Abstract
Recurrent metastatic breast cancer may arise in part due to the presence of drug resistant adult stem cells such as Side Population (SP) cells, whose phenotype has been demonstrated to be due to the expression of ABCG2. We hypothesised that SP may be identified in Fine Needle Aspirates (FNAs) and their presence may be determined by expression of ABCG2 in breast tumours. SP and non-side population cells (NSP) were isolated using dual wavelength flow cytometry combined with Hoechst 33342 dye efflux and analysed for expression of ABCG2 and chemoresistance. FNA samples used in SP analysis were matched with paraffin-embedded tissue which was used in immunohistochemical analysis to assess ABCG2 expression. Results were correlated to the pathobiology of the tumour. MCF7 and MDA-MB-231 cell lines contain SP cells. MCF7 SP have increased expression of ABCG2 and increased resistance to mitoxantrone compared to NSP cells. The presence of SP in FNAs were significantly associated with ER-negative (p=0.008) and with triple negative breast cancers (p=0.011) which were also found to have a significant increase in ABCG2 protein expression. ABCG2 transcript was detected in some but not all SP cell populations isolated from FNA samples.
Keywords: Breast cancer, drug resistance, cancer stem cells, side population, ABCG2
1. Introduction
In recent years a number of studies have identified Side Population (SP) cells in normal tissues, several solid tumours and in cancer cell lines [1; 2; 3]. SP are identified using dual-wavelength flow cytometry combined with Hoechst 33342 dye efflux and are a subpopulation of cells that have an elevated rate of Hoechst efflux [4]. SP isolated from both malignant and non-malignant tissue sources have been shown to exhibit stem cell characteristics such as unlimited self-renewal, multipotent potential and drug resistance [1; 2; 3]. SP cells in normal tissue have been phenotypically defined by their expression of ABC transporters, predominantly ABCG2, an ATP-Binding Cassette half-transporter involved in the efflux of lipophilic chemotherapeutic agents [5].
Many studies have shown that breast cancer is not just “one disease”, but rather tumour behaviour is driven by many different factors. There is a clear role for the ovarian steroids; oestrogen and progesterone in breast carcinogenesis, and breast cancers are routinely screened for the expression of oestrogen receptor (ER) and progesterone receptor (PR) as well as over-expression of Her2. Molecular characterisation of tumours based on hormone status directs patients to the appropriate targeted therapy. Tumours that express the steroid hormone receptors usually respond to endocrine therapy and therefore tend to have a better prognosis than those that do not [6]. Those tumours not expressing these markers are termed “triple negative” (ER−, PR−, HER2-negative) and generally are associated with a more aggressive clinical course. It has been proposed that many cancers may be the product of cancer stem cells and would therefore retain the ability for sustained tumour growth and persist after treatment increasing the risk of relapse [7; 8; 9].
In both mice and humans, SP cells have been identified in mammary gland tissue and constitute approximately 0.2-3.0% of the total cell population in the mouse [10; 11] and between 0.2-5.0% of the total cell population in humans [10; 12; 13]. Jonker et al. demonstrated that both the ABC transporters, ABCG2 and ABCB1 (MDR1), contribute to the SP phenotype in the normal murine mammary gland [14]. In vivo studies have shown that normal mammary gland SP cells have been shown to form lobuloalveolar and ductal-lobular outgrowths [10; 12]. Gene expression analysis on murine mammary gland SP cells has shown an up-regulation of genes associated with multidrug resistance, cell cycle regulation and basal epithelial markers [15].
Following on from this, SP cells identified in the MCF7 breast carcinoma cell line were found to display stem cell properties, express genes commonly associated with stem cells and have a more tumourigenic phenotype than non-SP cells ((NSP),cells that do not readily efflux vital dyes) [1; 3]. Studies on cancer cell lines have demonstrated that SP have an up-regulation of stem cell and ABC transporter genes, have increased invasive potential and exhibit multidrug resistance [1; 2; 3; 16; 17; 18; 19; 20].
At present it is unknown what role SP cells have in breast cancer and if they would be a useful tool in the management of breast cancer. In this study we tested the hypothesis that FNAs could be utilised for SP analysis allowing rapid analysis of patient samples and that the expression of ABCG2 may be used as a marker of SP in patient breast tumours.
2. Methods
2.1 Ethical Approval
At the Royal Victoria Infirmary Newcastle, UK, FNA samples were obtained from a cohort of 49 chemotherapy naive patients together with matched formalin-fixed paraffin-embedded ‘normal’ breast and tumour samples in 25 of the cohort. Prior to surgery, informed consent was obtained from all patients, all of whom had operable invasive breast cancer and all protocols were reviewed by the local Research and Development Department. Ethical approval was sought and approved by the local Research Ethics Committee which allowed the collection and analysis of Fine Needle Aspirates (FNA) taken from palpable breast tumours under anaesthesia but immediately prior to resection and for the study of resected ‘normal’ breast and tumour tissue.
2.2 Preparation and Hoechst staining of breast cancer cell lines
The concentration of Hoechst 33342 used in SP analysis was optimised on both MCF7 and MDA-MB-231 cell lines (ECACC) using a modified version of the technique first described by Goodell et al. (1996) [4]. Breast cancer cells were resuspended at 1×106 cells per ml in pre-warmed complete DMEM and DNase I was added to a final concentration of 0.5 Units to prevent cell aggregation. For both breast cancer cell lines 5μg/ml Hoechst 33342 dye was shown to be optimal (optimal Hoechst 33342 dye concentration was determined using a titration ranging from 1.25-10μg/ml Hoechst). The specific ABCG2 transporter inhibitor, Fumitremorgin C (10μm FTC; Axxora) was added prior to the addition of Hoechst to inhibit dye efflux. Cells were incubated at 37°C for 90 minutes in the dark then washed in ice-cold 1× PBS and pelleted at 400G for 5 minutes. Cells were resuspended in ice-cold 1× PBS to a final concentration of 1×106 cells per ml and filtered through 70μm cell strainers (BD) into sterile FACS tubes. Cells were maintained on ice in the dark for FACS analysis. Prior to cell analysis, non-viable cells were excluded by the addition of 2μg/ml Propidium Iodide. For all analysis, cell analysis and sorting was performed on a Becton Dickinson FACS Digital Vantage (Diva) (BD Biosciences, San Jose, CA) cell sorter. Hoechst was excited at 355nm and a fluorescent profile was generated for dual-wavelength analysis (450/50 nm and 675/20 nm).
2.3 Quantitative Real-Time PCR
Total RNA was isolated from 5,000 FACS-sorted SP and NSP using the Ambion RNAqueous®-Micro Kit (Applied Biosystems, Warrington, UK) according to the instructions of the manufacturer. cDNA was synthesised using the Bioline cDNA synthesis kit (Bioline, London, UK) according to the manufacturer’s instructions. Real-Time PCR was performed using 3μl cDNA and 2X TaqMan Gene Expression Mastermix (Applied Biosystems, Warrington, UK) in 20μl reaction volumes as per the manufacturer’s protocol. Real-Time PCR was performed on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, USA) which consisted of one 2 minute step at 50°C, 10 minutes at 95°C followed by 45 cycles of 95°C for 15 seconds followed by 60°C for 1 minute. All samples were amplified in triplicate and the relative mRNA expression was calculated using the ΔΔCt-method. To quantify differences between samples the relative fold changes were determined by normalising with β-actin mRNA expression. The primer/probes were purchased from Applied Biosystems, ABCG2 gene expression assay ID Hs01053790_m1 and Beta-actin endogenous control 4352935E (Applied Biosystems, Warrington, UK, see supplementary table 1).
2.4 Immunocytochemistry on SP and NSP cell populations
Sorted SP and NSP cells were resuspended in PBS and allowed to air dry onto plus coated glass slides. Slides were fixed in ice-cold methanol overnight, allowed to air-dry and were stored at −20°C prior to analysis. Slides were thawed to room temperature and rehydrated for 5 minutes in 1× PBS. Sections were permeabilised for 5 minutes with 0.6% Triton X-100 (Sigma, Poole, UK). Slides were then washed 3 times, 5 minutes with 1× PBS. Permeabilised cells were then incubated for 1 hour at room temperature in a 1 in 20 dilution of ABCG2 antibody (Abcam plc, Cambridge, UK) containing 1% serum. Slides were washed 3 times 1× PBS and stained with 1 in 25 dilution of FITC-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Europe Ltd., Suffolk, UK) for 30 minutes, in the dark, at room temperature. Sections were then washed 4 times with 1× PBS and mounted with Vectashield anti-fade mounting media (Vector Laboratories Ltd, Peterborough, UK) and stored at 4°C in the dark. Slides were assessed with a Leica TCS-SP2UV confocal laser scanning microscope (Leica Lasertechnik GmbH, Heidelberg, Germany) using Leica TCS-NT software (version 2.0). Images were acquired using a 40× objective lens with a numerical aperture of 1.2. FITC was excited by an excitation beam of 488nm and emission was collected at 530nm. Light emission was collected via a pinhole aperture in front of a photomultiplier and light was collected from one plane of focus. Images were collected sequentially as Z sections, and combined to produce the final image. The mean channel pixel intensity of staining was assessed using Leica Confocal Software (LCS) version 2.61 (Leica Microsystems GmbH Wetzlar, Germany).
2.5 Mitoxantrone Cytotoxicity Assay
It has been reported that MCF7 SP are more resistant to the anthracycline antineoplastic agent mitoxantrone, an ABCG2 substrate, often used to treat metastatic breast cancer [1]. For this reason, in order to determine the effect of chemotherapy on SP and NSP cells from breast cancer cell lines a mitoxantrone cytotoxicity assay was used. 1,000 SP or NSP were sorted into a 96 well plate and cultured overnight in 200μl complete DMEM in a humidified incubator at 37°C, 5% CO2. Cell media was removed and cells were incubated in 200μl complete DMEM (control samples) or 200μl complete DMEM containing 2μg/ml mitoxantrone for a further 48 hours. All experiments were performed in triplicate. After culturing for 48 hours, 20μl of MTS was added to each sample (100μl of cells in culture media) and the plate was incubated for 2 hours at 37°C in a humidified, 5% CO2 atmosphere. The absorbance was read at 490nm and cell viability was determined by expressing the mean absorbance of drug treated wells as a percentage of untreated controls. A one-way ANOVA was performed to determine the significance between different treatment groups and individual student t-tests were performed to compare the effect of different treatments between the SP and NSP populations of both cell lines, P<0.05 was considered to be statistically significant and is denoted by * in figures.
2.6 Preparation and Hoechst staining of Fine Needle Aspirates (FNA)
FNA samples (n=49) were flushed with 1ml 1× PBS and centrifuged at 400G for 5 minutes and red blood cells (RBC) were lysed twice in RBC lysis buffer (2% Tris, 0.83% NH4Cl, pH 7.5). Cells were centrifuged at 400G for 5 minutes and cultured overnight in a humidified incubator in an atmosphere of 5% CO2 at 37°C in complete DMEM. After culturing overnight, cells were resuspended to a final concentration of 1×106 cells per ml in complete DMEM and incubated in a final concentration of 2.5μg/ml Hoechst 33342 dye (optimal Hoechst 33342 dye concentration was determined using a titration ranging from 1.25-10μg/ml Hoechst), in the presence or absence of 50μm verapamil, for 45 minutes at 37°C in the dark. Verapamil is a non-specific calcium channel inhibitor which is a competitive substrate for ABCB1, an ABC transporter known to confer resistance to paclitaxel on ovarian cancer cells [21] whilst verapamil is thought to a less potent inhibitor of ABCG2. Cells were washed and analysed as described previously for the breast cancer cell lines.
2.7 Statistical analysis of Hoechst staining of FNA samples
Chi-square and binary logistic regression was used to determine the probability of SP cell presence/absence, (the dependent variable) being due to the pathobiology of the tumour, for example, the steroid receptor status/grade/lymph node involvement, which are all referred to as binary variables (covariates). SP status was compared to each individual binary variable to determine the significance of each factor alone (SPSS Statistics 17 Statistical Package, SPSS Inc, Chicago, USA).
2.8 Immunohistochemistry using monoclonal antibodies on paraffin sections
The horseradish peroxidase staining technique was performed using Vectastain® Elite ABC kit (Vector Labs Ltd., Peterborough, UK) as per the manufacturer’s protocol. All ‘normal’ and malignant breast samples (n=25) were obtained from breast cancer patients undergoing surgery at the Royal Victoria Infirmary (RVI), Newcastle-upon-Tyne, UK. 5μm serial sections were obtained from formalin-fixed (10% neutral buffered) paraffin embedded tissues. Tissue sections were deparaffinised in xylene for 5 minutes and rehydrated through a series of decreasing ethanol concentrations from 99% to 95% to 70%. Endogenous peroxidases were quenched in 0.6% methanol in water for 10 minutes. Sections were subjected to heat-mediated antigen retrieval in citrate buffer pH 6.0. Sections were hydrated in Tris buffered saline (TBS) pH 7.6 for 5 minutes and blocked with the appropriate blocking serum for 10 minutes. Excess block was replaced with a 1/50 dilution of mouse monoclonal antibody to ABCG2 (ab3380, Abcam, Cambridge, UK) diluted in TBS for 60 minutes. Sections were washed in TBS for 5 minutes twice and then incubated in biotinylated secondary antibody for 30 minutes. Slides were washed twice in TBS and incubated in tertiary antibody for 30 minutes. Slides were washed twice in TBS for 5 minutes and developed in NovaRED peroxidase substrate (Vector Laboratories, Peterborough, UK). Slides were washed in water and dehydrated through 70%, 95% and 99% ethanol and permanently mounted in VectaMount (Vector Laboratories, Peterborough, UK).
Each immunostained section was analysed using a modified “quickscore” method which semi-quantitatively assessed protein expression taking into account the intensity of staining (0=none. 1=weak, 2=moderate, 3=strong and 4=intense) and the percentage of positive cells for each staining intensity (1= 0-25%, 2= 26-50%, 3=51-75% and 4=76-100%) [22]. All scoring was performed blindly, twice by one person with five fields of view being assessed for each sample. The intensity and percentage scores were multiplied to give total scores ranging from 0-16). For example, moderate staining in 10% of the section (2x1) and strong staining in 40% of the section (3×2) would give a total score of 2+6= 8.
Immunohistochemical analysis involved using a Krusal-Wallis one-way analysis of variance and was used to determine if there were any significant relationships between protein expression and lesional grade/steroid receptor status. If the p value was less than 0.05 results were deemed significant and Mann-Whitney U tests (non-parametric tests) were performed to determine if two samples of observations came from the same distribution.
2.9 RT-PCR on SP isolated from FNA samples
SP cells were sorted from FNA samples using a Becton Dickinson FACS Digital Vantage (Diva) cell sorter (BD, San Jose, CA) into Qiagen RNA lysis buffer. Total RNA was isolated using the RNAeasy Plus Micro kit (Qiagen, West Sussex, UK) as per the manufacturer’s instructions which included a step to eliminate genomic DNA contamination. 2μl of RNA was used in one step RT-PCR reaction (Qiagen one-step RT-PCR kit, Qiagen, West Sussex, UK). Reverse-transcription was performed at 50°C for 30 minutes, HotStarTaq DNA polymerase was activated at 95°C for 15 minutes and PCR was performed to determine the expression of ABCB1 and ABCG2 at 94°C for 1 minute, then 35-40 cycles of denaturation at 94°C for 30 seconds, annealing at the temperature indicated in supplementary table 1 for 30 seconds and extension at 72°C for 1 minute, followed by a final extension stage at 72°C for 10 minutes. PCR products were visualised using ethidium bromide using a UV gel documentation system (UVP Ltd, Cambridge, UK).
3. Results
3.1 Assessment of ABCG2 expression in SP isolated from breast cancer cell lines
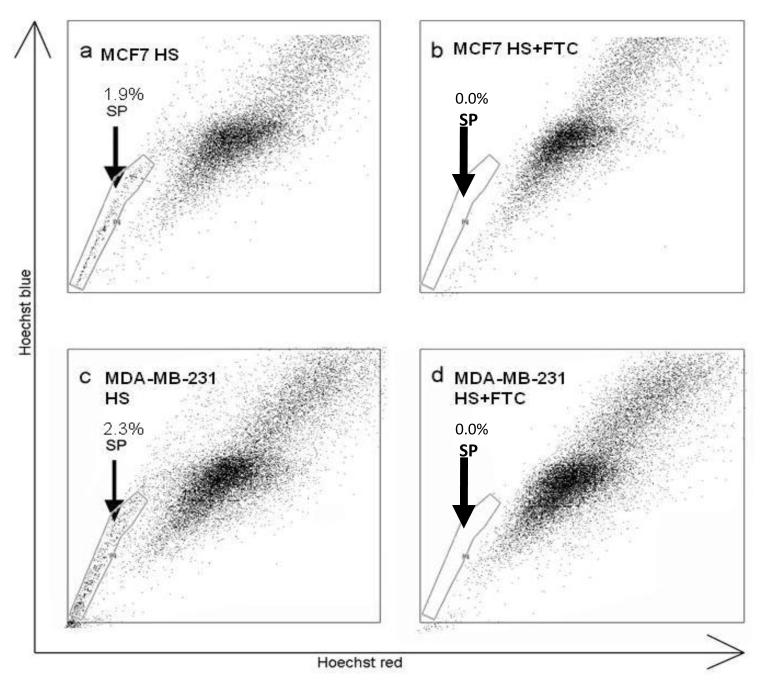
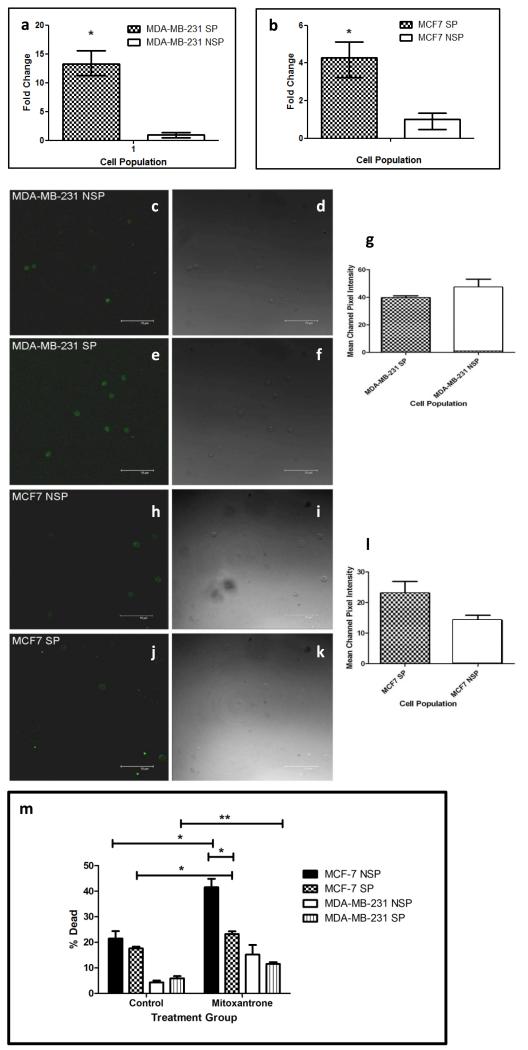
Examination of the oestrogen responsive MCF7 and oestrogen non-responsive MDA-MB-231 human breast cancer cell lines identified SP populations constituting 1.9% (Fig 1a) and 2.3% (Fig 1c) of the total cell population of the MCF7 and MDA-MB-231 cell lines respectively. The SP phenotype was confirmed by addition of the specific ABCG2 inhibitor FTC (Fig, 1b and 1d). Real-time PCR was used to determine the expression of ABCG2 mRNA in both the MCF-7 and MDA-MB-231 SP and NSP populations. Both SP populations have increased ABCG2 mRNA expression compared to their representative NSP populations (Fig, 2a and 2b). Figure 2a demonstrates that MDA-MB-231 SP have a 13.238-fold increase (p=0.0022) in the level of ABCG2 mRNA expressed compared to MDA-MB-231 NSP whilst MCF7 SP have a 4.277-fold increase (p=0.0209) in ABCG2 mRNA compared to NSP (figure 2b). The expression of ABCG2 protein was assessed in SP and NSP isolated from breast cancer cell lines using immunocytochemistry (Figure 2c-l). Similar levels of ABCG2 protein was detected in both populations of the MDA-MB-231 cell line (Figure 2g). Conversely, ABCG2 protein was shown to be increased in the MCF7 SP cell population compared to the NSP population (Figure 2l) as previously documented [1; 3; 23].
Figure 1.
Characterisation of SP in breast cancer cell lines. MCF-7 (a) and MDA-MB-231 (c) cell lines contained SP of 1.9% and 2.3% respectively. SP phenotype was confirmed by addition of FTC (b and d).
Figure 2.
ABCG2 expression and chemoresistance in SP and NSP cells. Quantitative Real-Time PCR was used to determine the level of ABCG2 mRNA expression in SP cells versus NSP in MDA-MB-231 (a) and MCF7 (b). Immunohistochemical analysis for ABCG2 protein expression in MDA-MB-231 NSP cells (c) MDA-MB-231 SP cells (e) MCF7 NSP cells (h) and MCF7 SP cells (J). The mean channel pixel intensity for the ABCG2 staining was assessed for both the MDA-MB-231 SP and NSP (g) and the MCF7 SP and NSP cells (l). Images (d, f, i and k are bright field images of those stained cells shown in panels (c, e, h and j) respectively. Function of ABCG2 was assessed using a mitoxantrone cytotoxicity assay (m). Graph compares the percentage of dead cells in each treatment. (* represents statistical significance using two-sided t-test (n=3) with a p-value < 0.05 and ** denotes p< 0.01 and error bars represent SEM).
A mitoxantrone cytotoxicity assay was used to assess the chemoresistance of both MCF7 and MDA-MB-231 SP and NSP cell populations. Figure 2m compares the percentage of dead cells in mitoxantrone treated cells relative to cells treated with media alone. The addition of mitoxantrone significantly decreased cell viability in all cell populations examined with the exception of MDA-MB-231 NSP cells (see supplementary Table 2). Comparison of cell viability of mitoxantrone-treated SP and NSP cells showed a significant difference between MCF7 SP and NSP populations with NSP having increased levels of cell death compared to SP cells (p=0.0185). In contrast, no significant difference was observed between mitoxantrone-treated MDA-MB-231 SP and NSP populations (p=0.1868). Overall, results from the mitoxantrone cytotoxicity assay suggest that SP cells in the MCF7 cell line are significantly more chemoresistant than MCF7 NSP cells.
3.2 Identification of SP cells in FNA samples
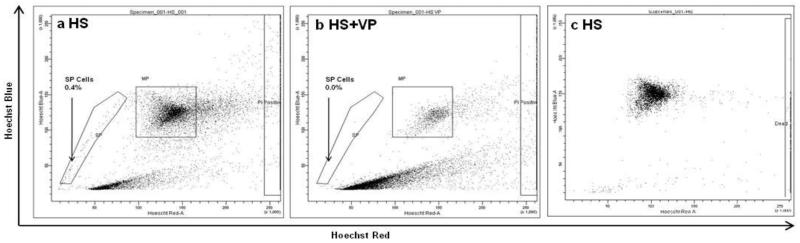
FNA samples were screened for the presence of SP cells within 24 hours of surgery. Figure 3 demonstrates the presence of an SP population of 0.4% of the total cell population in an FNA sample and this could be inhibited by the addition of 50μm verapamil prior to the Hoechst stain (Figure 3B). Figure 3C demonstrates a typical Hoechst profile for an SP-negative FNA sample. Data on additional FNA SP profiles can be found in Supplementary Figure 1.
Figure 3.
Identification of SP cells in Fine Needle Aspirates (FNAs) taken from palpable clinical breast tumours. Staining FNA samples with 2.5μg/ml Hoechst 33342 dye identified an SP of 0.4% (A). Adding 50μm verapamil prior to the Hoechst incubation (HS VP) inhibited Hoechst efflux from the SP (B). An example of an SP-negative FNA sample (C).
3.3 Comparison of patient FNA SP status to patient diagnostics
The presence of an SP was to be analysed with respect to the tumours grade, ER status, PR status, HER2 status and nodal involvement. FNA samples were collected from 49 palpable breast tumours of these no tumour pathology data was available for 7 samples. For 10 the ER status (ER+) was determined but the PR status was not available, for 2 that were ER+PR+ the Her2 data was not available, for one that was ER+, the Her2 and PR status was not available and for one that was ER+PR− the Her2 status was not available. The ER, PR and Her2 status was available for 28 tumours, these were categorised as follows: 6 were ER-PR-Her2− (triple negative), 3 were ER+PR+Her2+, 5 were ER-PR-Her2+ and 14 were ER+PR+Her2-. SP cells were identified in 12 out of 49 FNA samples tested. The SP cell percentages varied between FNA samples and were 0.4, 0.4, 0.9, 3.9, 2.9, 1.8, 1.4, 3.3, 1.2, 0.6, 0.7 and 1.5 percent. Statistical analysis using binary logistic regression to compare SP status with one covariable demonstrated a statistically significant relationship between the FNAs SP status and the ER status of the tumour (p=0.008), with ER-negative samples tending to have an SP. No significant relationships were observed when comparing SP status to any other covariable. Pathology of all the tumours from all aspirate examined revealed 6 patients had tumours that where ER-PR-Her2−. Chi-square analysis comparing the SP status with the triple-negative patient status revealed an association with SP cells being more frequently associated with patient tumours that had an ER-negative, PR-negative and Her2-negative status, with 5 out of 6 of these tumours containing SP cells (p=0.011).
3.4 Expression of ABCG2 in FNA samples screened for the presence of SP cells
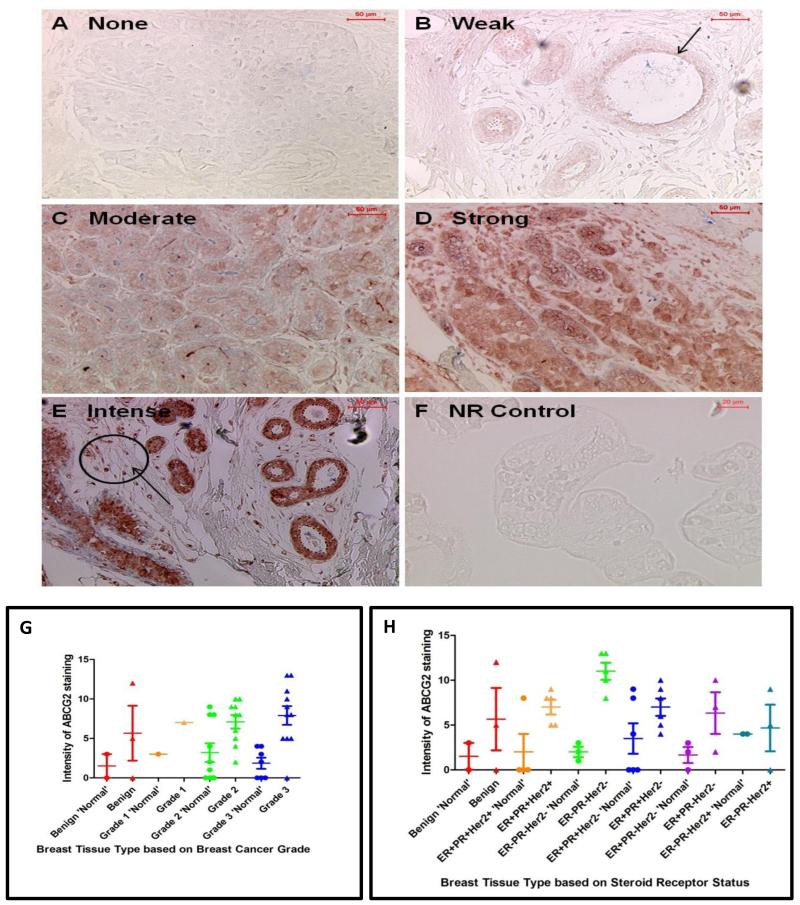
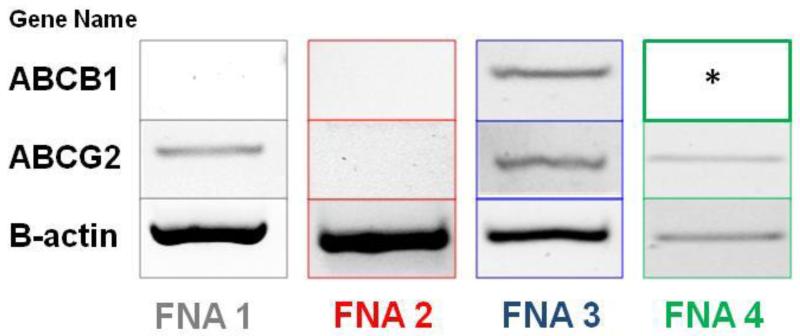
The intensity of ABCG2 and proportion of cells expressing ABCG2 protein was compared in paraffin embedded ‘normal’ and malignant tissue (Figure 4). The ‘normal’ breast tissue samples were subdivided by the grade of the patient’s tumour as this may have influenced the protein expression in the ‘normal’ breast. The modified “quickscore” value was then compared to the tumour grade (see Figure 4A-F for examples of different degrees of ABCG2 staining in breast cancer samples). ABCG2 was expressed by both ‘normal’ and malignant breast tissue as previously documented [24; 25; 26]. ABCG2 was expressed in both the cytoplasm and membrane of cells. There was a statistically significant difference in the expression of ABCG2 protein between grade 2 ‘normal’ breast and grade 2 tumours (p=0.0199) and between grade 3 ‘normal’ and grade 3 breast cancers (p=0.0031) and this trend was also observed in benign and grade 1 cancers although the sample size in these groups were too small to perform statistical analysis (Fig 4G). A trend was also observed with ABCG2 expression increasing with higher tumour grade. Comparing the modified “quickscore” to the steroid receptor status demonstrated a statistically significant difference in the expression of ABCG2 protein between ‘normal’ and malignant breast tissue from patients with ER−PR−HER2− tumours (p=0.0357) and this trend was also observed in all the steroid receptor status groups with cancers demonstrating greater ABCG2 protein expression than corresponding non-malignant areas (Figure 4H). There was also a statistically significant difference between the level of ABCG2 expressed in ER+PR+HER2+ tumours and ER−PR−HER2− tumours (p=0.0399) and between ER+PR+HER2− and ER−PR− HER2− cancers (p=0.0345), with ER−PR−HER2− tumours having the highest expression of ABCG2 protein observed. The expression of ABCG2 and ABCB1 transcripts in SP cells sorted from 4 SP-positive patient FNA samples was performed using reverse-transcriptase PCR. Results demonstrated the expression of ABCG2 transcripts within SP cell populations of FNA 1, FNA 3 and FNA 4 (Fig 5). No ABCG2 transcripts were observed in the SP population of FNA 2. Conversely, ABCB1 transcripts were only observed in SP cells from FNA 3 thus showing differential ABC transporter expression within patient SP populations.
Figure 4.
Scoring of ABCG2 antibody staining intensity. Staining intensity was graded relative to the level of staining observed in the no primary control (NR control: F). Intensity was graded as none (A), weak (B), moderate (C), strong (D) and intense (E) and can be observed as a red product. Images were taken at x10 magnification (A-E) in which scale bar denotes 50μm and at x20 magnification (F) in which scale bar represents 20μm. The modified “quickscore” method was used to compare ABCG2 protein expression to the lesion grade in ‘normal’ breast and breast tumour tissue (G) and to steroid receptor status (H). ‘Normal’ breast was segregated based on the patient’s tumour. Error bars denote standard error of the mean and arrows indicate examples of areas that are ABCG2-postive. ER= oestrogen receptor; PR= progesterone receptor; HER2= human epidermal growth factor receptor 2; SP= side population; − = negative; += positive. In (G) where staining intensity for ABCG2 is compared with lesion grade breast tissue type is represented as follows: Benign ‘Normal’ = red circles, Benign = red triangles, Grade 1 ‘Normal’ = orange circles, Grade 1 = orange triangles, Grade 2 ‘Normal’ = green circles, Grade 3 ‘Normal’ = blue circles, Grade 3 = blue triangles. In (H) breast tissue type was represented as follows: Benign ‘Normal’ = red circles, Benign = red triangles, ER+PR+Her2+ ‘Normal’ = orange circles, ER+PR+Her2+ = orange triangles, ER-PR-Her2− ‘Normal’ = green circles, ER-PR-Her2− = green triangles, ER+PR+Her2− ‘Normal’ = blue circles, ER+PR+Her2− = blue triangles, ER+PR-Her2− ‘Normal’ = purple circles, ER+PR-Her2− = purple triangles, ER-PR-Her2+ ‘Normal’ = seagreen circles, ER-PR-Her2+ = seagreen triangles.
Figure 5.
Transcriptional expression of ABCG2 and ABCB1 in SP cells sorted from patient FNA samples. * indicates inadequate levels of RNA left to perform this experiment.
4. Discussion
This study demonstrated that both oestrogen responsive and non-responsive breast cancer cell lines contain SP cells, and that SP of the MCF7 cell line expressed elevated levels of ABCG2 protein and were more drug resistant than the MCF7 NSP. Importantly SP cells detected in FNA samples were significantly associated with ER-negative and triple-negative breast cancers. IHC analysis of matched FFPE-breast tumour tissue demonstrated a significant increase in the expression of ABCG2 protein within ER-PR-HER2-negative tumours compared to other breast cancer subtypes which could indicate the increased prevalence of SP within this particular cohort. However, this study also demonstrated that not all patient-derived SP cells express ABCG2 transcripts, suggesting that ABCG2 alone may not be a suitable marker for the identification of SP cells in breast cancer.
In 2010, the first study to identify SP in primary human breast cancer cells demonstrated a predominance of SP within the luminal subtype and demonstrated the importance of Her2 signalling within this population [16]. HER2 is commonly used as a predictive marker of patient response in breast cancer to chemotherapeutic agents commonly effluxed by the ABCG2 transporter [27; 28], the putative SP marker. This led to the hypothesis that SP may be responsible for the poor response of Her2-positive breast cancers to conventional chemotherapy [16].
SP cells have been observed in several breast cancer cell lines [1; 3; 23; 29] and this study confirmed the presence of SP cells in both the MCF7 and MDA-MB-231 cell lines. The presence of SP cells in the MDA-MB-231 breast cancer cell lines is controversial with some studies documenting their presence [23; 30] and others their absence [3; 29; 31]. This discrepancy could be due to multiple factors including differences in the Hoechst 33342 dye staining protocol [32], the cell line passage number, the cell culture conditions, level of confluence and the FACSdata acquisition and analysis.
We also verified a differential increase in mRNA encoding ABCG2 in SP cells from these cell lines. However, the level of ABCG2 protein expressed by SP cells was shown to be increased only in the MCF7 SP when compared to the respective NSP cells. The discordance of ABCG2 mRNA and protein expression may indicate a rapid rate of protein turnover within the MDA-MB-231 SP or different post-translational modifications may be present in MDA-MB-231 SP cells that alter antibody specificity. The expression of ABCG2 mRNA has been shown to be significantly increased in SP cells when compared to the NSP population in both the MCF7 [3; 31; 33; 34] and MDA-MB-231 cell lines [23]. Moreover it has been reported that the majority of breast cancer cell lines contain a small population of ABCG2-positive cells but not all of these cell lines contain an SP [3]. Several studies have also shown that the MDA-MB-231 cell line has high expression of ABCG2 but does not contain an SP [3; 29; 31]. These data suggest that the identification of an SP does not reflect expression of ABCG2 protein.
Results from the cytotoxicity assay demonstrated that MCF7 cell populations are more sensitive than MDA-MB-231 cells to mitoxantrone. This contradicts a report that MDA-MB-231 cells are more sensitive to mitoxantrone than the MCF7 cell line [35]. This conflict could be due to many different factors such as the concentration of mitoxantrone used, the origin of the breast cancer cell lines, passage number of the cells tested or the effect of the cell sorting process.
Unlike previously published studies which have demonstrated that SP cells are resistant to mitoxantrone treatment [1; 18; 36; 37] we observed a significant difference in the level of cell viability between mitoxantrone-treated SP and NSP from the MCF7 cell line but not in the MDA-MB-231 cell populations. The MCF7 cell line data is consistent with previously published studies [1; 18; 36; 37]. One explanation for this difference could be the level of ABCG2 protein expressed by each population (figure 2). MDA-MB-231 SP and NSP cells were found to have similar levels of ABCG2 protein and had similar levels of cell viability when treated with mitoxantrone. Conversely MCF7 SP cells were shown to have increased levels of ABCG2 protein compared to NSP cells which may account for the difference in cell viability observed when both populations are treated with mitoxantrone. Another reason could be the differential expression of ABC transporters between SP of the two different cell lines. Using the Bcrp1− /Mdr1a−/Mdr1b− triple knockout mouse model, it has been demonstrated that all 3 ABC transporters are responsible for the SP phenotype in the murine mammary gland [14]. It has also been reported that MCF7 SP cells are more resistant to mitoxantrone and carboplatin [34], and that SP cells isolated from MCF7 and MDA-MB-231 cell lines have significantly increased survival when treated with doxorubicin, methotrexate and 5-FU [23]. The resistance of SP cells to this diverse array of chemotherapeutic agents suggests the involvement of several drug resistance transporters e.g., ABCB1, ABCC1, ABCC2, ABCC3, ABCC4, ABCC5, ABCC6, ABCC11, ABCC12 and ABCG2 which are capable of effluxing these agents [38] and may be responsible for the SP phenotype.
Overall, results from the mitoxantrone cytotoxicity assay suggest that the SP phenotype confers a survival advantage on SP in the MCF7 cell line. This warrants further investigation to determine if inhibition of ABC transporters responsible for the SP phenotype may render SP-positive breast cancer patients more sensitive to commonly used breast cancer therapies.
This study provides the first evidence that SP cells can be identified in FNAs and that the percentage of these SP is consistent with levels observed in other solid tumours[1, 18].
Studies investigating the transcriptional expression of ABC transporters within patient SP cells (Fig 5) demonstrated differential expression of ABC transporter transcripts. This is supported by studies that have shown that both ABCG2 and ABCB1 expression confers the SP phenotype within the murine mammary gland SP [14; 39] and SP from neuroblastoma cell lines have high levels of expression of ABCG2 and had an increased ability to efflux mitoxantrone compared to the NSP [1].
The data linking SP to ER-negative breast tumours contradict those of Nakanishi et al [16] but this could be due to differences in specimens used for analysis. We used cells analysed directly from the tumour while Nakanishi et al [16] prepared primary human breast cancer cell lines and characterised SP cells from these cell lines after several passages, the effects of this culturing process could change the SP characteristics. The data relating the presence of SP with ER-negative tumours are interesting as ER status is a major prognostic indicator in breast cancer, with ER-negative breast tumours tending to be more aggressive than ER-positive cancers. Wu and colleagues (2007) demonstrated that higher grade mesenchymal tumours contain higher proportions of SP than low grade lesions and postulated that if SP cells were characterised further that they may be used as a prognostic indictor of mesenchymal cancer [18].
We also used IHC analysis to determine ABCG2 protein expression in matched ‘normal’ and malignant breast tissue, and showed that higher levels of ABCG2 protein were expressed in the malignant areas compare with ‘normal’ areas and that ER−PR−HER2− tumours had the highest expression of ABCG2 protein. In the few studies that have evaluated the expression of ABCG2 in breast carcinomas, Diestra and colleagues demonstrated the expression of ABCG2 protein in 5 of 9 tumours [24]. Conversely, other studies have shown the low or absent expression of ABCG2 in breast tumours [25; 26] compared to the level expressed by normal breast ducts [25]. In contrast to our study, Faneyte and colleagues (2002) demonstrated that ABCG2 expression decreased with increasing breast cancer grade [26]. These discrepancies are probably due to inconsistencies with methodology with different pre-treatments being performed on paraffin-embedded tissue, or studies staining frozen rather than paraffin sections, the use of different ABCG2 antibodies with different specificities, differences in scoring methods and also the assessment of small numbers of patient samples.
This study is the first to identify SP cells in FNAs, this is exciting as FNA samples are taken routinely in the clinic and are a safe, minimally invasive procedure which provides an accurate representation of the tumour mass. The presence of SP cells can be screened immediately prior to evaluation of the solid tumour using more traditional IHC-based methods employed by the pathology laboratory. Further studies are needed to evaluate SP and links to the development of chemotherapeutic resistance, metastatic disease or disease-free survival.
Supplementary Material
Supplementary Figure 1- SP profiles for all FNAs containing an SP population. SP percentages of 0.4% (a), 3.9% (b) 2.9% (c) and 3.3% (d) of the total cell populations were observed.
Acknowledgments
The authors would like to acknowledge the Breast Cancer Surgical Team and participating patients at the Royal Victoria Infirmary (RVI), Newcastle upon Tyne, and also the RVI pathology department who provided pathobiology information. The authors would also like to thank the RVI Breast Cancer Appeal that funded this project and Dr Tom Chadwick, Newcastle University, UK, for his statistical advice.
This work was funded by the RVI Breast Cancer Appeal, Royal Victoria infirmary, Newcastle-upon-Tyne, UK, Gateshead NHS Trust and Susan B. Komen grant KG081099.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
We confirm that all authors fulfil all conditions required for authorship. We also confirm that there is no potential conflict of interest or financial dependence regarding this publication, as described in the instruction for authors. All authors have read and approved the manuscript.
References
- [1].Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proceedings of the National Academy of Science. 2004;1010:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. PNAS. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Research. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- [4].Goodell M, Brose K, Paradis G, Conner A, Mulligan R. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. Journal of Experimental Medicine. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proceedings of the National Academy of Sciences. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumourigenesis. Breast Cancer Research. 2002;4:197–201. doi: 10.1186/bcr452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dick JE. Breast cancer stem cells revealed. Proceedings of the National Academy of Sciences. 2003;100:3547–3549. doi: 10.1073/pnas.0830967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- [9].Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Current Opinion in Biotechnology. 2007;18:460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- [10].Alvi A, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco M.d., Dale T, Smalley M. Functional and molecular characterisation of mammary side population cells. Breast Cancer Research. 2003;5:R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Scal-1pos cells in the mouse mammary gland represent an enriched progenitor cell population. Developmental Biology. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- [12].Clayton H, Titley I, Vivanco M. Growth and Differentiation of progenitor/stem cells derived from the human mammary gland. Experimental Cell Research. 2004;297:444–460. doi: 10.1016/j.yexcr.2004.03.029. [DOI] [PubMed] [Google Scholar]
- [13].Clarke R, Spence K, Anderson E, Howell A, Okano H, Potten C. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Developmental Biology. 2005;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- [14].Jonker JW, Freeman J, Bolscher E, Musters S, Alvi AJ, Titley I, Schinkel AH, Dale TC. Contribution of the ABC Transporters Bcrp1 and Mdr1a/1b to the Side Population Phenotype in Mammary Gland and Bone Marrow of Mice. Stem Cells. 2005;23:1059–1065. doi: 10.1634/stemcells.2005-0150. [DOI] [PubMed] [Google Scholar]
- [15].Behbod F, Xian W, Shaw C, Hilsenbeck S, Tsimelzon A, Rosen J. Transcriptional profiling of mammary gland side population cells. Stem Cells. 2006;24:1065–1074. doi: 10.1634/stemcells.2005-0375. [DOI] [PubMed] [Google Scholar]
- [16].Nakanishi T, Chumsri S, Khakpour N, Brodie AH, Leyland-Jones B, Hamburger AW, Ross DD, Burger AM. Side-population cells in luminal-type breast cancer have tumour-initiating cell properties, and are regulated by HER2 expression and signalling. British Journal of Cancer. 2010;102:815–826. doi: 10.1038/sj.bjc.6605553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, MacLaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proceedings of the National Academy of Sciences. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu C, Wei Q, Utomo V, Nadesan P, Whetstone H, Kandel R, Wunder JS, Alman BA. Side Population Cells Isolated from Mesenchymal Neoplasms Have Tumor Initiating Potential. Cancer Research. 2007;67:8216–8222. doi: 10.1158/0008-5472.CAN-07-0999. [DOI] [PubMed] [Google Scholar]
- [19].Shi G, Xu Y, Fan J, Zhou J, Yang X, Qiu S, Liao Y, Wu W, Ji Y, Ke A, Ding Z, He Y, Wu B, Yang G, Qin W, Zhang W, Zhu J, Min Z, Wu Z. Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. Journal of Cancer Research and Clinical Oncology. 2008;134:1155–1163. doi: 10.1007/s00432-008-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ho M, Ng A, Lam S, Hung J. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Research. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- [21].Yusa K, Tsuruo T. Reversal Mechanism of Multidrug Resistance by Verapamil: Direct Binding of Verapamil to P-Glycoprotein on Specific Sites and Transport of Verapamil Outward across the Plasma Membrane of K562/ADM Cells. Cancer Research. 1989;49 [PubMed] [Google Scholar]
- [22].Detre S, Jotti G. Saclani, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. Journal of Clinical Pathology. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Steiniger S, Coppinger J, Krüger J, Yates J.r., Janda K. Quantitative mass spectrometry identifies drug targets in cancer stem cell-containing side population. Stem Cells. 2008;26:3037–3046. doi: 10.1634/stemcells.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Diestra J, Scheffer G, Català I, Maliepaard M, Schellens J, Scheper R, Germà-Lluch J, Izquierdo M. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. Journal of Pathology. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- [25].Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg ACLM, Schinkel AH, van de Vijver MJ, Scheper RJ, Schellens JHM. Subcellular Localization and Distribution of the Breast Cancer Resistance Protein Transporter in Normal Human Tissues. Cancer Research. 2001;61:3458–3464. [PubMed] [Google Scholar]
- [26].Faneyte IF, Kristel PMP, Maliepaard M, Scheffer GL, Scheper RJ, Schellens JHM, van de Vijver MJ. Expression of the Breast Cancer Resistance Protein in Breast Cancer. Clinical Cancer Research. 2002;8:1068–1074. [PubMed] [Google Scholar]
- [27].Menard S, Valagussa P, Pilotti S, Gianni L, Biganzoli E, Boracchi P, Tomasic G, Casalini P, Marubini E, Colnaghi M, Cascinelli N, Bonadonna G. Response to cyclophosphamide, methotrexate, and fluorouracil in lymph node-positive breast cancer according to HER2 overexpression and other tumor biologic variables. Journal of Clinical Oncology. 2001;19:329–335. doi: 10.1200/JCO.2001.19.2.329. [DOI] [PubMed] [Google Scholar]
- [28].Xie Y, Xu K, Linn D, Yang X, Guo Z, Shimelis H, Nakanishi T, Ross D, Chen H, Fazli L, Gleave M, Qiu Y. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. Journal of Biological Chemistry. 2008;283:3349–3356. doi: 10.1074/jbc.M707773200. [DOI] [PubMed] [Google Scholar]
- [29].Christgen M, Ballmaier M, Bruchhardt H, von Wasielewski R, Kreipe H, Lehmann U. Identification of a distinct side population of cancer cells in the Cal-51 human breast carcinoma cell line. Molecular and Cellular Biochemistry. 2007;306:201–212. doi: 10.1007/s11010-007-9570-y. [DOI] [PubMed] [Google Scholar]
- [30].Liu Y, Lu W-L, Guo J, Du J, Li T, Wu J-W, Wang G-L, Wang J-C, Zhang X, Zhang Q. A potential target associated with both cancer and cancer stem cells: A combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. Journal of Controlled Release. 2008;129:18–25. doi: 10.1016/j.jconrel.2008.03.022. [DOI] [PubMed] [Google Scholar]
- [31].Yin L, Castagnino P, Assoian RK. ABCG2 Expression and Side Population Abundance Regulated by a Transforming Growth Factor {beta}-Directed Epithelial-Mesenchymal Transition. Cancer Research. 2008;68:800–807. doi: 10.1158/0008-5472.CAN-07-2545. [DOI] [PubMed] [Google Scholar]
- [32].Golebiewska A, Brons NHC, Bjerkvig R, Niclou SP. Critical Appraisal of the Side Population Assay in Stem Cell and Cancer Stem Cell Research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- [33].Engelmann K, Shen H, Finn OJ. MCF7 Side Population Cells with Characteristics of Cancer Stem/Progenitor Cells Express the Tumor Antigen MUC1. Cancer Research. 2008;68:2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- [34].Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proceedings of the National Academy of Sciences. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vibet S, Maheo K, Gore J, Dubois P, Bougnoux P, Chourpa I. Differential Subcellular Distribution of Mitoxantrone in Relation to Chemosensitization in Two Human Breast Cancer Cell Lines. Drug Metabolism and Disposition: the biological fate of chemicals. 2007;35:822–828. doi: 10.1124/dmd.106.013474. [DOI] [PubMed] [Google Scholar]
- [36].Loebinger M, Giangreco A, Groot K, Prichard L, Allen K, Simpson C, Bazley L, Navani N, Tibrewal S, Davies D, Janes S. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. British Journal of Cancer. 2008;98:380–387. doi: 10.1038/sj.bjc.6604185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang J, Guo L, Chen L, Zeng Y, Lu S. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Research. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- [38].Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature Reviews Drug Discovery. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- [39].Tadjali M, Zhou S, Rehg J, Sorrentino BP. Prospective Isolation of Murine Hematopoietic Stem Cells by Expression of an Abcg2/GFP Allele. Stem Cells. 2006;24:1556–1563. doi: 10.1634/stemcells.2005-0562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1- SP profiles for all FNAs containing an SP population. SP percentages of 0.4% (a), 3.9% (b) 2.9% (c) and 3.3% (d) of the total cell populations were observed.