Abstract
As colorectal cancer remains the second highest cause of cancer-related deaths in much of the industrialised world, identifying novel strategies to prevent colorectal tumour development remains an important challenge. BAG-1 is a multi-functional protein, the expression of which is up-regulated at relatively early stages in colorectal tumorigenesis. Importantly, BAG-1 is thought to enhance colorectal tumour progression through promoting tumour cell survival. Here we report for the first time a novel role for BAG-1, establishing it as a suppressor of transforming growth factor beta [TGF-β1] expression in colorectal tumour cells. Microarray analysis first highlighted the possibility that BAG-1 may regulate TGF-β1 expression, a key cytokine in normal colonic tissue homeostasis. Q-RT-PCR and ELISA demonstrated TGFB1 mRNA and protein expression to be significantly increased when BAG1 levels were reduced by siRNA; additionally, induction of BAG-1L caused suppression of TGFB1 mRNA in colorectal tumour cells. Using reporter and ChIP assays, a direct association of BAG-1 with the TGFB1 gene regulatory region was identified. Immunohistochemistry and Weiser fraction data indicated levels of BAG-1 and TGF-β1 are inversely correlated in the normal colonic epithelium in vivo, consistent with a role for BAG-1-mediated repression of TGF-β1 production. In vitro studies showed that the change in TGF-β1 production following manipulation of BAG-1 is functionally relevant; through induction of anchorage-independent growth in TGF-β1 dependent NRK fibroblasts and regulation of SMAD2 phosphorylation in TGF-β1 sensitive adenoma cells. Taken together, this study identifies the anti-apoptotic protein BAG-1 as a suppressor of the inhibitory growth factor TGF-β1, suggesting that high expression of BAG-1 can impact on a number of the hallmarks of cancer, of potential importance in promoting the early stages of colorectal tumorigenesis. Establishing BAG-1 as a repressor of TGF-β1 has important biological implications, and highlights a new role for BAG-1 in colorectal tumorigenesis.
Keywords: BAG-1, TGF-β1, colorectal cancer, adenoma, transcriptional repression
INTRODUCTION
As the fourth most common cancer worldwide, reducing colorectal cancer incidence remains a significant challenge. A key signalling pathway commonly disrupted in many cancers, including colorectal cancer, is that of transforming growth factor beta (TGF-β, reviewed in [1]). TGF-β proteins are highly conserved, however only TGF-β1-3 are expressed in mammals, with tissue- and cell-type specificity in both developing and adult organisms. TGF-β1 has a plethora of functions according to the cellular context, but in normal epithelial systems TGF-β1 acts as a tumour suppressor protein and has been implicated in cell cycle control, differentiation and apoptosis [2]. Binding of TGF-β1 to Type II receptor induces hetero-oligomerisation with the Type I receptor and formation of this receptor complex transmits the signal to the intracellular signal transduction molecules, SMADs. The receptor SMAD2/3 is phosphorylated by the active receptor complex, and following association with the common partner SMAD4, translocates to the nucleus where it binds SMAD-binding elements in the regulatory regions of target genes [3].
In the normal colon, TGF-β1 is expressed in the upper region of the crypt, implicating TGF-β1 in maintaining colorectal epithelial homeostasis [4,5]. In normal epithelial cells, TGF-β1 signalling elicits a G1-phase cell cycle arrest and direct transcriptional responses of TGF-β1 signalling include induction of cyclin-dependent kinase inhibitors, as well as repression of the proto-oncogene, c-MYC (reviewed in [6]). As a secreted protein, TGF-β1 may exert autocrine, paracrine or endocrine responses, therefore its levels and biological activity must be tightly regulated.
Further, highlighting the potential importance of TGF-β1 signalling in colorectal carcinogenesis, expression is deregulated in colorectal cancer cells, with increased TGF-β1 expression associated with the malignant phenotype [4]. A change in functional response to TGF-β1 has been associated with colorectal carcinogenesis: in the early stages of tumorigenesis, colorectal adenomas retain sensitivity to TGF-β1 signalling, and the tumour suppressor functions of TGF-β1 pose an important barrier to transformation that cells must over-come in order to allow tumour progression. In the later stages of tumour progression, cells develop resistance to the inhibitory effects of TGF-β1 (through SMAD4 deletion or mutation of TGBR2 receptor, for example), and increased levels of TGF-β1 are associated with colorectal cancer. This is likely to be important in the switch of TGF-β1 from tumour suppressor to tumour promoter (described in a number of tumour types, as reviewed in [1]), although whether TGF-β1 assumes pro-oncogenic activities in colorectal cancer cells is yet to be established [7]. However, despite its altered expression in a number of pathologies, including in the colonic mucosa of patients with ulcerative colitis and Crohn’s disease [8] the regulation of TGFB1 expression in normal tissues and in tumorigenesis remains poorly understood.
BAG-1 is a multi-functional protein, implicated in heat shock response, cell signalling, cell survival and apoptosis (reviewed in [9]). There are three major isoforms of human BAG-1 (BAG-1L, M and S with molecular weights of 50, 46 and 36kDa respectively) generated from alternative translation initiation sites in a single BAG1 transcript [10]. The longest isoform contains additional domains that are partially or completely absent in the shorter isoforms, for example, the bipartite nuclear localisation signal and putative DNA binding domain. BAG-1 protein is up-regulated in a variety of human cancers (reviewed in [11]), and studies show that this may be a relatively early event as increased BAG-1 levels have been detected in colorectal adenomas [12]. Previous in vitro studies in this laboratory highlight an important role for nuclear BAG-1 in colorectal tumour cell survival [13, 14]. This is consistent with analysis of BAG-1 sub-cellular localisation in colorectal tumour tissue in which high nuclear BAG-1 is associated with distant metastasis, poor prognosis and decreased overall patient survival [15]. Depletion of BAG-1 in colorectal cancer cells has been shown to sensitise cells to TNFα and TRAIL-induced apoptosis, as well as decrease NF-κB transcriptional activity [12], providing a link for the first time between BAG-1 function and NF-κB signalling.
Importantly, the nuclear isoform of BAG-1 (BAG-1L) has been implicated in the control of transcription (reviewed in [16]). This may occur through direct interaction of BAG-1 with DNA [17,18], or through interaction with DNA-binding proteins such as nuclear hormone receptors [19,20], the retinoblastoma protein [13] or p73 [21]. More recently we have shown that, through direct interaction with homodimeric p50 NF-κB complexes, BAG-1 can regulate NF-κB dependent transcription at a sub-set of NF-κB target genes including the epidermal growth factor receptor (EGFR) and COX-2 (PTGS2) [22]. This finding further emphasises the importance of BAG-1 in regulating the transcription of genes whose protein products have established roles in colorectal carcinogenesis. Microarray analysis performed in this laboratory has identified a number of genes that were differentially regulated in HCT116 colorectal carcinoma cells following depletion of BAG1 levels by RNAi. Of potential importance was the finding that a number of TGF-β-related genes, such as SNON, TGIF (expression of both was suppressed by BAG-1 knock down, approximately 1.5 fold), and TGFB1 itself (an approximate 2 fold increase, unpublished observations) were regulated [12].
Given the importance of TGF-β1 in normal colonic tissue homeostasis and the fact that BAG-1 levels are dramatically increased in colorectal adenoma tissue, we hypothesised that expression of BAG-1 in the developing adenoma may allow the cells to overcome the tumour suppressive functions of TGF-β1, promoting conditions within the adenoma that are permissive for growth. Data presented show that BAG-1 acts as a direct repressor of TGFB1 expression in colorectal tumour cells and that the change in TGF-β1 production following manipulation of BAG-1 is functionally relevant. This investigation establishes BAG-1 as a negative regulator of TGF-β1 signalling, of potential importance at the early stages of colorectal tumorigenesis and highlights a new role for BAG-1 in colorectal tumorigenesis.
RESULTS
BAG-1 negatively regulates TGF-β1 production in colorectal carcinoma cells
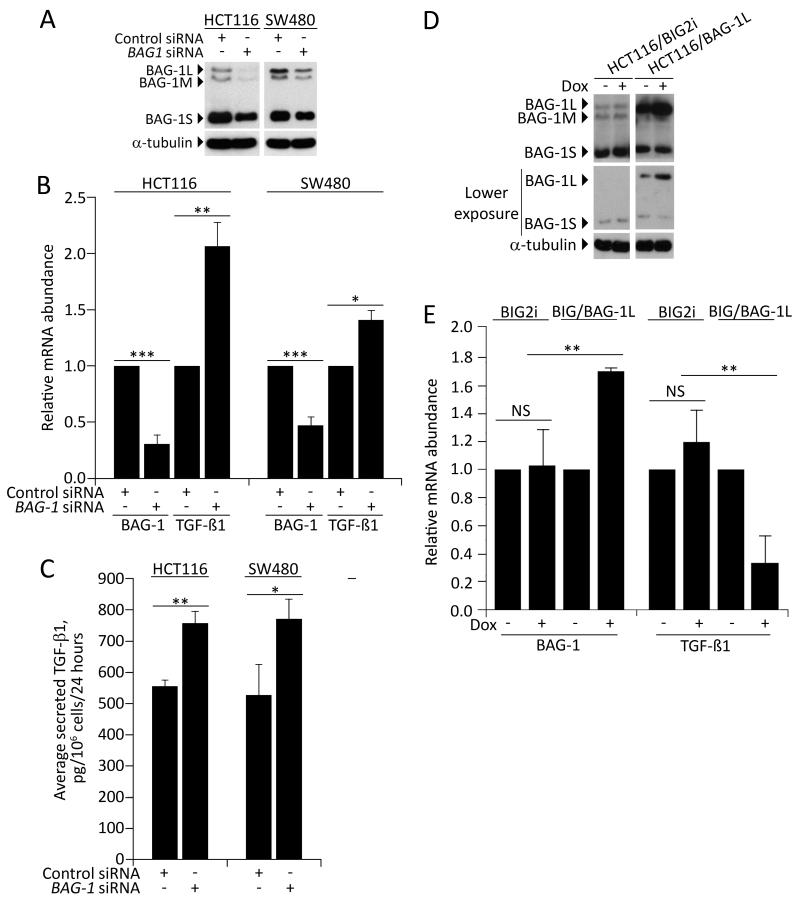
Experiments investigating the role of BAG-1 as a regulator of TGFB1 gene expression were carried out in colorectal cancer derived cell lines, SW480 and HCT116 (both insensitive to growth inhibition by TGF-β1 due to defective signalling; SW480 lacks SMAD4 expression, whereas HCT116 harbours a TGF-β receptor mutation). Colorectal cancer derived cell lines were used for these experiments as the relatively low transfection frequency makes it is difficult to regulate protein expression using RNAi or expression plasmids in adenoma derived cells. Of note, HCT116 cells were previously used to generate the unpublished microarray data which indicated the suppression of BAG-1 using siRNA might increase expression of TGFB1. To further investigate the role of BAG-1 in the regulation of TGFB1 expression, the array result was verified using quantitative real time PCR (Q-RT-PCR) and the effects of depleting BAG-1 expression on TGF-β1 protein production were examined by ELISA in HCT116 and SW480 colorectal tumour cells; the results are summarised in Figure 1. Suppression of BAG-1 expression by siRNA was confirmed by both Western blot analysis and Q-RT-PCR (Figure 1A and 1B). Q-RT-PCR revealed that following reduction of BAG1 expression (involving all three BAG-1 isoforms as they are generated from a single mRNA transcript; Figure 1A), TGFB1 mRNA levels were significantly increased compared to controls (Figure 1B). Importantly, secreted TGF-β1 protein was also increased in the BAG1 siRNA treated cells (Figure 1C).
Figure 1. BAG-1 negatively regulates TGFB1 expression.
(A) Western blot analysis confirmed that BAG-1 protein levels are reduced by BAG1 siRNA compared to control siRNA in HCT116 and SW480 colorectal carcinoma cells. Q-RT-PCR (B) and ELISA (C) were used to assess the effects of depleting BAG-1 expression on TGFB1 mRNA and TGF-β1 protein levels in HCT116 and SW480 cells. (C) Conditioned medium was collected from approximately 70% confluent cultures in serum-free medium containing 0.1% BSA. 24h after siRNA transfection, cell culture supernatants were collected following a further 24 hour incubation, centrifuged to remove debris and the amount of TGF-β1 was measured by ELISA, and normalised to cell counts from a parallel flask. Data points show the mean of three independent experiments ±SD bars, * p≤0.05; ** p≤0.01; *** p≤0.001 (D) HCT116 cells stably transfected with either the empty vector pBIG2i (HCT116/BIG2i) or pBIG2i/BAG-1L expression construct (HCT116/BAG-1L) were incubated with or without 2μg/mL Doxycycline (Dox) for 24 hours then BAG-1 protein levels were determined by immunoblot; equal loading was confirmed by α-tubulin. Note the BAG-1L expression above endogenous levels in the pBIG2i/BAG-1L transfected cells is due to the inherent leakiness of the Dox inducible system. (E) HCT116 cells stably expressing either the vector only (BIG2i) or the inducible BAG-1L construct (BIG/BAG-1L) were treated for 24 hours with 2μg/mL Dox. BAG1 and TGFB1 mRNA expression levels were then assessed by Q-RT-PCR and presented as the fold-change in mRNA abundance in Dox-treated cells relative to the non-Dox-treated controls. Data points show the mean of three independent experiments ±SD, ** p≤0.01.
Further, as BAG-1 expression is increased in the developing colorectal adenoma [12], it was necessary to show that increased expression of the BAG-1 could suppress TGFB1 mRNA expression. In contrast to the knock-down studies, isoform-specific expression plasmids allow the function of specific BAG-1 isoforms to be investigated (refer to [13]). As studying a possible transcriptional mechanism, the regulation of TGF-β1 production by the constitutively nuclear BAG-1L isoform [10,13,14,23] was investigated. An inducible system was employed to selectively over-express BAG-1L in HCT116 cells and its effect on TGFB1 mRNA expression determined. HCT116 cells stably expressing either the vector only (HCT116/BIG2i) or the Doxycycline (Dox)-inducible pBIG2i/BAG-1L expression construct (HCT116/BAG-1L) were treated with 2μg/mL Dox for 24 hours and BAG-1 levels assessed by Western blot (Figure 1D): this analysis showed that Dox treatment increased BAG-1L expression in the HCT116/BAG-1L cells. Parallel samples were prepared for quantification of BAG1 and TGFB1 mRNA levels by Q-RT-PCR (Figure 1E). Dox-treatment of HCT116/BAG-1L cells resulted in an approximate 2-fold increase in BAG1 mRNA above non-Dox-treated controls, which corresponded to approximately a 70% reduction in TGFB1 mRNA abundance. Taken together, these data implicate BAG-1 as a negative regulator of TGFB1 expression in colorectal tumour cells.
BAG-1 occupies the TGFB1 gene regulatory region, and suppresses TGFB1 transcriptional activity
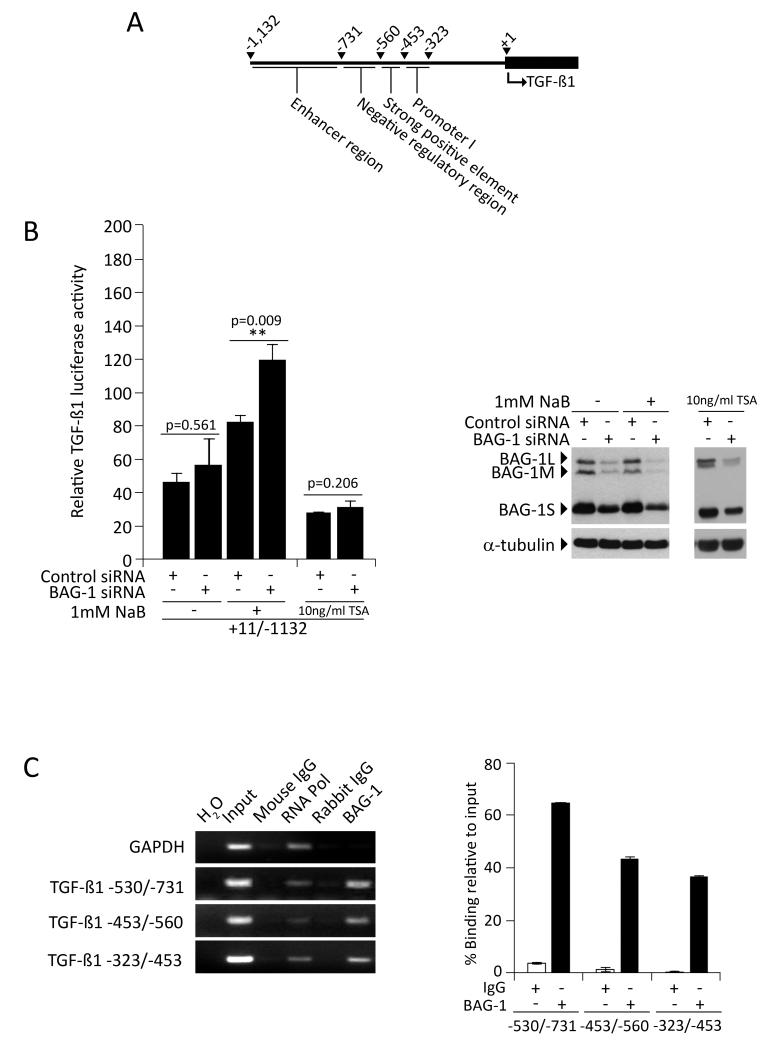
As BAG-1 has recently been shown to directly regulate transcription through binding to gene regulatory regions (reviewed in [16,22]), we investigated whether BAG-1 inhibited TGFB1 mRNA expression through interaction with the 5′-flanking region of the TGFB1 gene (regulatory regions identified in the 5′-flanking region of the TGFB1 gene [24] are depicted in Figure 2A). Initially, HCT116 cells were transfected with a TGFB1 promoter reporter that spans the different regulatory regions between +11/−1132 within a promoter-less pGL3 vector in order to identify whether any regions of the 5′-flanking sequences were implicated in BAG-1 binding (Figure 2B). Of note, under basal conditions, reduction of BAG-1 levels by RNAi caused a small but reproducible induction of TGFB1 reporter activity although this failed to reach statistical significance. Therefore, to increase the activity of the TGFB1 reporter assay, the HCT116 cells were treated with the histone deacetylase (HDAC) inhibitor sodium butyrate (NaB), previously shown to increase TGF-β1 expression [25,26]. NaB is a naturally occurring short chain fatty acid (SCFA), and although the mechanism by which NaB enhances TGF-β1 transcription is not known, it is a physiologically relevant reagent present in mM concentrations in the colon [27]. On exposure of the transfected cells to 1mM NaB for 24h, the luciferase activity of the TGFB1 reporter was significantly increased in cells transfected with BAG1 siRNA when compared to control siRNA (Figure 2B; p=0.009). To ensure the effect of decreased BAG-1 on the TGF-β1 reporter was not due to an indirect effect on histone acetylation in NaB treated cells, the experiment was repeated using TSA (a more specific HDAC inhibitor than NaB), and reporter activity measured +/− BAG-1 siRNA. Under these conditions neither TSA nor suppression of BAG-1 in TSA treated cells increased the TGF-β1 reporter activity, confirming that the regulation of TGF-β1 activity in the presence of 1mM NaB +/− BAG-1 siRNA was not due to changes in HDAC activity alone (results shown for 10ng/mL TSA treatment; no induction of reporter activity was seen with TSA treatment up to 50ng/mL, data not shown). These data further support our previous findings that BAG-1 negatively regulates TGFB1 expression, and indicate that this occurs via the region upstream of the TGFB1 transcriptional start site.
Figure 2. BAG-1 is a transcriptional regulator of TGFB1 gene expression.
(A) Schematic of the regulatory regions previously identified in the 5′ flanking region of the TGFB1 gene [adapted from 24]. (B) Luciferase activity of the TGFB1 reporter was assessed in HCT116 colorectal cancer cells transfected with either control siRNA or BAG1 siRNA, and treated with 1mM NaB to stimulate TGFB1 reporter activity (normalised to untransfected controls 100%). Depletion of BAG-1 had a small but non-significant effect on luciferase activity under basal conditions. However, on exposure to 1mM NaB for 24h, the luciferase activity of the +11/−1132 TGFB1 reporter was significantly increased in cells transfected with BAG1 siRNA when compared to control siRNA, which was not detected in cells treated with 10ng/mL TSA (an HDAC inhibitor). Data points show the mean of three independent experiments ±SD, ** p≤0.01. (C) Chromatin immunoprecipitation assays showed BAG-1 occupies the TGFB1 gene regulatory region between −323/−731 (−323/−453, −453/−560 and −560/−731) in HCT116 colorectal cancer cells. The GAPDH gene promoter was examined as a negative control for non-specific binding. Input DNA comprising 10% of total ChIP volume was used as a PCR control. Quantification represents BAG-1 binding as percent enrichment relative to input chromatin, IgG included as a negative control; mean of 3 independent measurements of a representative result, ±SD. Similar results were obtained in two independent experiments.
As BAG-1 is able to bind DNA [17], chromatin immunoprecipitation assay was used to investigate whether the BAG-1-mediated regulation of TGFB1 expression involves interaction of BAG-1 with a region upstream of the TGFB1 transcriptional start site. As shown in Figure 2C, ChIP assay revealed that BAG-1 is present at the TGFB1 gene regulatory region, between bases −323/−731 upstream of the +1 position. As this region encompasses negative regulatory elements [26], these results support a role for BAG-1 in the suppression of TGFB1 expression through interaction with the 5′-flanking region of the TGFB1 gene.
Expression patterns of BAG-1 and TGF-β1 in vitro and in vivo support a role for BAG-1 in suppressing TGF-β1 production in colorectal epithelial cells
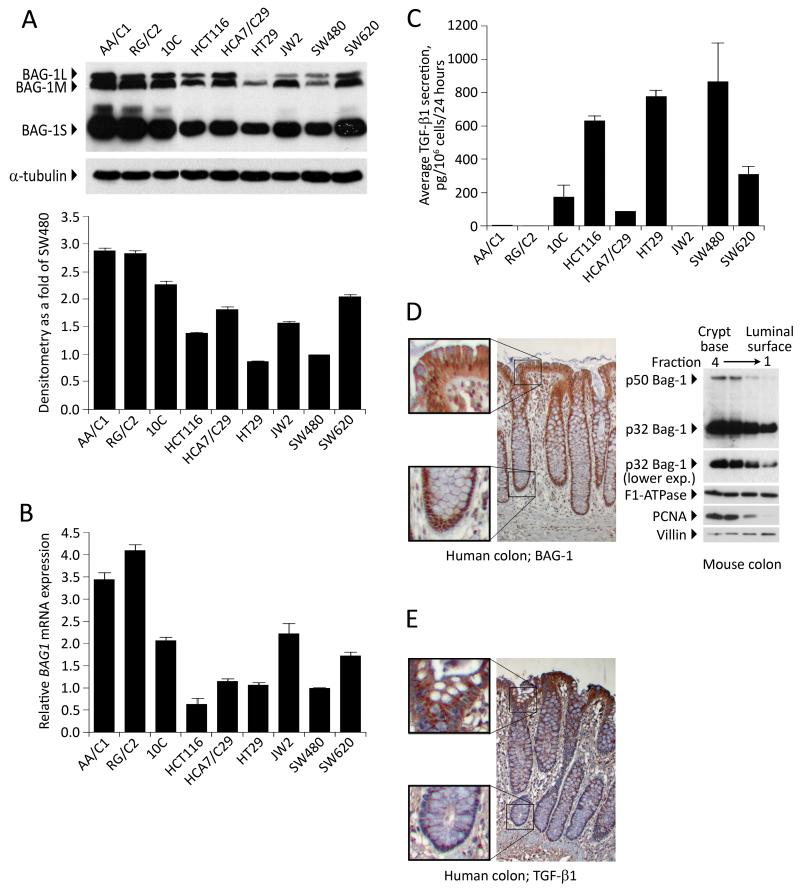
Having established BAG-1 as a negative regulator of TGF-β1 production in colorectal carcinoma cell lines, it was of interest to determine whether basal expression of BAG-1 and TGF-β1 reflected this regulatory role in a panel of colorectal cell lines (including adenoma derived cell lines). Immunoblot (Figure 3A) and Q-RT-PCR (Figure 3B) analysis of BAG-1 levels revealed variable BAG-1 levels across the panel of cell lines. ELISA was used to investigate levels of TGF-β1 protein secreted from these cell lines (Figure 3C) and it was noted that in cell lines where BAG-1 expression was lowest (HCT116, HT29 and SW480), TGF-β1 secretion was highest (600-900pg TGF-β1 per 106 cells per 24 hours). Furthermore, in the highest BAG-1 expressing cell lines, the AA/C1 and RG/C2 adenoma derived lines, there was very low secretion of TGF-β1 (below the detection level of the assay), supporting a role for BAG-1 as a negative regulator of TGF-β1.
Figure 3. BAG-1 and TGF-β1 protein levels are negatively correlated in vitro and in vivo.
(A) BAG-1 protein levels were assessed in a panel of colorectal adenoma (AA/C1, RG/C2), transformed adenoma (10C) and carcinoma-derived cell lines (HCT116, HCA7/C29, HT29, JW2, SW480, SW620) by Western blot, using α-tubulin to indicate equal loading. Quantification of total BAG-1L expression levels was carried out using Image J Software, and depicted as fold change relative to basal BAG-1 levels in the SW480 cells. Quantification is the mean of 3 independent measurements of a representative result ±SD. Similar results were obtained in three independent experiments. (B) BAG1 mRNA levels were also quantified by Q-RT-PCR. Data show the mean from three independent experiments ±SD bars. (C) TGF-β1 production by these cell lines was also measured by ELISA and is presented as the mean from three independent experiments ± SD. (D) BAG-1 expression levels were assessed in normal human colonic epithelium by immunohistochemistry. Formalin-fixed, paraffin-embedded tissue sections were stained with the TB3 BAG-1 antibody (brown staining) and counterstained with Gill’s haematoxylin (blue); objective ×10, insets show higher magnification at base of crypt and luminal surface. Western blot of Weiser fractions of the mouse colonic epithelium in which Fraction 1 is enriched for cells from the luminal surface of the crypt and Fraction 4 is enriched for cells from the crypt base. This approach demonstrated a gradient of mBAG-1 expression (as shown by the two murine isoforms) along the crypt axis. Protein loading was assessed by F1-ATPase, fractionation confirmed using PCNA and Villin expression. (E) TGF-β1 expression levels were assessed in normal human colonic epithelium by immunohistochemistry. Formalin-fixed, paraffin-embedded tissue sections were stained with a TGF-β1 antibody (brown staining) and counterstained with Gill’s haematoxylin (blue); objective ×10, insets show higher magnification at base of crypt and luminal surface.
In order to further investigate a regulatory role in vivo, levels of BAG-1 and TGF-β1 protein were examined by immunohistochemistry in the normal colonic epithelium (Figure 3D and E). In the colonic crypt, BAG-1 staining pattern changes along the crypt axis (Figure 3D, upper panel) as described previously [12,23], with predominant nuclear localisation at the crypt base. However because the BAG-1 antibody can not distinguish between the different isoforms, BAG-1 expression along the crypt axis was also investigated using intestinal epithelial cell fractionation. This technique, using normal mouse tissue, produces cell populations enriched in four consecutive fractions containing predominantly epithelial cells from the luminal surface of the crypt and the base of the crypt (fractions 1 and 4 respectively) [28,29]; protein expression was determined by Western blotting (Figure 3D). Of note, only two BAG isoforms are detectable in murine cells (50kDa and 32kDa) [23]. This approach showed that there is a strong gradient of BAG-1 protein expression in which the level of total BAG-1 decreases as the crypt is ascended. Alternatively, consistent with previous findings [4,5], TGF-β1 staining was exclusively cytoplasmic in the epithelial compartment, and an expression gradient was observed in which highest expression was detected in the upper third of the crypt (Figure 3E, lower panel). These data indicate that there is an inverse relationship in BAG-1 and TGF-β1 expression in the normal intestinal epithelium. Although correlative, the relative subcellular distribution of the proteins in vivo supports the in vitro findings that BAG-1 acts as a negative regulator of TGF-β1.
Manipulation of BAG-1 levels causes physiologically relevant changes in TGF-β1 production in colorectal tumour cells
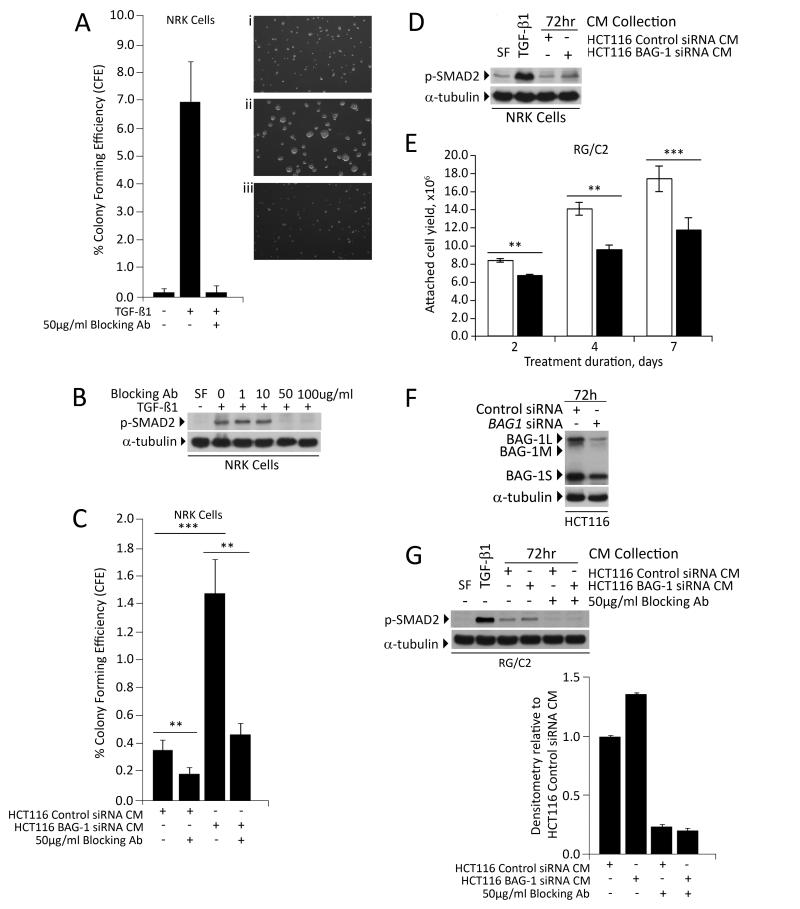
Having established BAG-1 as an inhibitor of TGF-β1 expression, it was asked whether the change in TGF-β1 production following manipulation of BAG-1 was physiologically relevant. As previously stated, either SMAD4 deletion (SW480) or receptor mutation (HCT116) renders the carcinoma derived lines insensitive to the growth inhibitory effects of TGF-β1. Therefore we used growth of the TGF-β1 sensitive NRK cell lines to assay for functional changes in TGF-β1 production from the HCT116 cells with depleted BAG-1 expression. In standard conditions, NRK cells exhibited poor growth in soft agar indicated by colony size and low colony forming efficiency (CFE <1%), whereas addition of TGF-β1 to cultures increased anchorage independent growth, which was completely blocked by addition of 50μg/mL TGF-β1 blocking antibody (Figure 4A). Efficacy of the blocking antibody was demonstrated by inhibition of SMAD2 phosphorylation in the NRK cells (figure 4B). Addition of CM from the HCT116/negative control siRNA to NRK cultures increased anchorage-independent growth which was blocked by 2h pre-incubation of the CM with of 50μg/mL TGF-β1 blocking antibody, indicating basal TGF-β1 secretion (Figure 4C). Further, there was a highly significant increase in CFE when the NRK cells were treated with the HCT116/BAG-1 siRNA CM (p≤0.001, Figure 4C) which again was blocked by pre-incubation with the blocking antibody. Stimulation of TGF-β1 signalling was further demonstrated by an increase in p-SMAD2 in the NRK cells treated with the HCT116 BAG-1 siRNA CM (Figure 4D). Taken together, these results demonstrate that the increase in TGF-β1 expression on suppression of BAG-1 in the colorectal tumour cells was sufficient to stimulate growth of the NRK cells.
Figure 4. The change in TGF-β1 production following manipulation of BAG-1 is physiologically relevant.
NRK cells were used to assess the levels of TGF-β1 in 72h conditioned culture medium from HCT116 cells, following manipulation of BAG-1 levels, through induction of their anchorage-independent growth. Colonies exceeding the threshold size after 7 days were counted in 10 fields on each plate and colony forming efficiency (CFE) calculated. (A) NRK cultures in sea plaque agarose were supplemented with (i) vehicle control, (ii) 5ng/mL TGF-β1 and (iii) 5ng/mL TGF-β1 plus 50μg/mL TGF-β1 blocking antibody. The graph shows average % CFE from three independent experiments each done in triplicate ±SD. (B) Western blot showing efficacy of the blocking antibody as demonstrated by inhibition of SMAD2 phosphorylation (p-SMAD2) in the NRK cells. α-tubulin was included as a loading control. (C) NRK cultures in sea plaque agarose were treated with conditioned medium from HCT116 cells transfected with control siRNA or BAG1 siRNA [24h after siRNA transfection, 72h cell culture supernatants were collected] +/− 50μg/mL of the TGF-β1 blocking antibody. Colonies exceeding the threshold size were counted in 10 fields on each plate and colony forming efficiency (CFE) calculated. The graph shows average % CFE from three independent plates ±SD; a t-test was performed to determine statistical significance (** p≤0.01; *** p≤0.001). (D) Western blot showing SMAD2 phosphorylation in NRK cells treated with conditioned medium from HCT116 cells transfected with control siRNA or BAG1 siRNA [24h after siRNA transfection, 72h cell culture supernatants were collected]. (E) RG/C2 colorectal adenoma-derived cells were treated with (+) or without (−) 10ng/mL TGF-β1 in serum free medium (SF). Adherent cells were counted after 2, 4 or 7 days and showed growth inhibition by TGF-β1, graph shows the mean of three independent experiments ±SD (** p≤0.01; *** p≤0.001). (F) Western blot to show that BAG-1 expression was reduced in HCT116 cells transfected with BAG1 siRNA compared to control siRNA. Protein loading was assessed by α-tubulin (G) Conditioned culture medium was collected after 72h from HCT116 cells transfected with control siRNA or BAG1 siRNA, and applied to parallel cultures of RG/C2 colorectal adenoma-derived cells, with or without 50 μg/mL TGF-β1 blocking antibody. After 2 hours, total protein was harvested and activation of the TGF-β1 signalling pathway was assessed by phosphorylation of SMAD2 (p-SMAD2). Protein loading was assessed by α-tubulin. Similar results were obtained in three independent experiments. Quantification of p-SMAD2 expression levels was carried out using Image J Software, represented as relative to p-SMAD2 levels in the RG/C2 cells treated with conditioned medium from the HCT116 cells transfected with the negative control siRNA. Quantification is the mean of 3 independent measurements of a representative result ±SD. Similar results were obtained in three independent experiments.
At the out set of this study we hypothesised that expression of BAG-1 in the developing tumour may allow the cells to overcome the tumour suppressive functions of TGF-β1, promoting conditions within the adenoma that are permissive for growth. Above we have shown that BAG-1 acts as a suppressor of TGF-β1 expression, capable of regulating the growth of the TGF-β1 dependent NRK cell line. To test whether suppressing BAG-1 expression was sufficient to promote inhibitory signalling in TGF-β1 sensitive adenoma derived cells, we tested the effect of the HCT116 BAG-1 siRNA CM on p-SMAD2 signalling in the RG/C2 adenoma derived cells. Initially the RG/C2 cells were incubated with or without TGF-β1 for up to 7 days to confirm that these adenoma derived cells remained sensitive to TGF-β1-mediated growth inhibition (Figure 4E), findings consistent with previous reports [30]. Conditioned medium (CM) was harvested from the HCT116 cell line after transfection with the BAG-1 or negative control siRNAs as before (Figure 4F). The RG/C2 cells were incubated in the CM for 2 hours and activation of the TGF-β1 signalling pathway detected by the level of phosphorylated SMAD2 (Figure 4G). Importantly, treatment with the HCT116 BAG-1 siRNA CM increased phosphorylation of SMAD2 when compared to cells treated with CM from HCT116 cells transfected with the negative control siRNA (Figure 4G). Further this induction of p-SMAD2 in the cells treated with the HCT116/BAG-1 siRNA CM was blocked by addition of the TGF-β1 blocking antibody (Figure 4G), confirming that it was the increase in TGF- β1 secreted from the BAG-1 transfected cells that promoted downstream signalling in the adenoma derived cells. Although the use of CM in long term growth assays was not feasible due to depletion of other factors in the CM, the enhanced TGF- β1 signalling in the adenoma cells is consistent with BAG-1 causing functionally relevant changes in TGF-β1 secretion in colorectal tumour derived cells.
Importantly, these data indicate that the change in TGF-β1 production following manipulation of BAG-1 in colorectal tumour cells (shown in Figure 1C) is physiologically relevant, impacting on the growth of TGF-β1 sensitive cells, which could potentially include both adjacent normal and adenoma derived cells as well as cells within the tumour associated stoma.
DISCUSSION
TGF-β1 is an anti-inflammatory cytokine recognised as important for the regulation of cell growth, differentiation and apoptosis in a wide range of tissues including the intestinal epithelium (reviewed in [2,31]). In normal epithelial cells, TGF-β1 signalling elicits a G1-phase cell cycle arrest and direct transcriptional responses of TGF-β1 signalling include induction of cyclin-dependent kinase inhibitors [32-34], as well as repression of the proto-oncogene, c-MYC [35]. As colorectal adenomas retain sensitivity to TGF-β1 signalling in the early stages of tumorigenesis, the tumour suppressor functions of TGF-β1 pose an important barrier to transformation that cells must over-come in order to allow tumour progression. Importantly, in this report, we have established BAG-1 as a transcriptional repressor of TGFB1, and have shown that manipulation of BAG-1 expression brings about physiologically relevant changes in TGF-β1 production. Our findings suggest that the increase in BAG-1 expression previously reported in colorectal adenoma tissue [12] would suppress TGF-β1 mediated growth inhibition, creating an environment permissive for rapid adenoma growth and progression. These findings now indicate that further analysis of both BAG-1 and TGF-β1 protein expression in adenoma tissue (preceding de-regulation associated with carcinogenesis) is now warranted.
Interestingly, the finding that BAG-1 suppresses TGFB1 expression in tumour cell lines is supported by the reciprocal expression patterns of the proteins in vivo; in normal colonic tissue, at the base of the crypts where BAG-1 expression is highest, TGF-β1 is not detected. Furthermore, in agreement with previous studies [23], BAG-1 expression was found to be predominantly nuclear at the base of the crypt, consistent with a role for BAG-1 in the transcriptional regulation of TGFB1. In contrast, where BAG-1 expression is lowest at the luminal surface of the crypt, TGF-β1 protein is expressed. Taken together, reciprocal expression in the normal tissue and in the cell lines is consistent with a role for BAG-1 as a transcriptional repressor of TGFB1 in colorectal epithelial cells.
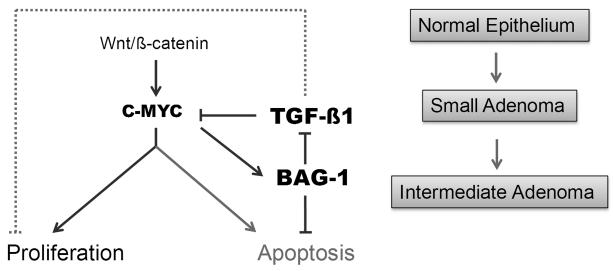
The possible functional importance of increased BAG-1 expression at the very early stages of colorectal tumorigenesis is extended by recently reported findings by Zhang and colleagues [36]. They describe BAG-1 as a C-MYC target and show that BAG-1 expression is required for the inhibition of MYC driven apoptosis in breast and lung epithelial cells [36]. This is of particular interest in the context of colorectal cancer since deregulation of Wnt/MYC signalling is thought to be central to the initiation of colorectal tumorigenesis (reviewed in [37]). Taken with results from this study, we would hypothesise that BAG-1 expression in adenoma tissue (driven by de-regulated wnt/C-MYC signalling) would have a dual role in promoting progression of the colorectal tumour (summarised in Figure 5). Increased BAG-1 expression would not only prevent C-MYC induced apoptosis [36], but as TGF-β1 has previously been associated with repression of c-MYC [38], increased BAG-1 expression (as a repressor of TGF-β1 expression) would further promote de-regulated c-MYC activity. Importantly, high BAG-1 expression in the adenoma cells would also release the epithelial cells from other growth inhibitory effects of TGF-β1, further promoting tumour cell growth and survival (Figure 5).
Figure 5. Dual role for increased BAG-1 expression in promoting progression of colorectal tumorigenesis.
Increased expression of BAG-1 in the adenoma [12] (potentially due to de-regulation of C-MYC expression [36]) leads to suppression of TGF-β1 resulting in loss of growth inhibition and increased c-MYC activity. Increased BAG-1 expression also suppresses c-MYC induced apoptosis hence further promoting tumour cell survival. [Dotted line represents MYC-independent regulation of proliferation by TGF-β1.]
As stated previously, in the early stages of colorectal tumour development, TGF-β1 can act as a tumour suppressor through its effects on cell cycle arrest and apoptosis. However, at later stages in tumour progression, genetic alterations in the canonical TGF-β1 signalling pathway cause the cells to acquire resistance to the growth inhibitory effects of TGF-β1, an event that is likely to be important in the switch of TGF-β1 from tumour suppressor to tumour promoter (reviewed in [1]). Although BAG-1 is reported here to repress TGFB1 expression in colorectal cancer derived cells, it is important to point out that both BAG-1 [12] and TGF-β1 [39-42] levels are frequently up-regulated in colon cancer. Therefore, when considering BAG-1 activity as a possible novel target for the prevention of colorectal cancer, some thought must also be given to the role of TGF-β1 function in the wider context of tumour progression and metastasis. TGF-β1 has been reported to promote oncogenic transformation in a number of cancers, through activating a number of non-canonical signalling pathways independent of SMADs, including PI3K-AKT, ERK-MAPK and RHO-GTPases (reviewed in [43]). However, importantly, we have been unable to show any stimulation of non-canonical SMAD-independent signalling pathways by TGF-β1 in colorectal cancer cell lines (data not shown); in fact, whether TGF-β1 assumes pro-oncogenic activities in colorectal cancer cells is yet to be established [7].
Of note, data presented here show that BAG-1-mediated suppression of TGF-β1 production in colorectal epithelial cells involves a direct inhibition of TGFB1 gene expression, through association of BAG-1 with TGFB1 gene regulatory sequences. This is the first evidence that BAG-1 occupies the gene promoter of a key growth regulatory cytokine, although it is likely that the regulation of TGFB1 gene expression involves BAG-1 as part of a multi-protein complex. For example, recent data identified BAG-1 as part of a trimeric complex [44], where glucocorticoid-mediated transcription was regulated by BAG-1 mediated recruitment of co-repressors [45]. Interestingly, a previous study has shown TGFB1 expression to be modulated by the p65 subunit of NF-κB [46] and BAG-1 has recently been identified as a regulator of NF-κB signalling [12,22]. These findings raise the intriguing possibility that BAG-1-mediated suppression of TGFB1 gene expression could be in part dependent on NF-κB signalling.
BAG-1 has come under much attention in the last decade with regards to its potential use as a biomarker or a therapeutic target in neoplastic disease, and inhibitors of BAG-1 function are currently under investigation [47,48]. Whereas many studies have focused on the function of BAG-1 in resistance of cancer cells to apoptosis, this study has identified BAG-1 as a transcriptional repressor of TGFB1, identifying a novel mechanism to influence the growth of cells in the tumour niche. As such BAG-1 function impinges on a number of the hallmarks of cancer [49]: BAG-1 is an important anti-apoptotic factor, now shown to suppress production of the cytokine TGF-β1, a pleiotropic growth factor also important for immunological homeostasis and inflammatory responses. Establishing BAG-1 as a regulator of this key inhibitory cytokine has important biological implications and demonstrates that BAG-1 is a major player in colorectal tissue homeostasis and tumorigenesis. As early detection and efficient chemoprevention are such fundamental goals in reducing colorectal cancer-related mortality, combined with the fact that BAG-1 levels are increased relatively early in adenoma growth, it remains an exciting possibility that targeting BAG-1 to restore TGF-β1-mediated tumour suppression represents a novel chemo-preventative strategy.
Materials and methods
Cell lines and culture
The human colorectal carcinoma-derived cell lines HCT116 and SW480 were obtained from the American Type Culture Collection (Rockville, USA). HCT116 cells were maintained in McCoy’s 5A Medium (Invitrogen, Paisley, UK), while SW480 cells were maintained in Dulbecco’s Modified Eagles Medium (DMEM; Autogen Bioclear, Wiltshire, UK), both supplemented with 10% foetal bovine serum (FBS) and 2mM glutamine, as well as 100U/mL penicillin and 100μg/mL streptomycin. The S/RG/C2 cell line is a clonogenic, non-tumourigenic adenoma-derived cell line [50,51]. S/RG/C2 cells were maintained in DMEM supplemented with 20% FBS with glutamine, penicillin and streptomycin as previously described, as well as 0.2U/mL insulin and 1μg/mL hydrocortisone (Sigma, Poole, UK). The NRK anchorage-independent growth assay was carried out as previously described [29].
Depletion of BAG1 expression by RNA interference
Cells were reverse transfected using Lipofectamine 2000 (Invitrogen, Paisley, UK) with small interfering RNAs (siRNAs, Applied Biosystems, Warrington, UK) targeting BAG1 or a negative control sequence (50nM) as described previously[12].
Preparation of conditioned medium
Briefly, cells were grown under standard growth conditions until approximately 70% confluent. In parallel, cells were transfected with control siRNA or BAG1 siRNA and allowed to recover for 24 hours in standard growth conditions. Cells were washed twice with PBS before application of 2mL serum-free medium containing 0.1% BSA for a further 24h or 3mL of 10% DMEM plus 0.1% BSA for 72h (depending on the experiment). Conditioned medium was then collected, debris removed by centrifugation and the conditioned medium then subject to thermal activation (80°C for 5 min) and cooled again to 37°C. Blocking experiments were carried out using a 2h pre-incubation with 50μg/mL of the TGF-β1-specific polyclonal antibody (R and D Systems, Minneapolis, MA, USA).
Generation of cell lines stably expressing inducible BAG-1L
HCT116 cells were transfected with 2.5μg pBIG2i/BAG-1L expression construct (under the control of a TET-ON system) [52] using Opti-MEM medium and Lipofectamine 2000 transfection reagent and selected with 400mg/mL G418 to generate HCT116/BAG-1L cells. Stable cell lines were maintained in McCoy’s 5A Medium supplemented with 10% TET-free FBS and glutamine, penicillin and streptomycin as described above, as well as 200mg/mL G418 to maintain the G418-resistant population. BAG-1L expression was induced upon treatment with 2ng/mL Doxycycline (Dox) for 48h.
Luciferase reporter gene assay
A reporter construct containing +11 to −1132 of the TGFB1 gene regulatory region were kindly provided in a promoter-less pGL3 basic vector by S.-J. Kim (Lee Gil Ya Cancer and Diabetes Institute, Gachon University of Medicine and Science, Korea). Growing cells were transiently transfected with 2.5μg pGL3 control vector or the +11/−1132 TGFB1 reporter construct including the pRL-SV40 renilla plasmid (Promega, Southampton, UK) using Lipofectamine 2000 following the manufacturer’s protocol. After 24 hours in standard growth conditions, cells were treated with 1mM NaB (to stimulate TGF-β1 expression) or 10ng/mL TSA (as a control for inhibition of HDAC activity) or the relevant vehicle control in serum-free media for another 24 hours. Cells were harvested and following lysis, luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, Southampton, UK) according to the manufacturer’s instructions.
Quantitative Reverse Transcription Polymerase Chain Reaction (Q-RT-PCR)
Total RNA extraction and comparative quantitative real-time polymerase chain reaction (Q-RT-PCR) was performed as previously described [12]. QuantiTect Primer Assays were obtained from Qiagen Ltd, (Crowley, West Sussex, UK).
Immunoblot analysis
Whole cell lysates were prepared and subjected to immunoblotting as previously described [12] using BAG-1-specific mouse monoclonal antibody (G3E2 [53]; a kind gift from G. Packham, Southampton University, UK), rabbit polyclonal antibody (C16; sc-939 Santa Cruz Biotechnology, CA, USA), phospho-SMAD2-specific rabbit polyclonal antibody (Cell Signalling, Danvers, MA, USA) or α-tubulin mouse monoclonal antibody (Sigma, Poole, UK).
Intestinal epithelial cell fractionation
was carried out and validated as described previously [27,28]. Antibodies used for immunoblots include the BAG-1 rabbit polyclonal antibody C16 (Santa Cruz Biotechnology, CA, USA) and the mouse monoclonal antibodies for F1-ATPsae, PCNA from (Santa Cruz Biotechnology, CA, USA) and villin from (Leica Biosystems, Newcastle, UK).
Quantification of secreted TGF-β1 by ELISA
Total secreted TGF-β1 was activated in cell culture supernatant following addition of 1× protease inhibitor cocktail (Sigma, Poole, UK) by acidification with 1M HCl and subsequent neutralisation with 1.2M NaOH/0.5M HEPES. The concentration of TGF-β1 in each sample was determined using the Quantikine Human TGF-β1 Immunoassay Kit (R&D Systems, Abingdon, UK) according to the manufacturer’s instructions.
Chromatin immunoprecipitation (ChIP)
was carried out as previously described [54] using BAG-1 antibody (C16; as above) and species matched IgG control (Santa Cruz Biotechnology, CA, USA). Three sequences in the TGFB1 gene regulatory region were assessed: −323 to −453, −453 to −560 and −560 to −731, using primers (5′-3′): F>CTCCTGACCCTTCCATCCTT, R>GTCACCAGAGAAAGAGGACCA; F>CAGGGTGTTGAGTGACAGGA, R>CCAGAACGGAAGGAGAGTCA and F>CTCCACGTCACCACCATC, R>CAGCCTCCTGTCACTCAACA respectively. Each experiment was repeated at least twice.
Immunohistochemistry
was carried out as previously described [12]; formalin-fixed, paraffin-embedded normal human large intestinal sections were obtained from the Department of Histopathology, Bristol Royal Infirmary, Bristol, UK with local Ethics Committee approval. TGF-β1 was detected using the rabbit polyclonal antibody (sc-146 (V); Santa Cruz Biotechnology, CA, USA) and BAG-1 was detected using the TB3 antibody (G. Packham, Southampton University, UK).
NRK anchorage-independent growth assay for secreted TGF-β1
5×104 normal rat kidney (NRK) fibroblast cells were resuspended in 1.5mL 0.5% (w/v) SeaPlaque LMP agarose (BMA, Lonza, Slough, UK) in standard 10% DMEM growth medium containing 2ng/mL EGF (NRK growth medium) plus 5ng/mL TGF-β1; or in HCT116/control siRNA CM or HCT116/BAG1 siRNA CM supplemented with 2ng/mL EGF. This top layer was seeded over a 5mL base layer of 0.5% (w/v) agarose in NRK growth medium. After 1 week, colonies over the threshold size (of approximately 50 cells) were counted in at least 10 fields per dish. Each experiment was carried out in triplicate.
Statistical analysis
was carried out using a Student’s t-test in Microsoft Excel and represented as: * = P < 0.05; ** = P < 0.01; *** = P < 0.001
Acknowledgements
This work was funded by a Cancer Research UK programme grant, the Citrina Foundation, a CORE small grant and by the John James Bristol Foundation.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
- 1.Massagué J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikushima H, Miyazono K. TGF-beta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 3.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 4.Avery A, Paraskeva C, Hall P, Flanders KC, Sport M, Moorghen M. TGF-beta expression in the human colon: differential immunostaining along the crypt epithelium. Br J Cancer. 1993;68:137–139. doi: 10.1038/bjc.1993.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard JA, Warwick GJ, Gold LI. Localization of transforming growth factor beta isoforms in the normal murine small intestine and colon. Gastroenterology. 1993;105:67–73. doi: 10.1016/0016-5085(93)90011-z. [DOI] [PubMed] [Google Scholar]
- 6.Heldin CH, Landström M, Moustakas A. Mechanism of TGF-beta signalling to growth arrest, apoptosis and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Lampropoulos P, Zizi-Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. TGF-beta signalling in colon carcinogenesis. Cancer Lett. 2012;314:1–7. doi: 10.1016/j.canlet.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:798–802. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 9.Townsend PA, Stephanou A, Packham G, Latchman DS. BAG-1: A multi-functional pro-survival Protein. Int J Biochem Cell Biol. 2005;37:251–259. doi: 10.1016/j.biocel.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Packham G, Brimmell M, Cleveland JL. Mammalian cells express two differently localized Bag-1 isoforms generated by alternative translation initiation. Biochem J. 1997;328:807–813. doi: 10.1042/bj3280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharp A, Crabb SJ, Townsend PA, Cutress RI, Brimmell M, Wang XH, et al. BAG-1 in carcinogenesis. Exp Rev Mol Med. 2004;6:1–15. doi: 10.1017/S1462399404007537. [DOI] [PubMed] [Google Scholar]
- 12.Clemo NK, Collard TJ, Southern SL, Edwards KD, Moorghen M, Packham G, et al. BAG-1 is up-regulated in colorectal tumour progression and promotes colorectal tumour cell survival through increased NF-kappaB activity. Carcinogenesis. 2008;29:849–857. doi: 10.1093/carcin/bgn004. [DOI] [PubMed] [Google Scholar]
- 13.Arhel NJ, Packham G, Townsend PA, Collard TF, H-Zadeh AM, Sharp A, et al. The retinoblastoma protein interacts with Bag-1 in human colonic adenoma and carcinoma derived cell lines. Int J Cancer. 2003;106:364–371. doi: 10.1002/ijc.11257. [DOI] [PubMed] [Google Scholar]
- 14.Barnes JD, Arhel NJ, Lee SS, Sharp A, Al-Okail M, Packham G, et al. Nuclear BAG-1 expression inhibits apoptosis in colorectal adenoma-derived epithelial cells. Apoptosis. 2005;10:301–311. doi: 10.1007/s10495-005-0804-8. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi R, Noguchi T, Takeno S, Funada Y, Moriyama H, Uchida Y. Nuclear BAG-1 expression reflects malignant potential in colorectal carcinomas. Br J Cancer. 2002;87:1136–1139. doi: 10.1038/sj.bjc.6600579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehring U. Multiple, but concerted cellular activities of the human protein Hap46/BAG-1M and isoforms. Int J Mol Sci. 2009;10:906–928. doi: 10.3390/ijms10030906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niyaz Y, Zeiner M, Gehring U. Transcriptional activation by human Hsp70-associating protein Hap50. J Cell Sci. 2001;114:1839–1845. doi: 10.1242/jcs.114.10.1839. [DOI] [PubMed] [Google Scholar]
- 18.Zeiner M, Niyaz Y, Gehring U. The hsp70-associating protein Hap46 binds to DNA and stimulates transcription. Proc Natl Acad Sci U S A. 1999;96:10194–10199. doi: 10.1073/pnas.96.18.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cutress RI, Townsend PA, Sharp A, Maison A, Wood L, Lee R, et al. The nuclear BAG-1 isoform, BAG-1L, enhances oestrogen-dependent transcription. Oncogene. 2003;22:4973–4982. doi: 10.1038/sj.onc.1206688. [DOI] [PubMed] [Google Scholar]
- 20.Shatkina L, Mink S, Rogatsch H, Klocker H, Langer G, Nestl A, et al. The cochaperone Bag-1L enhances androgen receptor action via interaction with the NH2-terminal region of the receptor. Mol Cell Biol. 2003;23:7189–7197. doi: 10.1128/MCB.23.20.7189-7197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XH, O’Connor D, Brimmell M, Packham G. The BAG-1 co-chaperone is a negative regulator of p73-dependent transcription. Br J Cancer. 2009;100:1347–1357. doi: 10.1038/sj.bjc.6604985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Southern SL, Collard TJ, Urban BC, Skeen VR, Smartt HJ, Hague A, et al. BAG-1 interacts with the p50-p50 homodimeric NF-kappaB complex: implications for colorectal carcinogenesis. Oncogene. 2012;31(22):2761–2772. doi: 10.1038/onc.2011.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takayama S, Krajewski S, Krajewska M, Kitada S, Zapata JM, Kochel K, et al. Expression and location of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissues and tumour cell lines. Cancer Res. 1998;58:3116–3131. [PubMed] [Google Scholar]
- 24.Kim SJ, Glick A, Sporn MB, Roberts AB. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989;264:402–408. [PubMed] [Google Scholar]
- 25.Barnard JA, Warwick GJ. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Diff. 1993;4:495–501. [PubMed] [Google Scholar]
- 26.Wang G, Higgins PJ, Gannon M, Staiano-Coico L. Transforming growth factor-beta 1 acts cooperatively with sodium n-butyrate to induce differentiation of normal human keratinocytes. Exp Cell Res. 1992;198:27–30. doi: 10.1016/0014-4827(92)90144-w. [DOI] [PubMed] [Google Scholar]
- 27.Hague A, Singh B, Paraskeva C. Butyrate acts as a survival factor for colonic epithelial cells: further fuel for the in vivo versus in vitro debate. Gastroenterology. 1997;112:1036–40. doi: 10.1053/gast.1997.v112.agast971036. [DOI] [PubMed] [Google Scholar]
- 28.Mariadason JM, Nicholas C, L’Italien KE, Zhuang M, Smartt HJ, Heerdt BG, et al. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology. 2005;128:1081–1088. doi: 10.1053/j.gastro.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 29.Smartt HJ, Greenhough A, Ordóñez-Morán P, Talero E, Cherry CA, Wallam CA, et al. Beta-catenin represses expression of the tumour suppressor 15-prostaglandin dehydrogenase in the normal intestinal epithelium and colorectal tumour cells. Gut. 2011 doi: 10.1136/gutjnl-2011-300817. e-pub ahead of print 14 Nov 2011; doi:10.1136/gutjnl-2011-300817. [DOI] [PubMed] [Google Scholar]
- 30.Manning AM, Williams AC, Game SM, Paraskeva C. Differential sensitivity of human colonic adenoma and carcinoma cells to transforming growth factor beta (TGF-beta): conversion of an adenoma cell line to a tumorigenic phenotype is accompanied by a reduced response to the inhibitory effects of TGF-beta. Oncogene. 1991;6:1471–1476. [PubMed] [Google Scholar]
- 31.Skeen VR, Paterson I, Paraskeva C, Williams AC. TGF-β1 Signalling, Connecting Aberrant Inflammation and Colorectal Tumori-genesis. Curr Pharm Des. 2012 doi: 10.2174/138161212802083734. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor-beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci U S A. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massagué J, Roberts JM, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 34.Pardali K, Kurisaki A, Morén A, ten Dijke P, Kardassis D, Moustakas A. Role of Smad proteins and transcription factor Sp1 in p21(Waf1/Cip1) regulation by transforming growth factor-beta. J Biol Chem. 2000;275:29244–29256. doi: 10.1074/jbc.M909467199. [DOI] [PubMed] [Google Scholar]
- 35.Mulder KM, Levine AE, Hernandez X, McKnight MK, Brattain DE, Brattain MG. Modulation of c-myc by transforming growth factor-beta in human colon carcinoma cells. Biochem Biophys Res Commun. 1988;150:711–716. doi: 10.1016/0006-291x(88)90449-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XY, Pfeiffer HK, Mellert HS, Stanek TJ, Sussman RT, Kumari A, et al. Inhibition of the single downstream target BAG1 activates the latent apoptotic potential of MYC. Mol Cell Biol. 2011;31:5037–5045. doi: 10.1128/MCB.06297-11. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myant K, Sansom OJ. Wnt/Myc interactions in intestinal cancer: partners in crime. Exp Cell Res. 2011;317:2725–2731. doi: 10.1016/j.yexcr.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 39.Friedman E, Gold LI, Klimstra D, Zeng ZS, Winawer S, Cohen A. High levels of transforming growth factor beta-1 correlate with disease progression in human colon cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:549–554. [PubMed] [Google Scholar]
- 40.Tsushima H, Kawata S, Tamura S, Ito N, Shirai Y, Kiso S, et al. High levels of transforming growth factor beta 1 in patients with colorectal cancer: association with disease progression. Gastroenterology. 1996;110:375–382. doi: 10.1053/gast.1996.v110.pm8566583. [DOI] [PubMed] [Google Scholar]
- 41.Picon A, Gold LI, Wang J, Cohen A, Friedman E. A subset of metastatic human colon cancers expresses elevated levels of transforming growth factor beta1. Cancer Epidemiol Biomarkers Prev. 1998;7:497–504. [PubMed] [Google Scholar]
- 42.Xu WQ, Jiang XC, Zheng L, Yu YY, Tang JM. Expression of TGF-beta1, TbetaRII and Smad4 in colorectal carcinoma. Exp Mol Pathol. 2007;82:284–291. doi: 10.1016/j.yexmp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YE. Non-Smad pathways in TGF-beta signalling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong W, Baniahmad A, Liu Y, Li H. Bag-1M is a component of the in vivo DNA-glucocorticoid receptor complex at hormone-regulated promoter. J Mol Biol. 2008;384:22–30. doi: 10.1016/j.jmb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Hong W, Baniahmad A, Li J, Chang C, Gao W, Liu Y. Bag-1M inhibits the transactivation of the glucocorticoid receptor via recruitment of corepressors. FEBS Lett. 2009;583:2451–2456. doi: 10.1016/j.febslet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Lee KY, Ito K, Hayashi R, Jazrawi EP, Barnes PJ, Adcock IM. NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1beta-induced TGF-beta1 gene transcription. J Immunol. 2006;176:603–615. doi: 10.4049/jimmunol.176.1.603. [DOI] [PubMed] [Google Scholar]
- 47.Sharp A, Cutress RI, Johnson PW, Packham G, Townsend PA. Short peptides derived from the BAG-1 C-terminus inhibit the interaction between BAG-1 and HSC70 and decrease breast cancer cell growth. FEBS Lett. 2009;583:3405–3411. doi: 10.1016/j.febslet.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 48.Sharp A, Crabb SJ, Johnson PW, Hague A, Cutress R, Townsend PA, et al. Thioflavin S (NSC71948) interferes with Bcl-2-associated athanogene (BAG-1)-mediated protein-protein interactions. J Pharmacol Exp Ther. 2009;331:680–689. doi: 10.1124/jpet.109.153601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Paraskeva C, Finerty S, Mountford RA, Powell SC. Specific cytogenetic abnormalities in two new human colorectal adenomaderived epithelial cell lines. Cancer Res. 1989;49:1282–1286. [PubMed] [Google Scholar]
- 51.Williams AC, Browne SJ, Yeudal WA, Paterson IC, Marshall CJ, Lane DP, et al. Molecular events including p53 and k-ras alterations in the in vitro progression of a human colorectal adenoma cell line to an adenocarcinoma. Oncogene. 1993;8:3063–3072. [PubMed] [Google Scholar]
- 52.Strathdee CA, McLeod MR, Hall JR. Efficient control of tetracycline-responsive gene expression from an autoregulated bi-directional expression vector. Gene. 1999;229:21–29. doi: 10.1016/s0378-1119(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 53.Brimmell M, Burns JS, Munson P, McDonald L, O’Hare MJ, Lakhani SR, et al. High level expression of differentially localized BAG-1 isoforms in some oestrogen receptor-positive human breast cancers. Br J Cancer. 1999;81:1042–1051. doi: 10.1038/sj.bjc.6690805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]