Abstract
The knowledge about the bone cement is of paramount importance to all Orthopaedic surgeons. Although the bone cement had been the gold standard in the field of joint replacement surgery, its use has somewhat decreased because of the advent of press-fit implants which encourages bone in growth. The shortcomings, side effects and toxicity of the bone cement are being addressed recently. More research is needed and continues in the field of nanoparticle additives, enhanced bone–cement interface etc.
Keywords: Bone cement, Joint replacement, Arthroplasty, Antibiotic, Viscosity
1. Introduction
Polymethyl methacrylate (PMMA), is commonly known as bone cement, and is widely used for implant fixation in various Orthopaedic and trauma surgery. In reality, “cement” is a misnomer because, the word cement is used to describe a substance that bonds two things together. However, PMMA acts as a space-filler that creates a tight space which holds the implant against the bone and thus acts as a ‘grout’.1 Bone cements have no intrinsic adhesive properties, but they rely instead on close mechanical interlock between the irregular bone surface and the prosthesis. Other types of commercially available bone cement like calcium phosphate cements (CPCs) and Glass polyalkenoate (ionomer) cements (GPCs) are successfully used in a variety of orthopaedic and dental applications. CPCs are bio resorbable and biocompatible, but are mainly used in cranial and maxillo-facial surgeries because of their low mechanical strength.2
Even though the uses and availability of various types of bone cement has greatly evolved over the past century, further research still continues to develop its more clinical applications and to reduce the adverse effects associated with their use.
2. Historical perspective
Themistokles Gluck (1870), had fixed a total knee prosthesis made of ivory using cement made of plaster and colophony.3 Otto Rohm and Kulzer were early pioneers who worked extensively on the physical properties and uses of bone cement. The era of modern PMMA bone cements comes from the patent by Degussa and Kulzer (1943), who had described the mechanism of polymerization of methyl methacrylate (MMA) at room temperature if a co-initiator, such as a tertiary aromatic amine, is added.4
The first bone cement use in Orthopaedics is widely credited to the famous English surgeon, John Charnley, who in 1958, used it for total hip arthroplasty.5 He had used cold-cured PMMA to attach an acrylic cup to the femoral head and to seat a metallic femoral prosthesis. This was a significant milestone in the advancement of Orthopaedic surgical procedures. Also, Charnley was the first to realize that PMMA easily could be used to fill the medullary canal and is easy to blend with the bone morphology.
In the 1970's, the U.S. Food and Drug Administration (FDA) approved bone cement for use in hip and knee prosthetic fixation.6 Since then, while bone cement has become widely used for fixation of prostheses to living bone, the trends of bone cement usage have evolved.
3. PMMA constituents
PMMA is an acrylic polymer that is formed by mixing two sterile components (Table 1): a liquid MMA monomer and a powered MMA-styrene co-polymer.7 When the two components are mixed, the liquid monomer polymerizes around the pre polymerized powder particles to form hardened PMMA. In the process, heat is generated, due to an exothermic reaction.
Table 1.
Constituents of bone cement.
| Powder | Liquid |
|---|---|
|
|
|
|
|
|
|
PMMA, along with the presence of various additives, gives the mixture a set of physical and chemical properties.3 Exposure to light or high temperatures can cause premature polymerization of the liquid component. Hydroquinone therefore is added as a stabiliser or inhibitor to prevent premature polymerization. An initiator, di-benzoyl peroxide (BPO), is added to the powder, and an accelerator, mostly N, N-dimethyl-p-toluidine (DmpT), is added to the liquid to encourage the polymer and monomer to polymerise at room temperature (cold curing cement).
In order to make the cement radiopaque, a contrast agent is added. Commercially available cements use either zirconium dioxide (ZrO2) or barium sulphate (BaSO4). Zirconium dioxide is one hundred times less soluble than barium sulphate and has less effect on the mechanical properties of the cement.
During the exothermic free-radical polymerization process, the cement heats up. This polymerization heat reaches temperatures of around 82–86 °C in the body. The cause of the low polymerization temperature in the body is the relatively thin cement coating, which should not exceed 5 mm, and the temperature dissipation via the large prosthesis surface and the flow of blood.8
3.1. Antibiotic bone cement
Bone cement has proven particularly useful because specific active substances, e.g. antibiotics, can be added to the powder component. This makes bone cement a modern drug delivery system that delivers the required drugs directly to the surgical site. The local active substance levels of bone cements are significantly below the clinical routine dosages for systemic single injections. Research has shown that adding various types of antibiotics to bone cement, in quantities less than 2 g per standard packet of bone cement, does not adversely affect some of the cement's mechanical properties (compressive or diametrical tensile strengths), although quantities exceeding 2 g did weaken them.9 Various antibiotics have been successfully mixed and used with bone cements like Gentamycin, Tobramycin, Erythromycin, Cefuroxime, Vancomycin, Colistin etc. The basic requirement, being that the mixable antibiotic should be heat resistant and should last for longer duration of time.
Gentamycin, when used in combination with tobramycin, shows a synergistic effect, with a 68% greater elution of tobramycin (P = 0.024), and 103% greater elution of vancomycin from the bone cement (P = 0.007), compared to controls containing only one antibiotic.10
van Staden in his study reported that Bacteriocins may be a possible alternative to antibiotics incorporated into bone cement. The in vitro results of the study showed that bacteriocins incorporated into brushite cement did not significantly alter the characteristics of the matrix and that the peptides were released in an active form. Finally it was shown that nisin F-loaded brushite cement controlled S. aureus infection in mice.11
Silver containing nanoparticles have also shown promise as effective antibacterial agents which can be added to bone cement.12 Vitamin E additives (10%) have shown a positive effect on free radical oxidation and exothermic activity, with only modest reduction (<5%) in tensile strength.4
Compared to intramuscular administration, systemic concentration levels of Gentamycin are low with bone cement, usual maximum level being <1 μg/ml (<10%). There are no detectable systemic levels after seven days from administration. Gentamycin levels in urine after bone cement administration range from 10 μg/ml initially to 1–2 μg/ml after seven days.
Different bone cements have different chemical formulations, giving a range of antibiotic bone cements with varying handling characteristics, which are suited to a broad range of clinical requirements and surgical techniques.
3.2. Usage and properties
Since Charnley first began using acrylic bone cement in hip arthroplasty, there have been a number of developments in the usage and properties of bone cement.
3.3. Curing process
The curing process is divided into 4 stages: a) mixing, b) sticky/waiting, c) working, and d) hardening. The mixing can be done by hand or with the aid of centrifugation or vacuum technologies.
Bone cements are heat sensitive. Any increase or decrease in temperature (either ambient, and/or of the cement components and mixing equipment) from the recommended temperature of 73 °F (23 °C) affects the handling characteristics and setting time of the cement. Manual handling and body temperature reduces the final setting time. Variations in humidity affect the cement handling characteristics and setting time. It is recommended that the unopened cement components are stored at 73 °F (23 °C) for a minimum of 24 h before use. Vacuum mixing of cement can also accelerate the setting time of the cement.
High viscosity cements are sometimes pre-chilled for use with mixing systems for easier mixing and prolonged working phase. This will also increase the setting time. The relative humidity might also influence the handling properties. That is the reason why the working time and setting time of the cement might vary in winter and summer.
Unlike the polymerization reaction of PMMA, calcium phosphate cements are hardened through a dissolution and precipitation process that produces hardening with entanglement of precipitated crystals.2
4. Methods of application
Various methods exist for the application of cement into the bone or joint surface.13
4.1. Digital
All antibiotic bone cements can be applied digitally. The cement is mixed thoroughly but carefully to minimize the entrapment of air. Once dough is formed the surgeon should wait until the cement no longer adheres to the glove and the surface has become dull as opposed to shiny. The cement can then be taken into gloved hands and kneaded thoroughly. It is vital that premature insertion of cement is avoided as this may lead to a drop in the patient's blood pressure. Importantly, this stage will occur at different times for different cement types.
The time of cement application and prosthesis insertion is at the discretion of the surgeon and will depend upon the surgical procedure used. In general, implant insertion should be delayed until the cement has developed a sufficient degree of viscosity to resist excessive displacement by the implant. However, implant insertion should not be delayed such that there is a risk that the procedure cannot be completed due to cement hardening.
Following introduction the implant must be firmly held in position to avoid movement and pressurization must be maintained until the cement finally hardens. Excess bone cement must be removed before the cement has completely hardened.
4.2. Syringe application
Gentamycin antibiotic bone cements may be applied using a suitable cement gun and syringe (Fig. 1). The surgeon should use their experience to judge when the cement has reached an appropriate viscosity to be extruded. This will not occur until after the cement has formed dough. A small amount of cement should be extruded from the syringe and visually assessed to ensure that the surface of the cement appears dull and excessive flow under gravity has ceased.
Fig. 1.
Powder and liquid components of bone cement.
Prior to extrusion, it is recommended that a cement restrictor be inserted, at the required depth into, the prepared bone cavity.14 Introduction of bone cement into the prepared cavity should be carried out in a retrograde fashion. Once the cavity is filled it is advisable that adequate pressurization is applied and maintained up to the point of hardening.
4.3. Vacuum mixing & delivery
Vacuum mixing, which was adapted from the dental field, was developed for bone cement in the early 1980s. Vacuum mixing reduces bone cement porosity and reduces monomer evaporation and exposure in the operating room.
Mixing as well as collecting cement under vacuum yields a homogenous mix without affecting viscosity or other properties of the cement.15
4.4. Pressurization
The pressure applied to the cement has to be larger than the blood pressure so as not to be pushed out of the bone. Pressure should be applied until the viscosity of the bone cement has increased so it is high enough to resist blood pressure. Many studies state that pressurization results in greater penetration of the bone, improves bone–cement interface and increases fatigue strength of the cement. When pressurizing the cement in the femur, a positive sign of pressurization is marrow extrusion in the greater trochanter (the so-called sweating trochanter sign).16
4.5. Viscosity
Mixing together the powder and the liquid components marks the start of the polymerization process. During the reaction, the cement viscosity increases, slowly at first, then later more rapidly (Fig. 2). Studies have shown that high viscosity cements result in better prosthetic fixation, as compared to low viscosity cements.17
Fig. 2.
Mixing of bone cement.
Bone cements may be divided into three kinds: low, medium and high viscosity.
Low viscosity: these cements have a long-lasting liquid, or mixing phase, which makes for a short working phase. As a consequence, application of low viscosity cements requires strict adherence to application times.
High viscosity: these cements have a short mixing phase and lose their stickiness quickly. This makes for a longer working phase, giving the surgeon more time for application.
Ideal viscosity will be high enough to avoid any cement mixing with blood or fat/bony material from the implantation region yet low enough to penetrate the bone adequately.
4.6. Stem centralizer
Femoral stem centralizers were originally designed for double tapered, straight stems.18 A stem centralizer (Fig. 3) guides the femoral prosthesis to a neutral position within the cement and guarantees an even cement layer between the bone and prosthesis.19
Fig. 3.

Distal centralizer.
Thickness of the cement mantle around any shaft should be approximately 3 mm to provide sufficient mechanical strength. Spacers on the acetabular cup will ensure an even cement layer around the cup. The cement mantle should be 2–3 mm, yielding better stress distribution.
The distal cement centralizer is widely used and is assumed to be valuable in affecting the quality of cemented total hip replacement. The literature suggests that the use of the distal centralizer improves the quality of the distal mantle as well as improves stem position.19 However, some studies dispute the same.18
4.7. Cement restrictors
The use of intramedullary plugs in cemented total joint arthroplasty is now considered a routine practice by most surgeons. In order to achieve good filling and pressurization in hip, a small piece of bone or a cement restrictor may be used to plug the shaft. The restrictor should be placed no more than 2 cm distal to tip of the stem.
The primary goal of plugging the intramedullary canal during total hip arthroplasty is to increase penetration of cement into the cancellous bone proximal to the intramedullary plug. This recalls in greater penetration and may enhance prosthetic stability.
5. Bone bed preparation
5.1. Micro-interlock
The concept of micro-interlock is a positive contribution to the quality of fixations. The interface strength is not only affected by the degree of cement penetration but also by the quality of the cancellous framework. The addition of hydroxyapatite (HA) enhances the connection to the bone since HA is the main inorganic constituent of bone tissue, although this compromises the mechanical strength of the cement.20
There are a number of essential prerequisites for successful micro-interlock:
-
1.
Thoroughly cleaned bone bed, brush and lavage before applying the cement.
-
2.
Injecting the cement until it is of high viscosity- It prevents the blood from penetrating into the cement and thereby weakening it.
-
3.
Pressurize the cement by using a cement gun and sealing off the bone cavity.
Careful preparation of the bone cavity and bone bed with high-pressure pulse lavage and brushing is essential for achieving an effective micro-interlock between the bone and the cement.
5.2. Reaming
The bone cavity should be shaped to provide an even cement layer between the bone and prosthesis. Size of reaming should be determined at preoperative planning.
5.3. Brushing
Mechanical cleaning with a brush is recommended. Accidental introduction of blood and tissue debris into the cement may cause laminations, which can lower the effective strength of the bone cement. Acetabular and femoral brushes are used to remove soft tissue and loose cancellous bone from the cavity.
5.4. Pulse lavage
Using high-pressure pulse lavage (Fig. 4) to remove remaining bone particles and debris in a joint arthroplasty produces a clean surface. The risk of blood lamination is reduced and the mechanical strength of the cement is increased. Micro-interlock between the bone and the cement is achieved by high-pressure pulse lavage repeatedly.21
Fig. 4.

Pulse lavage.
A study concluded that meticulous high volume; high-pressure pulsatile lavage reduces both pulmonary physiological derangements and fat emboli.22
5.5. Anchorage holes in the acetabulum
The anchorage holes are made in order to remove as little bone as possible and they may be drilled and/or impacted. Anchorage holes increase the contact area between bone and cement, providing for better fixation (Fig. 5).
Fig. 5.
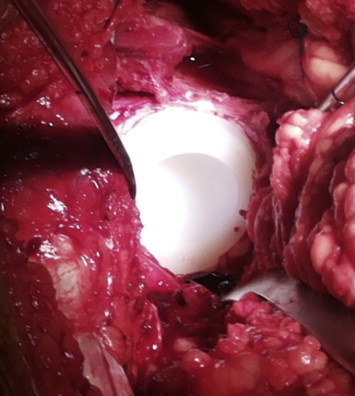
Cemented acetabular component.
6. Evolution of cementing techniques
Overall, advancements in cementing can be classified to have occurred from ‘first generation’ to ‘third generation techniques’, with changes occurring in bone bed preparation, cement preparation and cement delivery.23,24
6.1. First generation cementing technique
It involved the hand mixing of cement in bowels. There was only a minimal preparation of the femoral canal and cancellous bone was left in-situ. The canal was irrigated and suctioned prior to the digital application of cement. The prosthesis was then inserted into the femoral canal. During the 1980's these techniques were refined. Steps were taken to reduce the porosity of the cement and thereby increase the fatigue life. Pressurization of the cement was introduced to improve osseo-integration of the cement and the importance of a good cement mantle around the prosthesis was more clearly understood.
6.2. Second generation cementing techniques
All cancellous bone is removed as near to the endosteal surface and distal cement restrictor was also used. There is pulsatile irrigation, packing and drying of the femoral canal followed by retrograde insertion of cement with a cement gun. The prosthesis is again positioned manually.25 Further improvement lead to the development of third generation cementing techniques.
6.3. Third generation cementing techniques
Cement is now prepared using a vacuum-centrifugation, which further reduces porosity. The femoral canal is irrigated with pulsatile lavage and then packed with adrenaline soaked swabs. After insertion of the cement in a retrograde fashion, the cement is pressurised. Finally the prosthesis is inserted using distal and proximal centralizers to ensure an even cement mantle (4th generation).
7. Caution and adverse effects
Hypotensive episodes and cardiac arrest have been reported during cement insertion.26
Pressurization and thorough cleaning of the bone with expulsion of bone marrow has been associated with the occurrence of pulmonary embolisms, and this risk has been found to be increased in patients with highly osteoporotic bone and patients diagnosed with femoral neck fracture. Reaming of the marrow cavity can have similar effects on mean arterial pressure as the introduction of the bone cement. Marrow cavities should be vented when the cement is introduced digitally. The premature insertion of bone cement may lead to a drop in blood pressure, which has been linked to the availability of methyl methacrylate at the surface of the product,27 although this has not been proven. This drop in blood pressure, on top of hypotension induced either accidentally or intentionally, can lead to cardiac arrhythmias or to an ischaemic myocardium. However, according to a report, the possible risk of death associated with the use of cemented implant is confined to early postoperative and perioperative period.28
The hypotensive effects of methyl methacrylate are potentiated if the patient is suffering from hypovolaemia.
The most frequent adverse reactions reported with acrylic bone cements are:
-
•
Transitory fall in blood pressure.
-
•
Elevated serum gamma-glutamyl-transpeptidase (GGTP) upto 10 days post-operation.
-
•
Thrombophlebitis.
-
•
Loosening or displacement of the prosthesis.
-
•
Superficial or deep wound infection.
-
•
Trochanteric bursitis.
-
•
Short-term cardiac conduction irregularities.
-
•
Heterotopic new bone formation.
-
•
Trochanteric separation.
7.1. Other known adverse effects29,30
-
•
BCIS (Bone cement implantation syndrome) is characterized by a number of clinical features that may include hypoxia, hypotension, cardiac arrhythmias, increased pulmonary vascular resistance (PVR) and cardiac arrest. It is most commonly associated with, but is not restricted to, hip arthroplasty.29 It usually occurs at one of the five stages in the surgical procedure; femoral reaming, acetabular or femoral cement implantation, insertion of the prosthesis or joint reduction. It is an important cause of intraoperative mortality and morbidity in patients undergoing cemented hip arthroplasty and may also be seen in the postoperative period in a milder form causing hypoxia and confusion.
-
•
Hypoxaemia.
-
•
Cardiac arrhythmia.
-
•
Bronchospasm.
-
•
Adverse tissue reaction.
-
•
Haematuria.
-
•
Dysuria.
-
•
Bladder fistula.
-
•
Local neuropathy.
-
•
Local vascular erosion and occlusion.
-
•
Transitory worsening of pain due to heat released during polymerization.
-
•
Delayed sciatic nerve entrapment due to extrusion of the bone cement beyond the region of its intended application.
-
•
Intestinal obstruction because of adhesions and stricture of the ileum due to the heat released during cement polymerization.
8. Drawbacks of bone cement
One of the major drawbacks of bone cement in joint replacement is cement fragmentation and foreign body reaction to wear debris, resulting in prosthetic loosening and periprosthetic osteolysis. The production of wear particles from roughened metallic surfaces and from the PMMA cement promotes local inflammatory activity, resulting in chronic complications to hip replacements. Histologically, a layer of synovial like cells which line the bone cement interface supported by a stroma containing macrophages and wear particles, has been described in loose prostheses.31 A third of dense fibrous tissue contains polymethyl methacrylate, polyethylene and metallic debris. Activated macrophages express cytokines including interleukin-1, interleukin-6 and tumour necrosis factor alpha, which mediate periprosthetic osteolysis.
Bone cement generates heat as it cures and contracts and later expands due to water absorption. It is neither osteoinductive nor osteoconductive and does not remodel.
The monomer is toxic and there is a potential for allergic reactions to cement constituents.
9. Conclusion
The knowledge about the bone cement is of paramount importance to all Orthopaedic surgeons. Although the bone cement had been the gold standard in the field of joint replacement surgery, its use has somewhat decreased because of the advent of press-fit implants which encourage bone in growth. The shortcomings, side effects and toxicity of the bone cement are being addressed recently. More research is needed and continues in the field of nanoparticle additives, enhanced bone cement interface and other developments in quest for improving the quality and eliminating or reducing undesired side effects of bone cement.
Conflicts of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.Cluett J. What is Bone Cement? Available at: http://orthopedics.about.com/b/2008/05/18/what-is-bone-cement.htm.
- 2.Chow L.C., Eanes E.D., editors. vol. 18. Kerger; Basel: 2001. pp. 148–163. (Octacalcium phosphate). Monogr Oral Sci. [Google Scholar]
- 3.Ascherl R. Science of Bone Cement. Ortho Supersite. Available at: http://www.orthosupersite.com/view.asp?rID=3971.
- 4.Arora M., Chan E.K.S., Gupta S., Diwan A.D. Polymethylmethacrylate bone cements and additives: a review of the literature. World J Orthop. 2013;4(2):67–74. doi: 10.5312/wjo.v4.i2.67. http://www.wjgnet.com/2218-5836/full/v4/i2/67.htm Available from: URL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton P, Rampurada A, Qureshi F. Bone Cement, its History, its Properties, and Developments in it use. Available at: http://usmorthopaedic.wordpress.com/2009/08/24/bone-cement-its-history-its-properties-and-developments-in-its-use/.
- 6.Breusch S.J., Malchau H. Springer–Berlin; Heidelberg, NY: 2005. The Well-cemented Total Hip Arthroplasty: Theory and Practice. [Google Scholar]
- 7.Bowen B. Orthopedic Surgery. Alexander’s Care of the Patient in Surgery. In: Rothrock J.C., editor. 14th ed. Mosby; St. Louis, MO: 2011. pp. 741–742. [Google Scholar]
- 8.Available from URL: http://en.m.wikipedia.org/wiki/Bone_cement.
- 9.Clyburn TA, Cui Quanjun. Antibiotic laden cement: Current state of the art. Available at: http://www.aaos.org/news/bulletin/may07/clinical7.asp.
- 10.Penner M.J., Masri B.A., Duncan C.P. Elution characteristics of vancomycin and tobramycin combined in acrylic bone cement. J Arthroplasty. 1996;11:939–944. doi: 10.1016/s0883-5403(96)80135-5. PMID: 8986572. [DOI] [PubMed] [Google Scholar]
- 11.van Staden Anton Du Preez. Stellenbosch Univ.; 2011. Developing bone Cement Implants Impregnated with Bacteriocins for Prevention of Infections. [Dissertation] [Google Scholar]
- 12.Alt V., Bechert T., Steinrücke P. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. PMID: 15046929. [DOI] [PubMed] [Google Scholar]
- 13.Eveleigh R. Principles of bone cement mixing. Br J Perioper Nurs. 2001;11(1):18–20. doi: 10.1177/175045890101100103. [DOI] [PubMed] [Google Scholar]
- 14.Heisel C., Norman T., Rupp R., Pritsch M., Ewerbeck V., Breusch S.J. In vitro performance of intramedullary cement restrictors in total hip arthroplasty. J Biomech. 2003 Jun;36(6):835–843. doi: 10.1016/s0021-9290(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 15.Geiger M.H., Keating E.M., Ritter M.A., Ginther J.A., Faris P.M., Meding J.B. The clinical significance of vacuum mixing bone cement. Clin Orthop Relat Res. 2001 Jan;382:258–266. doi: 10.1097/00003086-200101000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Available at: http://www.bonecement.com/cementing-techniques/pressurization.
- 17.Mjöberg B., Rydholm A., Selvik G., Onnerfält R. Low- versus high-viscosity bone cement. Fixation of hip prostheses analyzed by roentgen stereophotogrammetry. Acta Orthop Scand. 1987 Apr;58(2):106–108. doi: 10.3109/17453678709146450. [DOI] [PubMed] [Google Scholar]
- 18.Bell C.A., Pilot P., van den Boogaart M., Verburg A.D. Effect of a distal centralizer on the positioning of an anatomical stem. Acta Orthop Belg. 2009 Feb;75(1):41–44. [PubMed] [Google Scholar]
- 19.Hanson P.B., Walker R.H. Total hip arthroplasty cemented femoral component distal stem centralizer: effect on stem centralization and cement mantle. J Arthroplasty. October 1995;10(5):683–688. doi: 10.1016/s0883-5403(05)80216-5. [DOI] [PubMed] [Google Scholar]
- 20.Gil Gonçalves, Sandra MA Cruz, José Grácio, Paula AAP Marques, Cecilia Ramírez-Santillán, María Vallet-Regí, María-Teresa Portolés. New bioactive PMMA-hydroxyapatite based bone cement reinforced with graphene oxide. Available at: http://www.phantomsnet.net/Graphene_Conf/2012/Abstracts/2012_Goncalves_Gil_ggoncalves@ua.pt_Graphene2012_Abstract.pdf.
- 21.Breusch S.J., Norman T.L., Schneider U., Reitzel T., Blaha J.D., Lukoschek M. Lavage technique in total hip arthroplasty: jet lavage produces better cement penetration than syringe lavage in the proximal femur. J Arthroplasty. 2000 Oct;15(7):921–927. doi: 10.1054/arth.2000.8098. [DOI] [PubMed] [Google Scholar]
- 22.Byrick R.J., Bell R.S., Kay J.C., Waddell J.P., Mullen J.B. High-volume, high-pressure pulsatile lavage during cemented arthroplasty. J Bone Joint Surg Am. 1989 Oct;71(9):1331–1336. [PubMed] [Google Scholar]
- 23.Barrack R.L., Mulroy R.D.J., Harris W.H. Improved cementing techniques and femoral component loosening in young patients with hip arthroplasty. A 12-year radiographic review. J Bone Joint Surg Br. 1992;74:385–389. doi: 10.1302/0301-620X.74B3.1587883. [DOI] [PubMed] [Google Scholar]
- 24.Eisler T., Svensson O., Iyer V. Revision total hip arthroplasty using third-generation cementing technique. J Arthroplasty. 2000 Dec;15(8):974–981. doi: 10.1054/arth.2000.9825. [DOI] [PubMed] [Google Scholar]
- 25.Mulroy W.F., Estok D.M., Harris W.H. Total hip arthroplasty with use of so called second generation cementing techniques. J Bone Joint Surg. 1995;77A:1845–1852. doi: 10.2106/00004623-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Leidinger W., Hoffmann G., Meierhofer J.N., Wolfel R. Reduction of severe cardiac complications during implantation of cemented total hip endoprostheses in femoral neck fractures. Unfallchirurgie. 2002;105:675–679. doi: 10.1007/s00113-001-0410-3. [DOI] [PubMed] [Google Scholar]
- 27.Ono Satoshi, Kadoma Yoshinori, Morita Sadao, Takakuda Kazuo. Development of new bone cement utilizing low toxicity monomers. J Med Dent Sci. 2008;55:189–196. [PubMed] [Google Scholar]
- 28.Yli-Kyyny T., Ojanpera J., Venesmaa P. Perioperative complications after cemented or uncemented hemiarthroplasty in hip fracture patients. Scand J Surg. June 2013;102(2):124–128. doi: 10.1177/1457496913482249. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson A., Thomson H., Harper N. Bone cement implantation syndrome. Br J Anaesth. 2009;102:12–22. doi: 10.1093/bja/aen328. [DOI] [PubMed] [Google Scholar]
- 30.Available at: http://www.medsafe.govt.nz/profs/datasheet/CMWGentamycinbonecement.pdf.
- 31.Goldring Steven R., Jasty M., Roelke Merrilee S. Formation of a synovial-like membrane at the bone–cement interface: Its role in bone resorption and implant loosening after total hip replacement. Arthritis Rheum. 1986;29(7):836–842. doi: 10.1002/art.1780290704. [DOI] [PubMed] [Google Scholar]


