–F1777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Xu H, Fink GD, Chen A, Watts S, and Galligan JJ. Nitric oxide-independent effects of tempol on sympathetic nerve activity and blood pressure in normotensive rats. Am J Physiol Heart Circ Physiol 281: H975–H980, 2001 [DOI] [PubMed] [Google Scholar]
- 326.Xu H, Fink GD, and Galligan JJ. Nitric oxide-independent effects of tempol on sympathetic nerve activity and blood pressure in DOCA-salt rats. Am J Physiol Heart Circ Physiol 283: H885–H892, 2002 [DOI] [PubMed] [Google Scholar]
- 327.Xu H, Fink GD, and Galligan JJ. Tempol lowers blood pressure and sympathetic nerve activity but not vascular O2- in DOCA-salt rats. Hypertension 43: 329–334, 2004 [DOI] [PubMed] [Google Scholar]
- 328.Xu L, Dixit MP, Chen R, Dixit NM, Collins JF, and Ghishan FK. Effects of angiotensin II on NaPi-IIa co-transporter expression and activity in rat renal cortex. Biochim Biophys Acta 1667: 114–121, 2004 [DOI] [PubMed] [Google Scholar]
- 329.Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, and Singhal PC. ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol 299: C488–C496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Yalavarthy R, Smith ML, and Edelstein C. Acute kidney injury complicating minimal change disease: the case for careful use of diuretics and angiotensin-converting enzyme inhibitors. Nephrology (Carlton) 12: 529–531, 2007 [DOI] [PubMed] [Google Scholar]
- 331.Yang H, Shi M, VanRemmen H, Chen X, Vijg J, Richardson A, and Guo Z. Reduction of pressor response to vasoconstrictor agents by overexpression of catalase in mice. Am J Hypertens 16: 1–5, 2003 [DOI] [PubMed] [Google Scholar]
- 332.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA, and Zeng C. Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens 30: 1176–1184, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Yang J, Pollock JS, and Carmines PK. NADPH oxidase and PKC contribute to increased Na transport by the thick ascending limb during type 1 diabetes. Hypertension 59: 431–436, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yang LE, Leong PK, and McDonough AA. Reducing blood pressure in SHR with enalapril provokes redistribution of NHE3, NaPi2, and NCC and decreases NaPi2 and ACE abundance. Am J Physiol Renal Physiol 293: F1197–F1208, 2007 [DOI] [PubMed] [Google Scholar]
- 335.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, and Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol 290: R96–R104, 2006 [DOI] [PubMed] [Google Scholar]
- 336.Ye S, Gamburd M, Mozayeni P, Koss M, and Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens 11: 723–728, 1998 [DOI] [PubMed] [Google Scholar]
- 337.Ye S, Zhong H, Yanamadala S, and Campese VM. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension 48: 309–315, 2006 [DOI] [PubMed] [Google Scholar]
- 338.Yip KP, Tse CM, McDonough AA, and Marsh DJ. Redistribution of Na+/H+ exchanger isoform NHE3 in proximal tubules induced by acute and chronic hypertension. Am J Physiol 275: F565–F575, 1998 [DOI] [PubMed] [Google Scholar]
- 339.Yoshimura M, Kambara S, Okabayashi H, Takahashi H, and Ijichi H. Effect of decreased dopamine synthesis on the development of hypertension induced by salt loading in spontaneously hypertensive rats. Clin Exp Hypertens A 9: 1141–1157, 1987 [DOI] [PubMed] [Google Scholar]
- 340.Yu L, Bao HF, Self JL, Eaton DC, and Helms MN. Aldosterone-induced increases in superoxide production counters nitric oxide inhibition of epithelial Na channel activity in A6 distal nephron cells. Am J Physiol Renal Physiol 293: F1666–F1677, 2007 [DOI] [PubMed] [Google Scholar]
- 341.Zatz R. and Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension 32: 958–964, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zelko IN. and Folz RJ. Extracellular superoxide dismutase functions as a major repressor of hypoxia-induced erythropoietin gene expression. Endocrinology 146: 332–340, 2005 [DOI] [PubMed] [Google Scholar]
- 343.Zeng C. and Jose PA. Dopamine receptors: important antihypertensive counterbalance against hypertensive factors. Hypertension 57: 11–17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, and Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res 99: 494–500, 2006 [DOI] [PubMed] [Google Scholar]
- 345.Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, and Jose PA. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension 45: 804–810, 2005 [DOI] [PubMed] [Google Scholar]
- 346.Zhan CD, Sindhu RK, Pang J, Ehdaie A, and Vaziri ND. Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: effect of antioxidant-rich diet. J Hypertens 22: 2025–2033, 2004 [DOI] [PubMed] [Google Scholar]
- 347.Zhang H, Luo Y, Zhang W, He Y, Dai S, Zhang R, Huang Y, Bernatchez P, Giordano FJ, Shadel G, Sessa WC, and Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol 170: 1108–1120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhang Z, Rhinehart K, Solis G, Pittner J, Lee-Kwon W, Welch WJ, Wilcox CS, and Pallone TL. Chronic ANG II infusion increases NO generation by rat descending vasa recta. Am J Physiol Heart Circ Physiol 288: H29–H36, 2005 [DOI] [PubMed] [Google Scholar]
- 349.Zhou Y. and Boron WF. Role of endogenously secreted angiotensin II in the CO2-induced stimulation of HCO3 reabsorption by renal proximal tubules. Am J Physiol Renal Physiol 294: F245–F252, 2008 [DOI] [PubMed] [Google Scholar]
- 350.Zoccali C. The endothelium as a target in renal diseases. J Nephrol 20Suppl 12: S39–S44, 2007 [PubMed] [Google Scholar]
- 351.Zou AP, Wu F, and Cowley AW, Jr., Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension 31: 271–276, 1998 [DOI] [PubMed] [Google Scholar]