Abstract
Significance: Reactive oxygen species (ROS) are produced during normal endoplasmic reticulum (ER) metabolism. There is accumulating evidence showing that under stress conditions such as ER stress, ROS production is increased via enzymes of the NADPH oxidase (Nox) family, especially via the Nox2 and Nox4 isoforms, which are involved in the regulation of blood pressure. Hypertension is a major contributor to cardiovascular and renal disease, and it has a complex pathophysiology involving the heart, kidney, brain, vessels, and immune system. ER stress activates the unfolded protein response (UPR) signaling pathway that has prosurvival and proapoptotic components. Recent Advances: Here, we summarize the evidence regarding the association of Nox enzymes and ER stress, and its potential contribution in the setting of hypertension, including the role of other conditions that can lead to hypertension (e.g., insulin resistance and diabetes). Critical Issues: A better understanding of this association is currently of great interest, as it will provide further insights into the cellular mechanisms that can drive the ER stress-induced adaptive versus maladaptive pathways linked to hypertension and other cardiovascular conditions. More needs to be learnt about the precise signaling regulation of Nox(es) and ER stress in the cardiovascular system. Future Directions: The development of specific approaches that target individual Nox isoforms and the UPR signaling pathway may be important for the achievement of therapeutic efficacy in hypertension. Antioxid. Redox Signal. 20, 121–134.
Introduction
The Endoplasmic Reticulum (ER) is present in all eukaryotic organisms and evolves in a context of metabolic compartmentation that is linked to special protein processing to keep intra-organelle, cell-to-cell interaction, and homeostasis (52). Perturbations in normal ER function may result in not only ER stress, which is mainly related to the accumulation of unfolded/misfolded proteins in the ER, but also calcium deregulation and redox imbalance. ER stress is related to numerous diseases (108, 115), including many cardiovascular conditions (62) and, more recently, to hypertension (28, 38, 45, 113).
Reactive oxygen species (ROS) are produced during normal cell metabolism in the ER (Fig. 1). However, there is accumulating evidence showing that during ER stress, ROS production is increased. While elevation of protein folding in the ER and mitochondria (39, 86) are thought to be important sources of ROS during ER stress, there is growing evidence showing that NADPH oxidase (Nox)(es) are important sources of ROS in the setting of ER stress (15, 50, 77, 86, 110). Nox(es) enzymes, particularly the Nox2 and Nox4 isoforms, are important regulators of blood pressure with a key role in the development of hypertension (5, 79, 91). A better understanding on the association of Nox-derived ROS and ER stress, and hypertension is currently of great interest, especially regarding the cellular mechanism in response to stress that can drive the adaptive versus maladaptive link to hypertension and other cardiovascular diseases (38). Here, we first summarize the main pathways involving the ER in association with ROS production and the emerging evidence related to Nox-mediated ER stress. We finally explore some examples on its potential contribution to hypertension involving the vasculature and peripheral organ systems, including the role in some other conditions that can lead to hypertension (e.g., insulin-resistance [IR], diabetes). The role of Nox and ER stress in the development of hypertension is also important in the brain and is reviewed in detail by Young et al. (113).
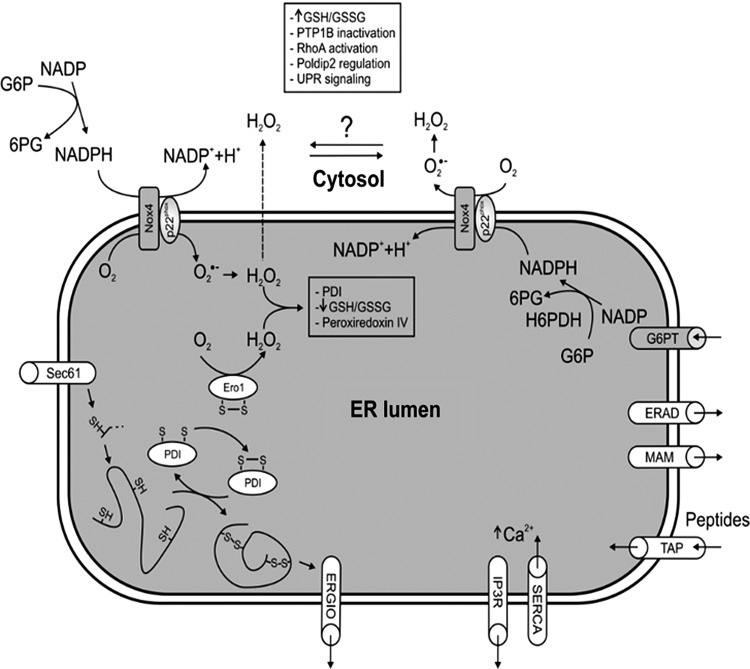
FIG. 1.
General depiction of the main endoplasmic reticulum (ER) communication with other cell compartments, and the main oxidative folding processes in the ER. Possible Nox4 topology and its role are also shown along with extra-ER and possible intra-ER sources of NADPH. G6PT, glucose-6-phosphate transporter; ERAD, ER-associated protein degradation; MAM, mitochondria-associated membrane; ERGIC, ER-Golgi and intermediate compartment.
ER and Basal ROS Production
The ER comprises a continuous interconnected network of tubules and sheets that extend from the nuclear envelope surroundings to spread throughout the cell. It is composed of a single membrane, which can account for more than a half of the total cell membrane complex. This architecture and expanded size reflects the diversity of ER function. Almost one third of total secreted proteins and proteins that belong to the endomembrane system are manufactured in the ER. They traffic in a dynamic way, reach the plasma membrane, and are transported from ER-exit sites to small coat protein II vesicles and then to the Golgi (56). There is also an independent route from the ER-Golgi in certain stages of development (68). The ER is linked to different aspects of lipid metabolism with a role in the synthesis of phospholipids, and triacylglycerol (27, 29), and the ER lumen also contains some enzymes of gluconeogenesis and fatty acid oxidation. The ER also stores high amounts of calcium that participate in protein N-glycosylation and protein folding (40). Calcium is also critical for ionic regulation and calcium cycles in and out of the ER to the cytosol to control second-messenger cascades and cell contraction. Calcium release from the ER can be sensed by mitochondria and induce cell death (9, 54). Indeed, the ER physically communicates with mitochondria at sites called mitochondria-associated membrane (MAM) (93); to other cell compartments, including the nucleus; to the proteasome through ER-associated protein degradation (ERAD); and to the Golgi apparatus ER-Golgi and intermediate compartment (ERGIC) (Fig. 1). The ER also fuses to lysosomes mediating phagocytosis (25), and it is an important source of autophagy vesicles (111).
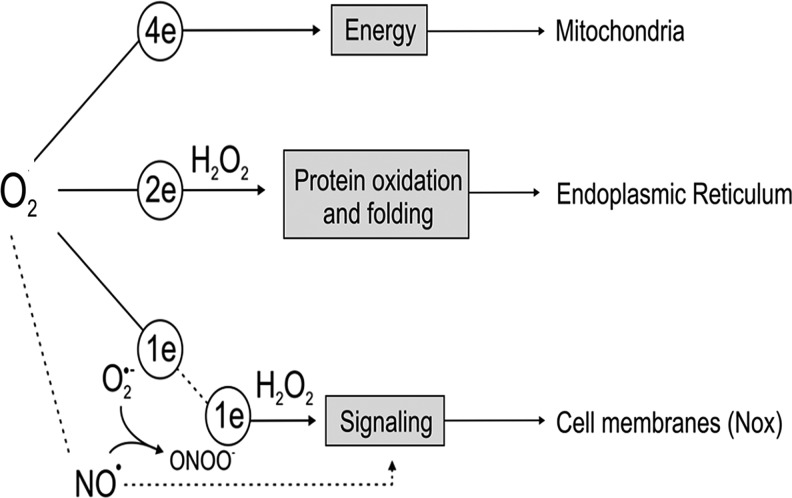
The ER has a distinctive feature compared with most cell organelles possessing a strong oxidizing lumen that is primarily related to protein oxidation and protein folding, and where oxygen plays a central role. For a long time, it has been known that oxygen utilization in the ER can mediate hydroxylation reactions that lead, for instance, to the oxidation of amino acids such as proline residues to hydroxyproline by prolyl-hydroxylase (PHD), a key step in the processing and synthesis of collagen and other adhesion molecules. From an evolutionary perspective, this ability is a hallmark in the transition of unicellular to multicellular organisms and metazoans to guarantee cell–cell contact and adhesion (11) (Fig. 2). PHD also mediates similar protein oxidation in the extra-ER space to regulate some transcriptional factors in response to stress, for example, the hypoxia-inducible factor.
FIG. 2.
Main destination of oxygen metabolism in eukaryotic organism. Total reduction of O2 to H2O in mitochondria yields energy to form ATP. Partial 2-electron (2e) reduction of O2 by ER oxireductin (Ero1) in the ER yields H2O2 and generates electrons to mainly oxidise thiols and to promote protein folding via protein disulfide isomerase. In contrast, partial reduction of O2 yields O2•− (and hence H2O2) by oxidases (e.g., Nox in cell membrane) and is key in cell signaling to regulate kinase/phosphatase and transcription factors. Partial reduction of O2 in mitochondria is also related to increased levels of superoxide and H2O2 within the cell, although it is not the main signaling route. On the other hand, oxygen in mitochondria is consumed to mediate other metabolic reactions that not only yield energy. In the cell membrane, oxygen is used by nitric oxide synthases to produce nitric oxide (NO•), a major regulator of vascular signaling and blood pressure. It should be noted that NO• reacts with O2•−, producing the strong oxidant peroxynitrite (ONOO−) that can induce nitration of aminoacids such as nitro-tyrosine, an important marker of the balance of NO• and O2•− in the cardiovascular system (50, 55). Finally, oxygen is a direct substrate of oxidases as prolyl or lysyl hydroxylase present in the ER (e.g., synthesis of collagen by prolyl hydroxylase dioxygenase (PDH4)) and cytosol (e.g., hypoxia-inducible factor hydroxylation by PHD1-3). In this case, an O2 molecule is added to the substrate proline or lysine.
In addition, the oxidizing environment of the ER promotes protein folding introducing disulfide bonds into nascent proteins (Eqs 1–4). The combined action of the ER oxireductin (Ero1) family of sulfhydryl oxidase and protein disulfide isomerase (PDI) is the main driving force for thiol oxidation in this process. Ero1 catalytic site has vicinal cysteine motifs (Cys-X-X-Cys, where X is a non Cys amino acid) and is bound to a flavin adenine dinucleotide (FAD), which is oxidized by molecular oxygen to produce H2O2 [Eq. 1] (33, 98, 102). In the process, oxidizing equivalents are used to form Ero1 disulfides [Eq. 2] that are further shuttled to form PDI disulfides [Eq. 3]. The oxidized PDI ultimately introduces disulfides to nascent proteins [Pt in Eq. 4] (Fig. 2). PDI is an abundant ER chaperone bearing special redox features (i.e., low pKa of the cysteine that is within two thioredoxin-like motifs, Trp-Cys-Gly-His-Cys), which, at relatively oxidizing conditions such as in the ER, favors PDI isomerase/oxidase activity (48, 69).
Ero1-(FADH2)+O2→Ero1-(FAD)+H2O2 [1]
Ero1-(FAD+2R-SH)→Ero1-(FADH2+RS-SR) [2]
Ero1-(FADH2+RS-SR)+PDI-(2R-SH)→Ero1-(FADH2+R-SH)+PDI-(RS-SR) [3]
PDI-(RS-SR)+Pt (2R-SH)→PDI-(2R-SH)+Pt (RS-SR) [4]
Although the final destination of H2O2 generated in the process [Eq. 1] is elusive, H2O2 may participate in oxidative folding in different ways (48, 60, 98, 99). For example, H2O2 removal hinders oxidative folding and secretion of IgM by human embryonic kidney cells (HEK) and I29μ+ cells (60). Moreover, H2O2 can react directly with antioxidants such as glutathione (GSH) and ascorbate, and overall contribute to keep an oxidative threshold state within the ER (48, 98). The H2O2 also oxidizes ER-located peroxiredoxin IV (PrxIV), and recently, it was shown that PDI can reduce the PrxIV disulfide (99). In this process, PDI becomes oxidized and is able to further promote disulfide formation of nascent proteins. This is thought to increase efficiency of disulfide formation by H2O2 generated by Ero1 (Eq. 1) or by other alternative sources of H2O2 (48, 59, 98, 120). Indeed, even in the absence of Ero1, protein folding still occurs, and it is suggested that other oxidases, including Nox(es), may compensate for the redox demand in the ER in some circumstances (59, 98). However, whether Nox(es) have a more direct impact on protein disulfide formation in the ER is not known. Nevertheless, the PDI-Ero1-dependent oxidative activity in the ER is balanced to cytosolic glutathione levels, supporting functional redox interplay between these two compartments (59, 86, 98). PDH-mediated oxidation and Ero1-PDI-mediated-disulfide bound formation show that the oxygen molecule is an important component in mediating protein oxidation and folding in the ER (e.g., an O2 molecule is added to the substrate proline in the collagen biomolecule or is converted to H2O2, generating electrons that are shuttled to form a disulfide formation, Eq1-Eq4). This reveals a role for oxygen beyond energy supply (Fig. 2).
General ROS Production During ER Stress
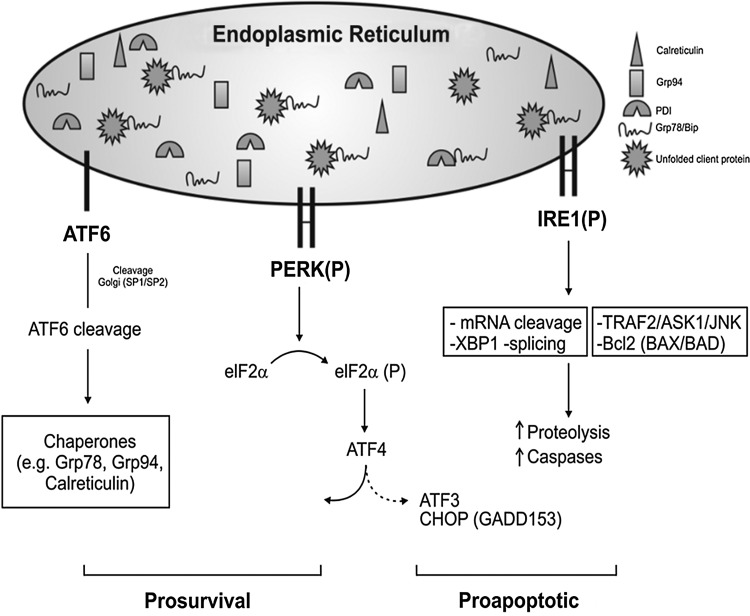
Changes in ER function induce ER stress, which is characterized by a global arrest in protein synthesis and diversion of the translational machinery to increase protein expression of the ER chaperones such as Glucose-related proteins Grp78 (BiP), Grp94, and calreticulin (Fig. 3). Dependent on the intensity or severity of stress, apoptosis is induced, especially via the ER-associated caspases (caspase-12 in many species and equivalent in humans) (65, 82, 83). Increased levels of such molecules are broadly used as ER Stress markers, although these changes may sometimes reflect homeostatic fluctuations of the ER rather than stress conditions per se (82). Common inducers of ER stress include tunicamycin (a potent inhibitor of transfer of N-actelyglucosamine-1-phosphate from UDP-N-acetylglucosamine to dolichol phosphate in the first step of glycoprotein synthesis in the ER) and thapsigargin (a non-competitive inhibitor of ER Ca ATP, known as sarcoplasmic reticulum calcium ATPase). Since the protein folding is strictly related to an oxidizing environment in the ER as discussed earlier, strong reducing agents such as dithiothreitol (DTT) also induce ER stress. The cellular response to ER stress is regulated by the unfolded protein response (UPR) signaling pathway that allows communication from the ER to the nucleus and to other cytoplasmic signaling pathways. There is also much evidence that places the UPR downstream of physiological stimuli (e.g., starvation, hypoxia, and differentiation) that is not necessarily acting via the accumulation of unfolded protein (82).There are excellent reviews on ER stress and the molecular details of the UPR (82, 97, 106, 109) and therefore will not be explored in detail here. Very briefly, the UPR is activated by three ER transmembrane proteins, two kinases (e.g., kinases the protein kinase RNA (PKR)-like ER kinase (PERK) and inositol-requiring protein-1 kinase, Ire1), and transcription factor 6 (or ATF6) (82, 97, 106, 109). Overall, the UPR integrates ER homeostasis to elicit an adaptive and/or proapoptotic response (Fig. 3).
FIG. 3.
A depiction of the unfolded protein response (UPR) signaling pathways. Inositol-requiring protein-1 kinase (Ire1) is the most ancient of all the UPR signaling conserved from yeast to mammalian. It works as an endoribonuclease, slicing the mRNA of the transcription factor X-box binding protein-1 (XBP1). Ire1 is closely linked to apoptosis, activating the c-Jun amino-terminal kinase (JNK) and also caspase-12 via interaction with TRAF2 and with proapoptotic Bcl-2-associated proteins (BAX/BAK), and it can also promote apoptosis via apoptosis signal regulating kinase 1 (ASK1)/JNK signaling. ATF6 is processed by SP1/2 proteins in the Golgi generating cleavage ATF6 that promotes ER stress-related chaperones. PERK: protein kinase RNA (PKR)-like ER kinase (PERK) autophosphorylation promotes the phosphorylation of the α subunit of the downstream eukaryotic initiation factor2 (eIF2α-P) to strongly inhibit mRNA translation and shuts down global protein synthesis (9, 25, 40, 54, 93). In a paradoxical way, this phosphorylation allows the synthesis of certain stress proteins such as the ATF4 transcriptional factor via an open reading frame (ORF)-dependent translation mechanism. Other kinases that promote eIF2α phosphorylation include heme-regulated inhibitor kinase, general control non-derepressible 2 kinase, PKR, and, thus, eIF2α-P is known as a part of the Integrated Stress Response. The ATF4, XBP1, and ATF6 regulate genes encoding ER chaperones, ERAD, and a broad range of metabolic process ranging from aminoacid transport to proteins and phospholipid synthesis and adaptive response. ATF4 along with the ATF6 activation is more associated to survival outcome in response to ER stress. In contrast, increased levels of CHOP (or Gadd153) expression along with IRE1 activation are related to more pro-apoptotic events in response to ER stress.
A common feature of the cellular ER stress and UPR activation is an increase in ROS generation due to a decrease in the level of antioxidants and an increase in the threshold of ROS generation. There are a vast number of publications showing that antioxidants usually attenuate the UPR (86). In addition, some antioxidants such as the lipid-soluble butylated hydroxyanisole were shown to improve protein secretion from the ER in mammalian cells loaded with misfolded proteins (55). The main molecular association between the UPR and cellular antioxidant response is via PERK. PERK autophosphorylation is well known to promote the phosphorylation of the α subunit of the eukaryotic initiation factor 2 (eIF2α-P) to strongly inhibit mRNA translation shutting down global protein synthesis (106). In a paradoxical way, eIF2α phosphorylation allows the synthesis of certain stress proteins such as activating ATF4 (Fig. 3) (106). ATF4 regulates genes related to cell metabolism, including those involved in antioxidant responses such as glutathione biosynthesis and glutathione transferase, the transport of cysteine amino acids, and homocysteine metabolism. Not surprisingly, cells lacking PERK are deficient in GSH synthesis and have increased basal levels of H2O2 accumulation (26, 35, 49). PERK has also been related to activation of the transcription factor Nrf2 (22, 23). Nrf2 is well known to activate antioxidant responses, and it was shown that during the UPR, Nrf2 plays a role in maintaining GSH levels which buffer the accumulation of ROS (22). Cells lacking Nrf2 have a deleterious outcome, which is attenuated by restoring GSH or reintroducing Nrf2 (22). In all these cases, the inhibition of ROS attenuates apoptosis induction after ER stress (22, 35, 49, 86). Another important redox aspect linked to ATF4, although not restricted to ATF4 (59), is the regulation of the transcription factor C/EBP homologous protein (CHOP, also known as GADD153) (58). CHOP is important for promoting Ero1 expression/activation, and CHOP−/− cells show a relative hypo-oxidizing environment and, therefore, preventing the formation of abnormal protein aggregates in response to ER stress (58). Such results are in tune with decreased levels of ROS when Ero-1 was knocked down in PERK-1-null worms (35). This is intriguing, as it was recently shown that chemical and physiological ER stressors also produce a hypo-oxidized ER environment in yeast cells (61).
Increases in ROS production have been detected under different conditions of ER stress or UPR signaling activation (4, 15, 22, 35, 77, 86, 110). There is always an early increase in ROS generation that possibly reflects a more direct response to ER stress inducers, but later, a secondary oxidative event occurs at more terminal stages of the UPR. Interestingly, ER-associated apoptosis can also feed back to ROS generation under circumstances in which caspase-12 was knocked down (86). While ER protein oxidation (discussed earlier) and mitochondria (39, 86) are thought to be important sources of ROS on ER stress, there is growing evidence showing that Nox(es) are important sources of ROS in the setting of ER stress (15, 77, 86, 110). Next, we review some of the emerging evidence showing the convergence of Nox-derived ROS with ER stress.
Nox-Derived ROS and ER Stress
Nox is a family of enzymes that generate ROS (O2•− and, hence, H2O2). It consumes oxygen and mediates electron transfer using NADPH (Fig. 1). There are seven oxidase family members; each isoform has a distinct catalytic subunit (i.e., Nox1–5 and Duox1 and 2) and also different requirements for additional protein subunits (8, 13, 47, 103, 104). The best studied member of the Nox family, Nox2 oxidase (aka gp91phox oxidase) is known for its role in phagocytic cells, where genetic defects in Nox2 causes chronic granulomatous disease, a condition that leads to recurrent severe fungal and bacterial infections due to defective phagocyte function (104). However, Nox2 is also expressed in many other cell types, and different Nox isoforms are widely expressed in a tissue-specific manner. Recent detailed reviews on the structure, biochemistry and function, and roles of Nox enzymes have been published (8, 47, 103). Nox-dependent redox signaling involves a controlled and local production of low levels of ROS in the surroundings of target proteins, and such effects have been implicated in signaling pathways that regulate almost all cellular processes (8, 47, 103).
The two Nox isoforms so far reported to be involved in ER stress are Nox2 (50) and Nox4 (15, 77, 86, 110) (Fig. 2). Each of these isoforms exists as a membrane-bound heterodimer with a lower molecular weight p22phox subunit, but there are several differences between these isoforms. Nox2 is usually quiescent and is acutely activated by stimuli such as G-protein-coupled receptor (GPCR) agonists (e.g., angiotensin II, endothelin-1), growth factors, phorbol myristate acetate, lipophosphoglycan (LPG), and cytokines in a tightly regulated process promoting cytosolic subunits (p47phox, p67phox, p40phox, and Rac1) to associate with the Nox2–p22phox heterodimer to initiate enzyme activity (22, 23, 26, 58). Nox2 also has electrogenic features (20) and in phagocytes, it is linked to the regulation of phagosome/lysosome pH and protease activity (57, 87, 96). Nox4, however, does not have a requirement for additional regulatory subunits, has constitutive low-level activity, and seems to be regulated largely by changes in expression (8, 47, 103). Thus, Nox4 is generally regarded as an inducible isoform. In addition, recent independent studies from several groups suggest that, in contrast to Nox2, Nox4 may generate H2O2 rather than O2•−, but several papers also show O2•− production (84). Such differences could be due to experimental conditions and methodological issues. Finally, it is clear that the intracellular location of the two isoforms in different cell types is very distinct. Generally, activated Nox2 is found predominantly in the plasma membrane, whereas Nox4 is found intracellularly in the cytoskeleton or focal adhesions (8) and in the mitochondria (1). However, several groups, including our own, have found Nox4 in an ER-related perinuclear location (43, 110, 114).
Nox4 was shown to physically interact with PDI (43), and the loss of PDI resulted in a decrease in Akt phosphorylation and increased cell death in response to Angiotensin-II in vascular smooth muscle cells (43). A functional and spatial/physical interaction between PDI and the p22phox oxidase subunit was shown in macrophages (85) and, more recently, between PDI and p47phox in neutrophils (24). The downstream role of ROS generated by PDI-Nox remains unknown but could be related to ER-mediated phagocytosis (25, 96) or, similar to PDI-Ero1, to protein folding in the macrophage ER to affect antigen processing (Fig. 1). Nox4 located in the ER has been linked to endothelial growth factor (EGF)-induced proliferation of endothelial cells (15). In this case, Nox4 mediated oxidation and inactivation of the protein tyrosine phosphatase PTB1B, which is also found in the ER (15) (Fig. 1). PTP1B can potentiate IRE1-dependent signaling during UPR and, thus, can be an important target of Nox4 during ER stress (34). Finally, using an ER-targeted fluorescent ROS sensor, Wu et al. showed that Nox4 promoted ROS generation in endothelial cells in response to ER stressors such as tunicamycin and HIV-1Tat protein, but not to thapsigargin or DTT (110). They found that Nox4 displayed at the cytosolic face of the ER promoted the activation of Ras-RhoA, a small GTPase protein known to regulate the actin cytoskeleton in the formation of stress fibers. This activation was correlated to an increase in autophagy levels, and this prosurvival response was further supported by increased levels of apoptosis after Nox4 or Atg5 knockdown (110). Thus, the ER surface seems to provide a locus related to Nox4 signaling, promoting a more homeostatic protective response (77, 110). Nox4 has also been related to RhoA regulation in vascular smooth muscle cells (53) (Fig. 1). In this case, Nox4/p22phox was found to interact with the protein polymerase delta interacting protein 2 to increase ROS formation and RhoA activation that had a specific role in focal adhesion and increases stress fiber formation (53).
The full role of Nox4 in the ER is unclear. One important aspect is that Nox4 requires the transmembrane topology characteristic of the Nox family (47, 103, 104), and assuming ER as an extracellular space and the well-known Nox2 topology in the plasma membrane, Nox4-derived ROS would be expected to face the ER lumen. Thus, basal Nox4-derived ROS generation could help the ER to promote protein folding, but whether Nox4 has an impact in protein folding has not yet been shown. An overstimulation of this system as that during ER stress is thought to result in a hyperoxidative ER environment that triggers upstream UPR signals (Figs. 1 and 3). In this pathway, electrons are transferred via NADPH originating from the pentose shunt in the cytosol that would also have redox consequences in the extra-ER space with increased NADPH consumption at the cost of glutathione and thioredoxin reductase activity. Whether Nox4 generates ROS facing the cytosol is not known but in this case, an NADPH source candidate could be hexose 6-phosphate dehydrogenase, a stress-inducible luminal enzyme of the ER that converts glucose-6-phosphate and NADP to 6-phosphogluconate and NADPH, the cofactor for ER luminal reductases such as 11-β-hydroxysteroid dehydrogenase type 1 (92). The different topology of Nox4 in the ER membrane can have an effect on the cytosolic or ER luminal NADPH pools, and thus with potential implications for cell metabolism. However, since H2O2 is permeable and diffuses through cell membranes, Nox4-derived H2O2 is expected to have effects in the surroundings of the ER membrane, especially with regard to the regulation of some general signaling events such as Nox4-mediated regulation of RhoA and PT1B (15, 110).
Nox4 activation in response to ER stress has also been shown in vascular smooth muscle cells (77, 86). Nox4 mRNA and protein levels were significantly induced by an oxidized derivative of cholesterol, 7-ketocholesterol, while the expression of Nox1 and Nox5 mRNA remained unaltered (77). 7-ketocholesterol increased the levels of ROS, an effect totally abrogated by Nox4 siRNA and also inhibited by PEG-superoxide dismutase (SOD) and PEG-catalase, and by the flavoprotein inhibitor diphenylene iodonium (DPI). The loss of Nox4 had early implications on calcium oscillation in response to 7-ketocholesterol (77). Moreover, the lack of Nox4 prevented the expression of several markers of the UPR, including proapoptotic CHOP and Bax proteins and was correlated with the onset of cell death involving c-Jun N-terminal kinase (JNK)-apoptosis signal regulating kinase 1 (ASK1), which is downstream of IRE1 activation (77) (Fig. 3). Similar findings were obtained more recently using rabbit aortic vascular smooth muscle cells (86). In this case, tunicamycin was used as an ER stress inducer and confirmed a marked time and concentration-dependent increase of the levels of Nox4 mRNA, while only marginal changes in the expression of Nox1. Nox4 expression levels were also correlated with increased levels of ROS using more specific methods of DHE-HPLC. The ROS generation was prevented by transfection of full-length GADD34 plasmid, a condition that abolished eIF2α phosphorylation and interrupted the UPR (86). These results suggest a possible participation of Nox4 in the UPR in smooth muscle cells.
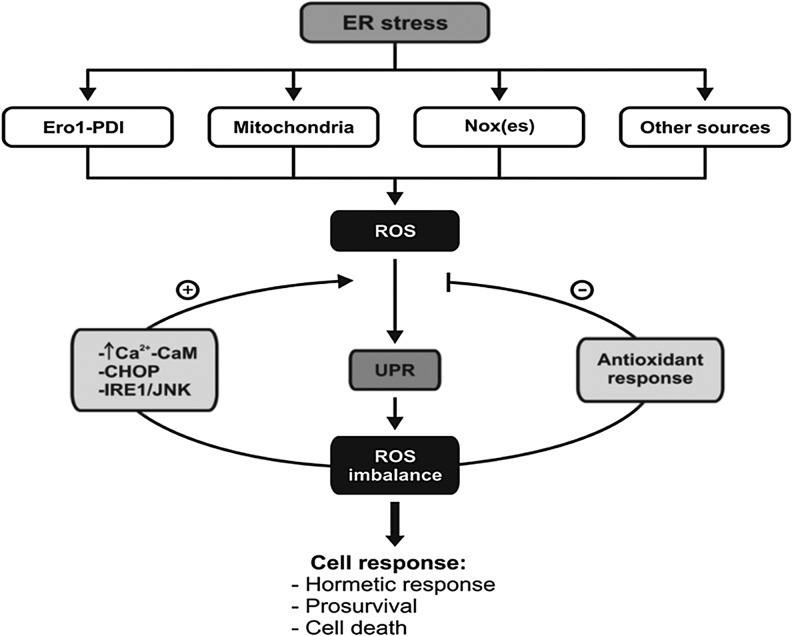
ER-stress-mediated ROS generation in response to cholesterol and 7-ketocholesterol have been shown in other cell types such as endothelial cells and macrophages (50, 100). In this case, however, Nox2 transcription and activation was correlated with increased calcium release from the ER and associated with UPR activation via PKR-eIF2α and CHOP pathway (Fig. 3) (50). Both lipids induced CHOP and calcium release from the ER through the activation of inositol 1, 2, 5-triphosphate receptor type 1 (IP3R1) and Ca2+/calmodulin-dependent protein kinases II (CaMKII), which ultimately resulted in Nox2 activation and ROS generation. Therefore, ER stress induction via CHOP leads to ER increased calcium release (IP3R) and, consequently, activation of Nox2 (50). However, the absence of Nox2 also suppressed CaMKII activation/phosphorylation, which is suggestive that Nox2-derived ROS could be important for sustaining PKR-eIF2α and CHOP induction, thus acting as a positive feedback mechanism in ER stress induction (50). In vivo, Nox2 deficiency protected ER-stressed mice from renal CHOP induction and apoptosis and prevented renal dysfunction (50). Thus, Nox2-derived ROS in association with calcium release is thought to be an important mechanism of ER-stress-mediated apoptosis. One striking observation is the effect of cholesterol and 7-ketocholesterol on the onset of apoptosis (77) that seems to be closely linked with the ER and calcium release effect. Although a better understanding of the UPR signaling and different Nox(es) is needed, these results could, in part, explain some opposing effects where Nox4 is likely more protective (15, 110). Another important aspect is that both mRNA of Nox2 and Nox4 are induced by 7-ketocholesterol (50, 77). There is no mechanism described yet, and it would be very interesting to know whether this regulation is more directly linked to some of the UPR transcription factors or via a Ca2+-dependent mechanism. Nevertheless, these results suggest that Nox4 and Nox2 are important sources of ROS generation in response to ER stress in most cardiovascular cell types. Overall, ROS production likely occurs both upstream and downstream of the ER stress response along with the coordinated expression of ATF4, Nrf2, and CHOP, and the oxidases Ero1, Nox2, and Nox4 may couple ER stress to cellular redox signaling and metabolism, with a prosurvival or proapoptotic outcome (Fig. 4).
FIG. 4.
Upstream and downstream reactive oxygen species (ROS) production in relation to ER stress and UPR. In response to ER stress, ROS production is increased due to different sources, including Nox(es). This ROS is associated to UPR signaling that can activate an antioxidant response (e.g., Nrf2) or increase ROS generation (e.g., Ero1, Nox). This redox balance determines a cell response that can be adaptive or induce cell death.
ER Stress and Nox in Hypertension
Hypertension is a major modifiable risk factor for renal failure, cardiovascular disease, and stroke (67, 101). Hypertension affects 30% of adults in the Western world and is the leading cause of morbidity and mortality worldwide. Although the exact etiology still remains largely unknown, it is clear that hypertension is a multifactorial, complex polygenic disorder with many interacting mechanisms contributing to its pathophysiology and involving many organ systems, including the heart, kidney, brain, vessels, and, more recently, the immune system (37, 67, 101). Factors implicated in the pathophysiology of hypertension include activation of the sympathetic nervous system, up-regulation of the renin-angiotensin-aldosterone system, altered G protein-coupled receptor signaling, and inflammation (36, 105). Common to these processes is oxidative stress, which is primarily due to excess ROS generation, decreased nitric oxide (NO•) levels, and reduced antioxidant capacity in the cardiovascular and renal systems (12, 88).
In the early phases of essential hypertension, cardiac output (CO) is increased and total peripheral resistance (TPR) is normal, but with time, CO drops to normal levels and TPR increases. Many factors contribute toward increasing TPR, including vasoconstriction, endothelial dysfunction, structural remodeling, and vascular inflammation. At the cellular and molecular levels, signaling pathways, including mitogen activated protein kinases, tyrosine kinases, calcium, and GTPases (Rac-Rho), involving cross talk between different receptors (GPCRs and tyrosine kinases) in different vascular cell types (endothelial, smooth muscle, adventitial fibroblasts, and nerve terminals), have been implicated in vascular changes, which contribute to increased TPR in hypertension. Common to these processes is the formation of ROS in the heart, kidney, and vasculature (e.g., endothelial and smooth muscle cells, and fibroblasts) (12). Nitric oxide is the prototype of a free radical regulating blood pressure (Fig. 2), but, more recently, it was discovered that different Nox(es) isoforms in many cell types of the cardiovascular system and brain may be important (8, 13, 103, 104, 118, 119). In pathological conditions, ROS production contributes to the activation of proinflammatory, profibrotic, and mitogenic signaling pathways, leading to oxidative damage in the vasculature, which, in turn, results in increased vasoreactivity, endothelial dysfunction, vascular remodeling, reduced vascular compliance, increased TPR, and elevated BP (6). Thus, ROS is highly regulated under physiological conditions; whereas in disease states, dysregulation of Nox(es) contributes to cardiovascular injury and hypertension. Nox-mediated hypertension has key components in the vasculature (101) and in the central nervous system (118, 119).
Hypertension has been recently linked to ER stress, and there is accumulating evidence that ER stress is an important factor in diabetes and other cardiovascular conditions such as cardiac hypertrophy, heart failure, and atherosclerosis (28, 38, 45, 62, 113). It is well established that increased arterial blood pressure is associated with cardiovascular complications, such as hypertrophy, fibrosis, renal failure, and vascular endothelial dysfunction. Cardiac hypertrophy and fibrosis are well documented in hypertensive animals and patients (72, 76). Hypertension-induced cardiac hypertrophy is a progressive event that is associated with myocardial remodeling which is characterized by fibrosis and alterations in cardiomyocyte size and function. A recent study demonstrated that ER stress is an important factor in vascular dysfunction and cardiac damage in AngII-dependent hypertension (64). AngII infusion also increased the levels of ATF4 and CHOP in aorta and mesenteric resistance arteries (MRA), an effect that was associated with impaired endothelial dependent relaxation (EDR) in both vessels. This effect was reversed after ER stress inhibition. Nox activity was increased in both aorta and MRA from mice injected with tunicamycin, an effect also abolished by ER stress inhibitors. The ER stress marker Grp78 was also increased in both the aorta and MRA. Transforming growth factor beta 1 (TGFβ-1) antagonists restored Grp78 levels and EDR only in the aorta, but no change was observed in MRA. On the other hand, apocynin, a nonspecific Nox inhibitor, completely restored EDR in mesenteric arteries, and no effect was observed in the aorta. The use of apocynin to inhibit Nox is controversial, as in order to inhibit Nox, apocynin requires oxidation and formation of a dimer derivative, which reacts with p47phox cysteine residues and thus inhibits Nox (64). This effect has been well shown in neutrophils which contain myeloperoxidase (MPO), but it is very unlikely that this would apply to Nox4 and it is debatable that this effect would be present in vascular cells which do not have MPO. In fact, apocynin effects on redox status in vascular cells may not be Nox specific, and actions of this methoxy-substituted catechol could vary depending on the cell type studied and whether MPO (or other peroxidases) is functionally present. Therefore, the use of apocynin as an inhibitor of Nox in nonphagocytic cells should be conducted with caution and with the knowledge that, in the absence of peroxidases and H2O2, effects on redox status may be through Nox-independent processes, such as through radical scavenging, which poses this compound as an antioxidant (41). In addition, the observed vasodilator effect of apocycin in aortas treated with ER stress inducers (45) could also be a consequence of the inhibition of Rho/Rho kinase pathway (89). Since RhoA has been shown to be redox modulated (44), it is possible that the decrease in ROS induced by apocynin ultimately contributes to restoring EDR in MRA submitted to ER stress. Therefore, ER stress induces vascular dysfunction through different signaling pathways by a TGFβ-1 dependent mechanism in the conduit arteries and through a ROS/Rho kinase-dependent mechanism in resistance arteries. In addition, studies using cultured cells demonstrated that ROS can also increase ER stress, which suggests that the convergence of these two processes could result in vascular endothelial dysfunction in hypertension (78) (Fig. 5). In fact, TGFβ-1 and ROS generation have been proposed as important factors in the development of vascular complications in hypertension (7, 78) and cardiovascular disease (19). The development of heart failure has neurohumoral and inflammatory components (10), and recent reports suggest that ER stress is involved in cardiac remodeling in Dahl salt-sensitive rats with hypertension, cardiac hypertrophy, and heart failure (42). Interestingly, these authors found a new ER protein PARM-1 (or prostatic androgen repressed message) that is specifically expressed in cardiac cells under hypertension induced by a high salt diet (42). The knockdown of PARM-1 induced apoptosis in cardiac cells in response to ER stress, repressing the expression of PERK and ATF6 to augment expression of CHOP, and without affecting IRE-1 expression or JNK and Caspase-12 activation. Thus, these results suggested that PARM-1 is a novel ER molecule that is involved in cardiac remodeling in hypertensive heart disease (42). ER stress was also involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice (42). Moreover, the inhibition of ER stress attenuates cardiovascular remodeling in aldosterone-salt-treated rats (2). A recent study demonstrated that ER stress is an important player in conduit and resistance vessel dysfunction related to enhanced Nox2 and Nox4 in hypertension (45). In this case, AngII-induced hypertension resulted in increased levels of eIF2α-phosphorylation and ATF4-CHOP that were associated with a marked increase in the levels of Nox4 mRNA. ER stress inhibitors such as the chemical chaperones 4-phenylbutyric acid (PBA) and tauroursodeoxycholic acid (TUDCA) attenuated the stress response and improved EDR and cardiac damage with a decrease in the levels of apoptosis. Interestingly, the levels of Nox4 were down-regulated, suggesting that Nox4 may have a role in the ER stress response in these circumstances (45). It should be noted that vascular dysfunction and cardiac damage are well characterized in aorta and mesenteric resistance vessels in hypertensive animal models and patients (45, 46, 75). It is worth noting that currently PBA and TUDCA are approved for use in humans (18), and PBA also alleviated ER stress response in the mice model of cardiac hypertrophy (81), a known condition related to ER stress (72, 76). PBA is a low-molecular-weight fatty acid and a nontoxic pharmacological compound that has been found to have chaperone-like activity. Its physiochemical properties enable it to stabilize peptide structures, improving the luminal folding capacity and traffic of aberrant proteins.
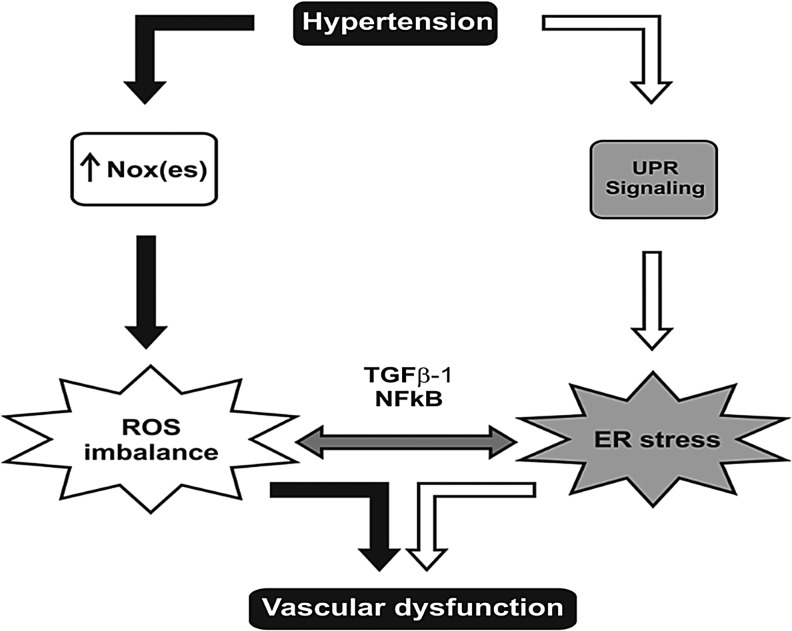
FIG. 5.
Converging pathways that possibly integrate hypertension, oxidative stress, and ER stress. Hypertension increases ER stress, triggering UPR. Nox activity is increased during hypertension, leading to oxidative stress with alterations in vasculature structure and culminating in vascular dysfunction. ROS imbalances can converge with ER stress through the activation of NFkB and transforming growth factor beta 1 (TGFβ-1), and both processes contribute to an increase in ROS generation, which also culminates in vascular dysfunction and hypertension development.
Increased renal Nox-derived ROS production and hypertension is closely associated with kidney damage as illustrated by different models of hypertension induced by a high salt diet in Dahl rats, deoxycorticosterone acetate (DOCA) salt rats, and stroke-prone spontaneously hypertensive rat (SHR) (14), or induced by AngII (63). Nox2 and Nox4 are present in the renal cortex, medulla, and renal vessels (66). A specific Nox2 inhibitor (Nox2ds-tat) (21) normalizes ROS and EDR with no change in blood pressure (116). The role of Nox-mediating ER stress in these models of renal dysfunction remains to be explored, but Nox2 was shown to be associated with ER-stress-induced apoptosis in the kidney (50). Thus, Nox2 may be a prominent link between ER stress and vascular and renal dysfunction in hypertension. The possibility that Nox4 association with ER stress is linked to renal dysfunction remains to be addressed.
ER Stress and Nox in IR and Diabetes
Hypertension also occurs as a part of the metabolic syndrome, which includes IR, type 2 diabetes, and obesity (16, 71). They share common pathogenic mechanisms (80, 90), and have causal roles in hypertension through the direct effects of hyperglycaemia, hyperlipidaemia, and hyperinsulinaemia, leading to vascular dysfunction (17, 95). Both ER stress and ROS production initiate IR, diabetes, and obesity (18, 30, 31, 73, 74), which go on to have direct effects on the peripheral vasculature and result in hypertension. To our knowledge, there are no studies examining the direct links between ER stress, ROS production, and hypertension in the context of metabolic syndrome. Therefore, what follows is an overview of the known mechanisms that are thought to underlie the link between the ER, ROS (with a focus on Nox-related ROS), and components of the metabolic syndrome.
ER stress, ROS, and pancreatic β-cell dysfunction
ROS has been associated with contributing to ER-stress-induced β-cell failure. There is evidence from in vivo models that CHOP may promote apoptosis via oxidative stress. H2O2 can act upstream of ER stress by inducing CHOP-mediated β-cell failure (3). ROS may also act downstream of ER stress. β-cells from CHOP-deficient diabetic mice were more resistant to oxidative stress (94). In addition, using a mouse model deficient in phosphorylated eIF2α, the same authors demonstrated that β-cells exhibited increased apoptosis and oxidative stress, which was attributed to the accumulation of unfolded protein with subsequent downstream ROS production and apoptosis (4). Giving these mice the antioxidant butylated hydroxyanisole reduced β-cell apoptosis and improved glucose homeostasis. Therefore, oxidative stress can act either upstream or downstream of the ER to initiate β-cell dysfunction. In the studies mentioned earlier, it is argued that ROS is generated through the UPR (Ero1-PDI system discussed in the introduction). More recently, a study has directly addressed the question of ROS source implicating Nox-related ROS in ER stress and β-cell dysfunction (51). Moreover, crossing the Akita mouse [a model of Chop-dependent ER stress-induced β-cell dysfunction leading to type 1 diabetes (107)] into a p47phox null background resulted in improvement in diabetes and β-cell function. Indeed, in non-diabetic p47phox knock-out mice, glucose tolerance was found to be better compared with their wild-type littermates and was independent of any effect on insulin sensitivity, thus suggesting that Nox-ROS is contributing to β-cell dysfunction caused by ER stress.
ER stress, ROS, and adipocyte dysfunction
It has been shown that ROS-mediated ER stress can initiate IR in adipocytes. In 3T3-L1 adipocytes [which are known to express Nox2 and Nox4 (8)], exposure to chronic insulin to mimic a hyperinsulinaemic state attenuated the phosphorylation of the insulin receptor and downstream targets such as Akt, GSK-3β, and p70S6K, which could be restored with N-acetyl-cysteine (NAC) treatment (32). In addition, superoxide and H2O2 generation were increased (as measured by DHE and CM-dichloro fluorescence (DCF) respectively), an effect attenuated by NAC. DPI treatment could also reduce free radical formation (as assessed by electron paramagnetic resonance spin trapping). In addition, treatment with NAC, SOD, catalase, or DPI could restore glucose uptake. Importantly, Grp78 and phosphorylated eIF2α were increased with chronic insulin exposure, which could be inhibited by NAC treatment. JNK activation was also reversed by NAC. In another study involving 3T3-L1 adipocytes exposed to advanced oxidation protein products (which accumulate in diabetes and obesity), it was shown that insulin-stimulated glucose uptake and Akt phosphorylation were reduced. ROS generation (assessed using intracellular fluoroprobe DCF) was increased and was prevented by DPI and apocynin but not with other ROS-source inhibitors, including rotenone, Nw-Nitro-L-arginine methyl ester hydrochloride (L-NAME), or allopurinol. Advanced oxidation protein products also led to increased phosphorylation of p47phox and its association with p22phox as well as increased Nox expression. In addition, DPI or apocynin were capable of abolishing the phosphorylation of PERK, eIF2α, IRE1, and JNK and Grp78 over-expression. They further go on to show that the activation of inflammation through phosphorlyation of NF-KB and overexpression of NF-KB and IL-6 were dependent on Noxs and ER stress (117). Another in vitro study has implicated ROS production in leading to ER stress and autophagy in a model of inflammation-induced adipogenesis (112). Data on adipose tissue involvement in IR and obesity are limited to in vitro studies, but point toward a role for Nox-mediated ROS in ER stress-induced metabolic disturbance. Altogether, these results are suggestive of a role for adipose tissue Nox in ROS generation during diabetes and obesity.
ER stress, ROS, and hepatocyte dysfunction
In Zucker obese fatty (ZOF) rats, hepatic steatosis and IR could be improved with ezetimibe treatment (which inhibits the cholesterol transporter, Niemann-Pick C1-like 1) (70). ZOF rats treated with ezetimibe showed reduced DHE-fluorescence as well as decreased phosphorylation of JNK and p38. A high fat diet fed ZOF rat liver had increased mRNA expression of Nox components p47phox and p67phox, which were inhibited by ezetimibe. PERK, ATF6, and CHOP were also down-regulated by ezetimibe treatment. In vitro studies using human primary hepatocytes treated with free cholesterol (co-administered with tumor necrosis factor or Lipopolysaccharide to mimic steatosis) showed that ezetimibe could restore insulin sensitivity (as assessed by Akt phosphorylation). Ezetimibe also caused a decrease in DHE fluorescence and down-regulation of the mRNA for subunits p47phox and p67phox. They also showed that ezetimibe reduced JNK and p38 activation. Cholesterol-treated hepatocytes had up-regulation of mRNA for PERK, ATF6, and CHOP, but to a lesser degree. However, to show that there was a direct involvement of Nox and ER stress due to cholesterol uptake via NPC1L1, it was knocked down in steatotic hepatic HuH7 cells using shRNA. This was able to not only reduce cholesterol accumulation but also increase Akt phosphorylation and reduce PERK, ATF6, and CHOP mRNA as well as reduce DHE-fluorescence and mRNA of p47phox and p67phox. These results, therefore, suggest that hepatic steatosis results in decreased insulin sensitivity that is associated with Nox2-derived ROS and ER stress. However, they fall short of demonstrating whether ROS was linked upstream or downstream to ER stress. In summary, ER stress can indirectly cause hypertension through IR, diabetes, and obesity, in which Nox-derived ROS can exert its effects upstream and downstream of the ER stress response (Fig. 5).
Conclusions
In this article, we have reviewed the main cellular and pathological pathways that are involved in the Nox-derived ROS production during ER stress. Nox2 and Nox4 isoforms are particularly involved in the modulation of the ER stress response, and various cellular signaling pathways that are associated with the UPR cascade are gradually being identified. Nox2 seems to have a role in inducing apoptosis (e.g., ER stress-CHOP-CaMKII-Nox activation-cell death) in response to ER stress with a role in atherosclerosis (Fig. 4). The role of Nox4 is less clear, but it appears to be protective in some settings, although the molecular mechanisms remain to be defined. It is important to note that the UPR evoked by ER stress can be adaptive as well as detrimental (Fig. 3). There is accumulating evidence supporting a role of ER stress and UPR in the pathophysiology of cardiac hypertrophy, IR and diabetes, and a more direct role in hypertension still needs to be better defined. Future work in the field needs to address several key aspects, which include (i) compartment-specific Nox regulation and signaling events in the settings of ER stress; (ii) mechanisms that regulate Nox-derived ROS integrated with ER function and ER stress (or the UPR); (iii) the detrimental and/or protective roles of specific antioxidants in ER stress, and the opposite, the impact of chemical chaperones (e.g., TUDCA and PBA) in Nox-derived signaling regulation; and thus (iv) the identification of key molecular mechanisms that could be therapeutically targeted. A better understanding of this complex regulatory system may allow the development of more specific therapeutic strategies for hypertension and its associated cardiovascular conditions.
Abbreviations Used
- ATF4
transmembrane transcription factor-4
- CAMKII
Ca2+/calmodulin-dependent protein kinases II
- CHOP
CCAAT/enhancer binding protein
- CO
cardiac output
- DCF
dichloro fluorescence
- DHE-HPLC
dihydroethidium-high performance liquid chromatography
- DPI
diphenylene iodonium
- EDR
endothelium dependent relaxation
- eIF2α
eukaryotic initiation factor2, subunit alpha
- EPR
electron paramagnetic resonance
- ER
endoplasmic reticulum
- ERAD
ER-associated protein degradation
- Ero1
ER oxireductin
- G6PDH
glucose-6-phosphate dehydrogenase
- GPCR
G-protein-coupled receptor
- Hif
hypoxia-inducible factor
- IP3R
inositol 1, 2, 5-triphosphate receptor type 1
- IR
insulin resistance
- Ire1
inositol-requiring protein-1 kinase
- JNK
c-Jun N-terminal kinase
- MPO
myeloperoxidase
- MRA
mesenteric resistance arteries
- NAC
N-acetyl-cysteine
- NOS
nitric oxide synthase
- Nox
NADPH oxidase
- Nrf2
NF-E2-related factor-2
- PARM-1
prostatic androgen repressed message
- PBA
4-phenylbutyric acid
- PDI
protein disulfide isomerase
- PERK
kinases the protein kinase RNA (PKR)-like ER kinase
- PHD
prolyl hydroxylase dioxygenase
- PrxIV
peroxiredoxin IV
- ROS
reactive oxygen species
- SERCA
sarcoplasmic reticulum calcium ATPase
- SOD
superoxide dismutase
- TGFβ1
transforming growth factor beta 1
- TNFα
tumor necrosis factor alfa
- TPR
total peripheral resistance
- TUDCA
tauroursodeoxycholic acid
- UPR
unfolded protein response
- XBP1
X-box binding protein-1
- ZOF
Zucker obese fatty
Acknowledgments
C.X.C.S. and A.M.S. are supported by the British Heart Foundation (RG/08/011/25922; CH/99001; RE/08/003), a Leducq Foundation Transatlantic Network of Excellence award, and a National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust. A.A.N. is supported by a UK Medical Research Council Clinical Research Training Fellowship (G1100441). L.R.L. is supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). L.L.C. is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- 1.Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias-Loza PA, Hu K, Dienesch C, Mehlich AM, Konig S, Jazbutyte V, Neyses L, Hegele-Hartung C, Heinrich Fritzemeier K, and Pelzer T. Both estrogen receptor subtypes, alpha and beta, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension 50: 432–438, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ariyama Y, Tanaka Y, Shimizu H, Shimomura K, Okada S, Saito T, Yamada E, Oyadomari S, and Mori M. The role of CHOP messenger RNA expression in the link between oxidative stress and apoptosis. Metabolism 57: 1625–1635, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, and Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab 10: 13–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, and Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 100: 1016–1025, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Berk BC. Redox signals that regulate the vascular response to injury. Thromb Haemost 82: 810–817, 1999 [PubMed] [Google Scholar]
- 7.Blum A. and Miller H. Pathophysiological role of cytokines in congestive heart failure. Annu Rev Med 52: 15–27, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Brandes RP, Weissmann N, and Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med 49: 687–706, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, Iglewski M, Chiong M, Simmen T, Zorzano A, Hill JA, Rothermel BA, Szabadkai G, and Lavandero S. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci 124: 2143–2152, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brilla CG. Renin-angiotensin-aldosterone system and myocardial fibrosis. Cardiovasc Res 47: 1–3, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Budd GE. The earliest fossil record of the animals and its significance. Philos Trans R Soc Lond B Biol Sci 363: 1425–1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cakir Y. and Ballinger SW. Reactive species-mediated regulation of cell signaling and the cell cycle: the role of MAPK. Antioxid Redox Signal 7: 726–740, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, and Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8: 691–728, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, and Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Kirber MT, Xiao H, Yang Y, and Keaney JF., Jr.Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung B. Hypertension as part of the metabolic syndrome. J Hum Hypertens 22: 871–874, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Cheung BM. and Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep 14: 160–166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cnop M, Foufelle F, and Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med 18: 59–68, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Conraads VM, Bosmans JM, and Vrints CJ. Chronic heart failure: an example of a systemic chronic inflammatory disease resulting in cachexia. Int J Cardiol 85: 33–49, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Cross AR. and Segal AW. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochim Biophys Acta 28: 1–22, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, and Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullinan SB. and Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem 279: 20108–20117, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Cullinan SB. and Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 38: 317–332, 2006 [DOI] [PubMed] [Google Scholar]
- 24.de APAM, Verissimo-Filho S, Guimaraes LL, Silva AC, Takiuti JT, Santos CX, Janiszewski M, Laurindo FR, and Lopes LR. Protein disulfide isomerase redox-dependent association with p47(phox): evidence for an organizer role in leukocyte NADPH oxidase activation. J Leukoc Biol 90: 799–810, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Desjardins M. ER-mediated phagocytosis: a new membrane for new functions. Nat Rev Immunol 3: 280–291, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Dickhout JG, Carlisle RE, Jerome DE, Mohammed-Ali Z, Jiang H, Yang G, Mani S, Garg SK, Banerjee R, Kaufman RJ, Maclean KN, Wang R, and Austin RC. Integrated stress response modulates cellular redox state via induction of cystathionine gamma-lyase: cross-talk between integrated stress response and thiol metabolism. J Biol Chem 287: 7603–7614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, Lavery GG, Bedendo O, Ray DW, Laing I, Malunowicz E, White PC, Hewison M, Mason PJ, Connell JM, Shackleton CH, and Stewart PM. Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat Genet 34: 434–439, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Dromparis P, Paulin R, Stenson TH, Haromy A, Sutendra G, and Michelakis ED. Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation 127: 115–125, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Fagone P. and Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res 50: 23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flamment M, Hajduch E, Ferre P, and Foufelle F. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab 23: 381–390, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Fu S, Watkins SM, and Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab 15: 623–634, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Ge X, Yu Q, Qi W, Shi X, and Zhai Q. Chronic insulin treatment causes insulin resistance in 3T3-L1 adipocytes through oxidative stress. Free Radic Res 42: 582–591, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, Thorpe C, and Fass D. Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci U S A 103: 299–304, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu F, Nguyen DT, Stuible M, Dube N, Tremblay ML, and Chevet E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem 279: 49689–49693, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, and Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Harris DM, Cohn HI, Pesant S, and Eckhart AD. GPCR signalling in hypertension: role of GRKs. Clin Sci 115: 79–89, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Harrison DG, Vinh A, Lob H, and Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol 10: 203–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasty AH. and Harrison DG. Endoplasmic reticulum stress and hypertension - a new paradigm? J Clin Invest 122: 3859–3861, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haynes CM, Titus EA, and Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15: 767–776, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Helenius A. and Aebi M. Intracellular functions of N-linked glycans. Science 291: 2364–2369, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Isodono K, Takahashi T, Imoto H, Nakanishi N, Ogata T, Asada S, Adachi A, Ueyama T, Oh H, and Matsubara H. PARM-1 is an endoplasmic reticulum molecule involved in endoplasmic reticulum stress-induced apoptosis in rat cardiac myocytes. PLoS One 5: 0009746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CX, and Laurindo FR. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem 280: 40813–40819, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Jin L, Ying Z, and Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287: H1495–H1500, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, and Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol 32: 1652–1661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kassan M, Montero MJ, and Sevilla MA. Chronic treatment with pravastatin prevents early cardiovascular changes in spontaneously hypertensive rats. Br J Pharmacol 158: 541–547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lassegue B, San Martin A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laurindo FR, Pescatore LA, and Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med 52: 1954–1969, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Lewerenz J. and Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 284: 1106–1115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li G, Scull C, Ozcan L, and Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol 191: 1113–1125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu GC, Fang F, Zhou J, Koulajian K, Yang S, Lam L, Reich HN, John R, Herzenberg AM, Giacca A, Oudit GY, and Scholey JW. Deletion of p47 (phox) attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse. Diabetologia 55: 2522–2532, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Garcia P. and Moreira D. Selective forces for the origin of the eukaryotic nucleus. Bioessays 28: 525–533, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malhotra JD. and Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol 3: a004424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, and Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A 105: 18525–18530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malhotra V. and Erlmann P. Protein export at the ER: loading big collagens into COPII carriers. Embo J 30: 3475–3480, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantegazza AR, Savina A, Vermeulen M, Perez L, Geffner J, Hermine O, Rosenzweig SD, Faure F, and Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 112: 4712–4722, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, and Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margittai E. and Banhegyi G. Oxidative folding in the endoplasmic reticulum: towards a multiple oxidant hypothesis? FEBS Lett 584: 2995–2998, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Margittai E, Low P, Stiller I, Greco A, Garcia-Manteiga JM, Pengo N, Benedetti A, Sitia R, and Banhegyi G. Production of H(2)O(2) in the endoplasmic reticulum promotes in vivo disulfide bond formation. Antioxid Redox Signal 16: 1088–1099, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Merksamer PI, Trusina A, and Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell 135: 933–947, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minamino T, Komuro I, and Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 107: 1071–1082, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Montezano AC. and Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med 44: 653393, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Mora-Pale M, Kwon SJ, Linhardt RJ, and Dordick JS. Trimer hydroxylated quinone derived from apocynin targets cysteine residues of p47phox preventing the activation of human vascular NADPH oxidase. Free Radic Biol Med 52: 962–969, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, and Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98–103, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, and Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A 88: 10045–10048, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narayan KM, Ali MK, and Koplan JP. Global noncommunicable diseases—where worlds meet. N Engl J Med 363: 1196–1198, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Nickel W. and Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol 10: 148–155, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Noiva R. Protein disulfide isomerase: the multifunctional redox chaperone of the endoplasmic reticulum. Semin Cell Dev Biol 10: 481–493, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Nomura M, Ishii H, Kawakami A, and Yoshida M. Inhibition of hepatic Niemann-Pick C1-like 1 improves hepatic insulin resistance. Am J Physiol Endocrinol Metab 297: E1030–E1038, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Oda E. Metabolic syndrome: its history, mechanisms, and limitations. Acta Diabetol 49: 89–95, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, and Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110: 705–712, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Ozcan L. and Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med 63: 317–328, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, and Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Panza JA, Quyyumi AA, Brush JE, Jr., and Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22–27, 1990 [DOI] [PubMed] [Google Scholar]
- 76.Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, and Kim do H. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun 421: 578–584, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, and Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Popovic N, Bridenbaugh EA, Neiger JD, Hu JJ, Vannucci M, Mo Q, Trzeciakowski J, Miller MW, Fossum TW, Humphrey JD, and Wilson E. Transforming growth factor-beta signaling in hypertensive remodeling of porcine aorta. Am J Physiol Heart Circ Physiol 297: 28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, and Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Romeo GR, Lee J, and Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation—mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol 32: 1771–1776, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 63: 423–432, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Rutkowski DT. and Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol 189: 783–794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rutkowski DT. and Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci 32: 469–476, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Santos CX, Anilkumar N, Zhang M, Brewer AC, and Shah AM. Redox signaling in cardiac myocytes. Free Radic Biol Med 50: 777–793, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santos CX, Stolf BS, Takemoto PV, Amanso AM, Lopes LR, Souza EB, Goto H, and Laurindo FR. Protein disulfide isomerase (PDI) associates with NADPH oxidase and is required for phagocytosis of Leishmania chagasi promastigotes by macrophages. J Leukoc Biol 86: 989–998, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Santos CX, Tanaka LY, Wosniak J, and Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11: 2409–2427, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, and Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126: 205–218, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Schiffrin EL. Vascular stiffening and arterial compliance. Implications for systolic blood pressure. Am J Hypertens 17: 39S–48S, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Schluter T, Steinbach AC, Steffen A, Rettig R, and Grisk O. Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res 80: 271–279, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Schmid-Schonbein GW. An emerging role of degrading proteinases in hypertension and the metabolic syndrome: autodigestion and receptor cleavage. Curr Hypertens Rep 14: 88–96, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sedeek M, Hebert RL, Kennedy CR, Burns KD, and Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens 18: 122–127, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Senesi S, Csala M, Marcolongo P, Fulceri R, Mandl J, Banhegyi G, and Benedetti A. Hexose-6-phosphate dehydrogenase in the endoplasmic reticulum. Biol Chem 391: 1–8, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Simmen T, Lynes EM, Gesson K, and Thomas G. Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM). Biochim Biophys Acta 8: 27, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song B, Scheuner D, Ron D, Pennathur S, and Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118: 3378–3389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol 286: H1597–H1602, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Stolf BS, Smyrnias I, Lopes LR, Vendramin A, Goto H, Laurindo FR, Shah AM, and Santos CX. Protein disulfide isomerase and host-pathogen interaction. ScientificWorldJournal 11: 1749–1761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tabas I. and Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tavender TJ. and Bulleid NJ. Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum. Antioxid Redox Signal 13: 1177–1187, 2010 [DOI] [PubMed] [Google Scholar]
- 99.Tavender TJ, Springate JJ, and Bulleid NJ. Recycling of peroxiredoxin IV provides a novel pathway for disulphide formation in the endoplasmic reticulum. Embo J 29: 4185–4197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, and Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest 119: 2925–2941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Touyz RM. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens 14: 125–131, 2005 [DOI] [PubMed] [Google Scholar]
- 102.Tu BP. and Weissman JS. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell 10: 983–994, 2002 [DOI] [PubMed] [Google Scholar]
- 103.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11: 1289–1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44: 938–955, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaziri ND. and Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 582–593, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Walter P. and Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, and Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 103: 27–37, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang S. and Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol 197: 857–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wiseman RL, Haynes CM, and Ron D. SnapShot: the unfolded protein response. Cell 140: 590–590, 2010 [DOI] [PubMed] [Google Scholar]
- 110.Wu RF, Ma Z, Liu Z, and Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30: 3553–3568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yorimitsu T. and Klionsky DJ. Eating the endoplasmic reticulum: quality control by autophagy. Trends Cell Biol 17: 279–285, 2007 [DOI] [PubMed] [Google Scholar]
- 112.Younce C. and Kolattukudy P. MCP-1 induced protein promotes adipogenesis via oxidative stress, endoplasmic reticulum stress and autophagy. Cell Physiol Biochem 30: 307–320, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, and Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, and Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao L. and Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol 18: 444–452, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, and Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension 47: 81–86, 2006 [DOI] [PubMed] [Google Scholar]
- 117.Zhou QG, Zhou M, Lou AJ, Xie D, and Hou FF. Advanced oxidation protein products induce inflammatory response and insulin resistance in cultured adipocytes via induction of endoplasmic reticulum stress. Cell Physiol Biochem 26: 775–786, 2010 [DOI] [PubMed] [Google Scholar]
- 118.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, and Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 119.Zimmerman MC, Lazartigues E, Sharma RV, and Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Zito E, Melo EP, Yang Y, Wahlander A, Neubert TA, and Ron D. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol Cell 40: 787–797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]