Abstract
Objectives
The application of an electric field has been shown to positively influence the impregnation of the resin monomers currently used in dentin bonding systems during hybrid layer formation. This study presents an experimental characterization of the electrical properties of these monomers with the aim of both correlating them to their chemical structures and seeking an insight into the mechanisms of the monomer migration under an applied electric field.
Methods
Some common monomers examined were TEGDMA (triethyleneglycol dimethacrylate), HEMA (2-hydroxyethyl methacrylate), UDMA (urethane dimethacrylate), 2-MP (bis[2-(methacryloyloxy)ethyl] phosphate, TCDM di(hydroxyethyl methacrylate) ester of 5-(2,5-dioxotetrahydrofurfuryl)-3-methyl-3-cyclohexenyl-1,2-dicarboxylic anhydride) and Bis-GMA [2,2-bis(4-2-hydroxy-3-methacryloyloxypropoxyphenyl)propane]. A customized cell produced for the measurement of the electrical properties of monomers was manufactured and electrical conductivity and permittivity of resin monomers were measured.
Results
The permittivity of the tested monomers is largely affected by electrical frequency. The large values of permittivity and dielectric losses observed as frequency decreased, indicate a dominant effect of ionic polarization, particularly evident in materials showing the highest conductivity. Permittivity and conductivity of the tested monomers showed a similar behavior, i.e. materials with the lowest permittivity also show small values of conductivity and vice versa.
Significance
The results of the present study revealed a good correlation between electrical properties and Hoy solubility parameters and, in particular, the higher the polar contribution (polar forces plus hydrogen bonding) the higher the permittivity and conductivity. The most relevant outcome of this study is that the electrophoretic mechanism prevails on the electroendoosmotic effect in determining the monomer migration under the application of electric fields.
Keywords: composite resins, dentin bonding systems, electrical conductivity, electrical permittivity
Introduction
Dentin bonding systems (DBS) allow restorative materials to bond to enamel and dentin [1,2]. Dentin bonding is achieved by infiltrating the substrate via passive diffusion of solvent and DBS monomers into the insoluble demineralized dentin collagen fibril network that is saturated with residual interfibrillar water [1]. The result is the formation of the hybrid layer, a structure composed by DBS monomers, residual solvent, dentin collagen fibrils and hydroxyapatite at different degrees in relation to the type of DBS used [1-3]. In the case of self-etch DBS, residual smear layer particles are embedded within the hybrid layer [2].
Silver nitrate-impregnated resin-bonded specimens analyzed under electron microscopy revealed different degree of nanoleakage (i.e. interfacial nanoporosities within the hybrid layer that are claimed to represent the weakest area of the adhesive interface) due to poor or incomplete resin infiltration of both etch-and-rinse and self-etch (or etch-and-dry) DBS leaving residual interfibrillar water [4,5]. High porosity hybrid layers lead to higher silver uptake along the adhesive interface, and result in reduced immediate bond strength and accelerated degradation [5-7].
The use of electricity has been proposed to facilitate resin monomers impregnation of the dentin [8-11]. Preliminary in vitro studies performed with different prototypes (ElectroBond, Seti, Italy) delivering current intensities ranging from 25-175 μA, confirmed that the application of DBS under the influence of an electric field increases bond strengths and reduces interfacial nanoleakage [8-11]. Initially iontophoresis was suggested to increase monomer impregnation, favoring the substitution of water by adhesive monomers [8-11]. However a recent study revealed that DBS monomer migration within an electric field can be affected by several parameters related either to the dentin substrate (buffer pH and ionic strength), or to the electric field (applied voltage), strongly affecting the prevalence of electromigration forces or electroendoosmotic flux (EEO) within the matrix [12]. Electromigration causes the migration of the negatively charged molecules toward the anode and the concurrent migration of mobile cations to the cathode (negative electrode) [13,14], thus determining monomers migration due to the pH-dependent ionization of molecules of weak acids [12]. The second effect, EEO, which is the water movement within organic matrices during electrophoresis as a result of the fixed negative charges and electromigration of positive counterions on the matrix, causes the migration of water and all dissolved substances towards the cathode, irrespective of charge [15,16]. If electromigration is higher than EEO monomers migrate toward the anode, while if electromigration is lower than EEO monomers migrate toward the cathode [12]. Thus monomer migration toward the anode or cathode can be achieved as desired by selective choice of pH and ionic strength of the substrate and the applied electric field [12].
However, despite these findings the interaction between the dentin substrate and electricity, the mechanism of how an electrical field can facilitate impregnation of monomers remains unclear since little is known about the electrical properties of monomers currently used in restorative dentistry (i.e. their electrical conductivity and electrical permittivity). The electrical conductivity describes the ability of a material to transport current when subjected to an electric field. In liquid dielectrics, conductivity is closely correlated to the ionization status of the material and to the electrical mobility of ions and electrons [17].
Permittivity is a physical quantity that describes how an electric field affects a dielectric medium [18]. It is determined by the ability of a material to polarize in response to electric fields, and thus reduce the total electric field inside the material. In general, the response of a solid medium to external fields depends on the frequency of the field. This frequency dependence reflects the fact that material polarization does not respond instantaneously to an applied field due to material inertia [19]. This leads to power losses generated by the polarization process (dielectric losses) and to a phase delay between polarization and electric field. When the applied electric field exhibits a sinusoidal behaviour, this delay can be mathematically expressed by considering a complex quantity for the electrical permittivity, ε̄ [20]:
| (1) |
The real part (ε′) and the imaginary part (ε″) of the permittivity are associated with the extent of polarization and polarization delay (thus, power losses), respectively. Here the relative electrical permittivity, ε̄r, is considered, dividing eq. (1) by the vacuum permittivity constant ε0 = 8.85 × 10−12 F/m.
The aim of the present study was to investigate the electrical conductivity and the electrical permittivity of resin monomers currently used in DBS blends, since such information is not currently available in the literature. Additionally these electrical properties will be correlated to the chemical properties of these monomers, such as their structure and Hoy's solubility parameters [21-24]. The tested null hypothesis was that no correlation exists between electrical conductivity, electrical permittivity and Hoy's solubility parameters.
Materials and Methods
Tested monomers
TEGDMA (triethylene glycol dimethacrylate), HEMA (2-hydroxyethyl methacrylate), UDMA (urethane dimethacrylate), 2-MP (bis“2-(methacryloyloxy)ethyl” phosphate, TCDM di(hydroxyethyl methacrylate) ester of 5-(2,5-dioxotetrahydrofurfuryl)-3-methyl-3-cyclohexenyl-1,2-dicarboxylic anhydride) and Bis-GMA “2,2-bis(4-2-hydroxy-3-methacryloyloxypropoxyphenyl)propane” were purchased from Sigma Chemical (St Louis, MO, USA) and used without further purification (Table 1).
Table 1.
Hoy's solubility parameters of the tested monomers. They represent index of the intermolecular forces that occurs between molecules. In particular, δd is the dispersive contribution (i.e. all non-polar contribution), δp the polar contribution, δh the hydrogen bond contribution and δt represents the total solubility parameter.
| Abbreviations | Chemical name | Molecular Structure | δd | δp | δh | δt |
|---|---|---|---|---|---|---|
| TEGDMA | Triethylene glycol dimethacrylate |
|
14.2 | 10.1 | 8.2 | 19.2 |
| HEMA | 2-hydroxyethyl methacrylate |
|
13.3 | 12.3 | 15.2 | 23.6 |
| UDMA | Urethane dimethacrylate |
|
17.0 | 12.4 | 2.8 | 21.3 |
| 2-MP | Bis[2-(methacryloyloxy)ethyl] phosphate |
|
14.9 | 14.8 | 14.3 | 25.4 |
| TCDM | Di(hydroxyethyl methacrylate)ester of 5-(2,5,-dioxotetrahydrofurfuryl)-3-methyl-3-cyclohexenyl-1,2-dicarboxylic anhydride |

|
18.7 | 15.1 | 7.4 | 25.1 |
| Bis-GMA | 2,2-bis[4-(2-hydroxy-3-methacryloyloxy propoxy)]-phenyl propane |
|
16.6 | 13.4 | 5.8 | 22.1 |
Cell Fixture
A customized cell fixture for the measurement of the electrical properties of monomers was manufactured, as no device was commercially available [25]. In fact, due to the high viscosity of some of these monomers, these liquids are not compatible with commercial measurement cells for liquid dielectrics [26, 27]. Moreover, the cell has been specifically designed for applied voltages much lower than those used in standard high voltage dielectric measurements. This is a key feature of the experimental setup, as it allows experiments to be performed at electric field levels similar to those applied in a clinical environment.
The material adopted for the cell is poly(methyl methacrylate) (PMMA) in order to ensure chemical compatibility with the monomers tested in the present study [28]. An image of the customized cell is shown in Fig. 1. The cell is composed of a hollow cylinder closed at both ends with two stainless steel circular electrodes having a diameter of 44 mm and separated by a 3 mm gap. The area of the electrodes is therefore equal to 1520 mm2. Two holes on the top part of the cylinder allow the test monomers to be poured in between the electrodes and for the trapped air to be expelled.
Figure 1.
Cell fixture used to measure the complex permittivity and electric conductivity of the monomers under test.
With this fixture, two-terminal impedance measurements were carried out by means of an Alpha dielectric analyzer (Novocontrol, Hundsangen, Germany) [29]. The complex permittivity and the electric conductivity were then measured for liquid monomers in the frequency range from 10-2 Hz to 6 MHz and for a supply voltage of 1 V rms.
Results
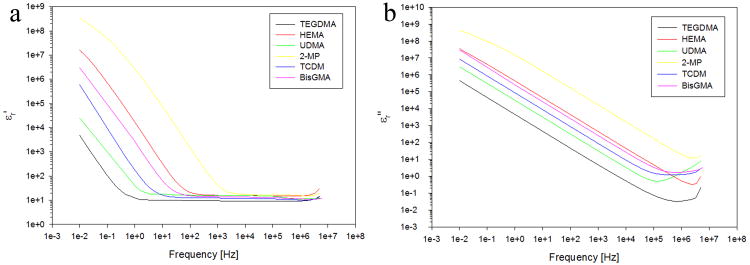
The measured electric properties of the tested liquid monomers are shown in Figs. 2 and 3.
Figure 2.
Real part εr′ (a) and imaginary part εr″ (b) of the relative electrical permittivity εr as a function of frequency for the tested monomers.
Figure 3.
Electrical conductivity σ as a function of frequency for the tested monomers.
The real part, εr′ (polarization, Fig. 2a) and imaginary part, εr″ (polarization delay, Fig. 2b) of the relative electrical permittivity as a function of frequency for the different monomers tested at room temperature are reported in Fig. 2. Both real and imaginary permittivity are largely affected by frequency. In particular, εr′ is constant at high frequencies, i.e. from 5 MHz down to a frequency characteristic for each material, ranging from 5kHz (max) to 1Hz (min) for the two monomers 2-MP and UDMA, respectively.
Figure 3 reports the frequency response of electrical conductivity, σ, for the same monomers as in Fig. 2 at room temperature. The electrical conductivity shown exhibits an opposite behaviour compared to permittivity, being constant in the low-frequency range and variable at high frequencies (Fig. 3). The frequency of transition between these two regimes depends on the material, ranging from 10 Hz to 100 kHz for UDMA and 2-MP, respectively.
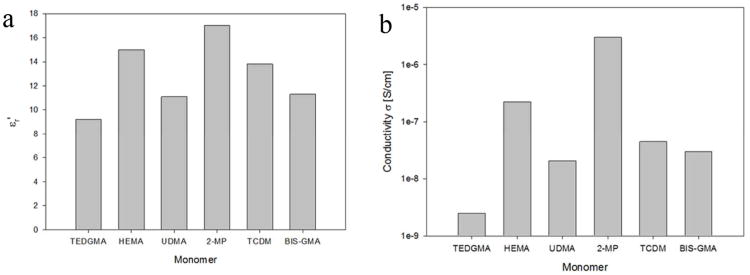
Figure 4 shows the comparison of real part of permittivity, εr′, (Fig. 4a) and conductivity, σ, (Fig. 4b) for the tested monomers obtained from plots of Figs. 2a and 3, at frequencies for which the permittivity and conductivity are frequency-independent, i.e. at high (10 MHz) and low (10 Hz) frequencies, respectively. Permittivity and conductivity showed a similar behaviour, i.e. materials having the lowest permittivity also showed small values of conductivity and vice versa. It should be noted that among the different materials, conductivity varied in a wider range with respect to permittivity. Interestingly 2-MP showed the largest values of electrical permittivity and conductivity, TEGDMA had both the lowest conductivity and permittivity (εr′<10) while UDMA and Bis-GMA exhibited similar values of conductivity and real permittivity.
Figure 4.
Comparison of the real part of the permittivity at 1 MHz (A) and conductivity at 10 Hz (B) for the tested monomers
Discussion
The results of the present study revealed that electrical properties can be correlated with the chemical structure and with the Hoy solubility parameters since they are correlated with the polarity of the molecules. Thus the tested null hypothesis was rejected.
Additionally, this study revealed a linear behaviour of electrical conductivity of the tested adhesive resinsat high frequencies in log-log scale (Fig. 3) in accordance with the fact that conductivity increases follow a power law of “universal dielectric response” developed by Jonscher [18]:
| (2) |
where f is the frequency, A and n are the model parameters.
It can also be seen that the large values of permittivity (Fig. 2a) and dielectric losses (Fig. 2b) observed as frequency decreases, indicate a dominant effect of ionic polarization, particularly evident in materials showing the highest conductivity, (Fig. 3). Polarization at the electrode/monomer interface could also play a role, increasing permittivity and losses in the low frequency range. This behaviour is quite typical of liquid dielectrics having large polar groups [18].
Hoy's solubility parameters shown in Table 1 (δd, δp, δh, δt) [22] are indices of the intermolecular forces that occur between molecules. In particular, δd is the dispersive contribution (i.e. all non-polar contribution), δp the polar contribution, δh the hydrogen bond contribution and δt the total solubility parameter that is calculated according to the formula δt2 = δd2 + δp2 + δh2. Hoy's parameters can be used to predict whether a given material is compatible with another one. Materials with similar Hoy's parameters behave similarly and are compatible. Hoy's solubility parameters have been calculated using the Computer Chemistry Consultancy software [30]. The molecules that exhibit higher δp (polar contribution) and δh (contribution from hydrogen bonds) had higher electrical permittivity and conductivity. This is to be expected since the dielectric constant of liquids can be calculated from their chemical group contributions [31]. Permittivity of the tested monomers showed a highly linear correlation with Hoy's total solubility parameters (δt) yielding an R2=0.89, p<0.005 (Fig. 5a). Conductivity of the tested monomers correlated with Hoy's δp+h values, producing R2=0.69, p<0.05 (Fig. 5b).
Figure 5.
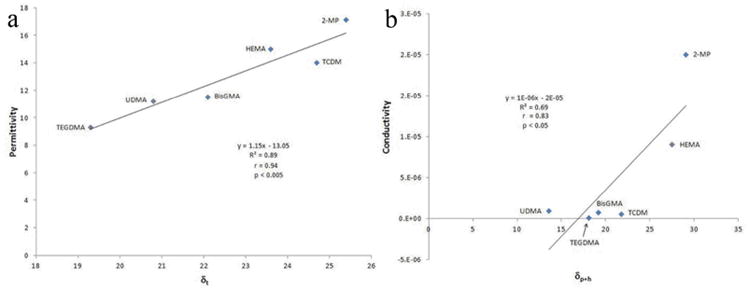
Correlation between permittivity (a) of the tested monomers with Hoy's total solubility parameters (δt, highly linear correlation, R2=0.89, p<0.005) and between conductivity (b) of the tested monomers and Hoy's δp+h values (R2=0.69, p<0.05).
Additionally also the size of the molecules can have an effect on electrical conductivity as the smaller molecules are more mobile and thus more conductive compared to larger ones. On the contrary, the δd (the dispersive contribution i.e. all non-polar contribution) does not have a significant effect on electrical properties.
The results of a previous study on electrical migration of DBS monomers in the presence of an electric field, showed that the migration properties depend on the buffer pH, ionic strength and applied electric field [12]. Two main factors involved in migration have been observed changing these parameters, namely a migration towards the anode due to electrophoretic migration or towards the cathode due to the EEO effect [12]. The overall velocity of molecule migration, v, is the sum of the average velocities of electrophoretic, vep, and EEO, veo, effects, which can be expressed in terms of the relative mobilities μep and μeo respectively:
| (3) |
where E is the applied electric field [20].
The previous findings [12] correlate well with the results of the present study, since both the electrophoretic and EEO effects are affected by the polarity and by the permittivity of the molecules involved. In particular, when considering these two types of migration as acting separately, vep is determined by the equilibrium of the force acting of the electric charge and of the drag force acting on the molecules [32]:
| (4) |
which yields:
| (5) |
where q is the charge of the molecule, η is the viscosity of the solution and r is the radius of the molecule.
An explicit dependence of the electrical conductivity, σ, on the flow velocity can be written as follows [17]:
| (6) |
where n is the number of molecules per unit volume.
Combining (5) and (6) the following expression of the electrophoretic flow velocity can be written:
| (7) |
It is noteworthy that the electrophoretic mechanism of migration is therefore mainly affected by the electrical conductivity and viscosity of the monomer.
For the EEO flow rate, veo, the following equation holds [33]:
| (8) |
where ε is the permittivity and ζ is the electrokinetic potential.
Equation (8) shows that the main parameters affecting EEO mechanism are dielectric permittivity and viscosity.
It can therefore be observed that monomers exhibiting a high conductivity are subjected to a strong electrophoretic effect [12-14], whereas materials with high permittivity are prone to a significant EEO migration [12,15,16]. These considerations, together with the measured values of ε and σ for the analyzed monomers, allow a better understanding of the previous results [12].
Moreover, the abovementioned results show a clear correlation of both electrophoretic and EEO effects with the electrical properties of the monomers (Figs. 2a and 4). This relation is particularly evident for 2-MP, that exhibited the highest electrical permittivity and conductivity combined δp + δh (29.1) and therefore presented the higher polarity among the monomers [24]. Interestingly 2-MPalso showed the maximal migration in an electric field among all tested monomers. This effect is consistently observed with different buffer pH, ionic strength (buffer concentration) of the organic matrix, and applied voltage [12].
Among the tested adhesive monomers, HEMA exhibited the highest hydrogen bonding δh = 15.2 and high polarity δp = 12.3 has the second higher conductivity and permittivity [24]. Indeed, due to its high permittivity and electrical conductivity, HEMA should exhibit a high mobility under the effect of the electric field. However previous findings revealed that HEMA has little mobility within the agarose gel [12]. It was speculated that due to its high hydrogen bonding Hoy value, strong hydrogen bonding might occur between the hydroxyl groups of HEMA and the hydroxyl groups of agarose not allowing HEMA monomers to migrate towards the electrodes.
TCDM and Bis-GMA have intermediate electrical properties and also medium/low hydrogen bonding characteristics [24]. TCDM has lower permittivity but slightly higher conductivity compared to Bis-GMA [24]. This behavior can be ascribed to the smaller dimension of TCDM since molecular dimensions influence the conductivity. Therefore, smaller molecules such as TCDM should have a higher conductivity respect to larger molecules such as Bis-GMA. This additionally confirms the previous findings that revealed significant migration for TCDM (which is the other most acidic monomer with 2-MP), with the exception of the experiment at pH 3.1 where migration was absent [12].
UDMA has the lowest hydrogen bonding Hoy parameter (δh=2.8) and low polarity (δp=12.4), while it presented the second lower conductivity and permittivity. TEGDMA having the lowest polarity Hoy value (δp=10.1), showed the lowest permittivity and conductivity even if its hydrogen bonding value, calculated using the Computer Chemistry Consultancy software, is not low. TEGDMA is a further proof of the correlation between electrical properties and migration as the lowest permittivity and electrical conductivity was associated in a previous study with little migration only in very peculiar conditions (i.e. at pH 12.3) regardless of the buffer ionic strength [12].
Considering these results, migration of monomers is independent of buffer ionic strength and pH only when the permittivity and electrical conductivity of the monomers are high. It must be emphasized that the permittivity values of Fig. 2a were obtained at a frequency of 1 MHz, whereas the experiments carried out in Colonna et al. [12] refer to a direct current voltage setup.
Conclusions
The results of the present study revealed a good correlation between electrical properties and Hoy solubility parameters and, in particular, the higher the polar contribution (polar forces plus hydrogen bonding) the higher the permittivity and conductivity. Molecular size can play a smaller role in the case of molecules with similar polar properties. In this case, smaller molecules present higher conductivity with respect to larger molecules.
Moreover, the current work, when viewed in the concept of the previous work [12] shows that the electrophoretic mechanism is dominant over the electroendosmotic effect in the migration of monomers under the application of electric fields. The electrophoretic effect is enhanced by high values of electrical conductivity, which should therefore be considered as the key parameter determining the impregnation of a resin monomer during hybrid layer formation under applied electric field.
Further studies are needed to establish the optimal electrical parameters to improve monomer migration within the dentin organic matrix and to select an appropriate mixture of monomers, capable to form a more stable and durable bond with respect to passive diffusion achieved by standard application with a microbrush currently used in adhesive procedures.
Acknowledgments
The authors wish to thank Mr. Aurelio Valmori for technical assistance. The study was partially founded with grants: Progetto Strategico di Ateneo IDeA of the University of Bologna, FIRB RBAP1095CR, PRIN 2009SAN9K5 from MIUR (Italy), R01-DE015306 from the NIDCR to DHP (PI).
References
- 1.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215–235. [PubMed] [Google Scholar]
- 4.Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20:18–25. [PubMed] [Google Scholar]
- 5.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002;81:472–476. doi: 10.1177/154405910208100708. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Tjaderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in Bonding to Dentin and Experimental Strategies to Prevent Bond Degradation. J Dent Res. 2011;90:953–968. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breschi L, Mazzoni A, Pashley DH, Pasquantonio G, Ruggeri A, Jr, Suppa P, et al. Electric Impulse-Assisted Application of Self-Etch Adhesives to Dentin. J Dent Res. 2006;85:1092–1096. doi: 10.1177/154405910608501205. [DOI] [PubMed] [Google Scholar]
- 9.Pasquantonio G, Tay FR, Mazzoni A, Suppa P, Ruggeri A, Jr, Falconi M, et al. Electric device improves bonds of simplified etch-and-rinse adhesives. Dent Mater. 2007;23:513–518. doi: 10.1016/j.dental.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Visintini E, Mazzoni A, Vita F, Pasquantonio G, Cadenaro M, Di Lenarda R, et al. Effects of thermocycling on bonds created by simplified one-step self-etch adhesives applied with ElectroBond. Eur J Oral Sci. 2008;116:564–570. doi: 10.1111/j.1600-0722.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- 11.Mazzoni A, Visintini E, Vita F, Pasquantonio G, Saboia V, Ruggeri A, Jr, et al. ElectroBond improves dentin impregnation of etch-and-rinse adhesives. J Adhes Dent. 2009;11:27–33. [PubMed] [Google Scholar]
- 12.Colonna M, Breschi M, Mazzoni A, Nato F, Ruggeri A, Jr, Nucci C, et al. Effects of pH, ionic strength, and applied voltage on migration of dental monomers in an organic matrix. Dent Mater. 2011;27:1180–1186. doi: 10.1016/j.dental.2011.08.399. [DOI] [PubMed] [Google Scholar]
- 13.Monning CA, Kennedy RT. Capillary electrophoresis. Anal Chem. 1994;66:280–314. doi: 10.1021/ac00084a013. [DOI] [PubMed] [Google Scholar]
- 14.Altria KD. Fundamentals of capillary electrophoresis theory. In: Altria KD, editor. Capillary Electrophoresis Guidebook: Principles, Operation, and Applications. New Jersey: Humana Press publisher; 1996. pp. 1–21. [Google Scholar]
- 15.Hierholzer JC. Effects of sulfate concentration, electroendosmotic flow, and electrical resistance of agars and agaroses on counterimmunoelectrophoresis with adenovirus antigens and antisera. J Immunol Methods. 1976;11:63–76. doi: 10.1016/0022-1759(76)90019-3. [DOI] [PubMed] [Google Scholar]
- 16.Walhagen K, Unger KK, Hearn MT. Capillary electroendoosmotic chromatography of peptides. J Chromatogr A. 2000;887:165–185. doi: 10.1016/s0021-9673(00)00460-x. [DOI] [PubMed] [Google Scholar]
- 17.Seanor DA. Electrical properties of polymers. Academic Press; New York: 1982. [Google Scholar]
- 18.Jonscher AK. Dielectric relaxation in solids. Chelsea Dielectrics Press; London (UK): 1983. [Google Scholar]
- 19.Cole RH. Progress in Dielectrics. III. Heiwood; London, UK: 1961. Theories of Dielectric polarization and relaxation. [Google Scholar]
- 20.Frolich H. Theory of dielectrics. Oxford Press; Oxford: 1949. [Google Scholar]
- 21.Hoy KL. New values of the solubility parameters from vapor pressure data. J Paint Techn. 1970;42:76. [Google Scholar]
- 22.Hoy KL. Solubility parameter as a design parameter for water-borne polymers and coatings. J Coated Fabrics. 1989;19:53–67. [Google Scholar]
- 23.Asmussen E, Hansen EK, Peutzfeldt A. Influence of the solubility parameter of intermediary resin on the effectiveness of the gluma bonding system. J Dent Res. 1991;70:1290–1293. doi: 10.1177/00220345910700091101. [DOI] [PubMed] [Google Scholar]
- 24.Nishitani Y, Yoshiyama M, Hosaka K, Tagami J, Donnelly A, Carrilho M, et al. Use of Hoy's solubility parameters to predict water sorption/solubility of experimental primers and adhesives. Eur J Oral Sci. 2007;115:81–86. doi: 10.1111/j.1600-0722.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 25.ASTM D924 – 08, “Standard Test Method for Dissipation Factor (or Power Factor) and Relative Permittivity (Dielectric Constant) of Electrical Insulating Liquids”
- 26.Baker-Jarvis J, Jones C, Riddle B, Janezic M, Geyer RG, Grosvenor JH, Jr, et al. Dielectric and magnetic measurements: a survey of nondestructive, quasi-nondestructive, and process-control techniques. Research in Nondestructive Evaluation. 1995;7:117–136. [Google Scholar]
- 27.Afsar MN, Birch JR, James RN, Clarke RN, Chantry GW. Measurement of the properties of materials. Proc IEEE. 1986;74:183–199. [Google Scholar]
- 28.Woishnis W, Ebnesajjad S. Chemical Resistance of Thermoplastics. Vol. 1. Elsevier; 2011. [Google Scholar]
- 29.Novocontrol Dielectric Analyzer Instruction Manual. 1999 [Google Scholar]
- 30.http://www.compchemconsul.com
- 31.Tropea AL, Yarin JF, Foss JF. Handbook of experimental fluid mechanics. Vol. 1. Springer; 2007. [Google Scholar]
- 32.Altria Kevin D. Capillary electrophoresis guidebook: principles, operation, and applications. Humana Press; 1996. [Google Scholar]