Abstract
Background
Neuroendocrine tumors of the small intestine commonly metastasize to regional lymph nodes (LNs). Single-institution reports suggest that removal of LNs improves outcome, but comprehensive data are lacking. We hypothesized that the extent of lymphadenectomy reported in a large administrative database would be associated with overall survival for jejunal and ileal neuroendocrine tumors.
Methods
A search of the Surveillance Epidemiology and End Results database was performed for patients with jejunal and ileal neuroendocrine tumors from 1977 to 2004. Descriptive patient characteristics were collected to include age at diagnosis, sex, race, grade, primary tumor size, LN status, number of LNs resected, presence of distant metastasis, and the type of operation. Statistical analyses were limited to patients with only one primary tumor to exclude patients with other malignancies. Univariate and multivariate analyses were performed to analyze the number of LNs resected and the LN ratio (number of positive LNs/total number of LNs removed) to determine the effect on cancer-specific survival.
Results
Altogether, 1,364 patients were included in this analysis. Removal of any LNs was associated with improved cancer-specific survival when compared to patients with no LN removal reported (p = 0.0027) on univariate analysis. Among those who had any LNs removed, a median of eight LNs were identified in resection specimens with a median LN ratio of 0.29 (range 0–1). On multivariate analysis (adjusting for age and tumor size), patients with ≤7 LNs removed experienced better cancer-specific survival than those with B7 LNs removed (median survival not reached vs. 140 months): hazard ratio and 95 % confidence interval were 0.573 (0.402, 0.817) (p = 0.002).
Conclusions
This review of a large number of surgical patients demonstrates that regional mesenteric lymphadenectomy in conjunction with resection of the primary tumor is associated with improved survival of patients with small bowel neuroendocrine tumors.
Introduction
Neuroendocrine tumors of the small intestine comprise one of the most common small bowel malignancies in the United States [1]. The prognosis is poor, with a 5-year survival rate near 60 % [2]. Unlike other malignancies, lymph node (LN) metastases associated with small bowel neuroendocrine tumors tend to be bulky and may cause intestinal obstruction and mesenteric ischemia [2, 3]. As many as 41 % of patients with jejunal or ileal neuroendocrine tumors have LN metastases at the time of diagnosis [3]. Consequently, a proposed treatment strategy includes surgical resection of the primary tumor and regional LNs to prevent bulky metastasis and its effects. Single-institution reports have demonstrated that resection of the primary tumor with regional mesenteric lymphadenectomy improves survival and reduces symptoms even in patients with liver metastasis [4].
The 2010 National Comprehensive Care Network (NCCN) guidelines recommend that patients with jejunal or ileal neuroendocrine tumors undergo small bowel resection with regional lymphadenectomy if it can be accomplished without compromising large amounts of small bowel. Because small bowel neuroendocrine tumors are often multifocal, complete examination of the small bowel is also recommended. These guidelines, however, do not specify the extent of lymphadenectomy (number of LNs removed) needed to achieve the largest survival benefit. Using a large, administrative database, we hypothesized that the analysis of lymph nodes removed at operation would provide a statistical means to determine the extent of lymphadenectomy (expressed in number of lymph nodes examined) necessary to achieve an improved overall survival for patients with jejunal and ileal neuroendocrine tumors.
Methods
A search of the Surveillance Epidemiology and End Results (SEER) database was performed to identify patients with jejunal and ileal neuroendocrine tumors from 1977 to 2004. Patients with the following histologic subtypes coded in the SEER database were included in this analysis: carcinoid tumor, enterochromaffin cell carcinoid, goblet cell carcinoid, composite carcinoid, neuroendocrine carcinoma. Descriptive patient characteristics were collected, including age at diagnosis, sex, race, grade, primary tumor size, LN status, number of LNs resected, presence of distant metastasis, and type of operation. Because this study was focused on cancer-specific survival from small bowel neuroendocrine tumors, patients with additional primary tumors of other disease sites, unknown or non-cancer-related causes of death, and unknown or no surgical intervention were excluded from the analysis (Fig. 1). Stage was determined according to the seventh edition of the American Joint Committee on Cancer (AJCC) cancer staging manual—not the stage provided by the SEER database because staging systems have changed over time [2].
Fig. 1.
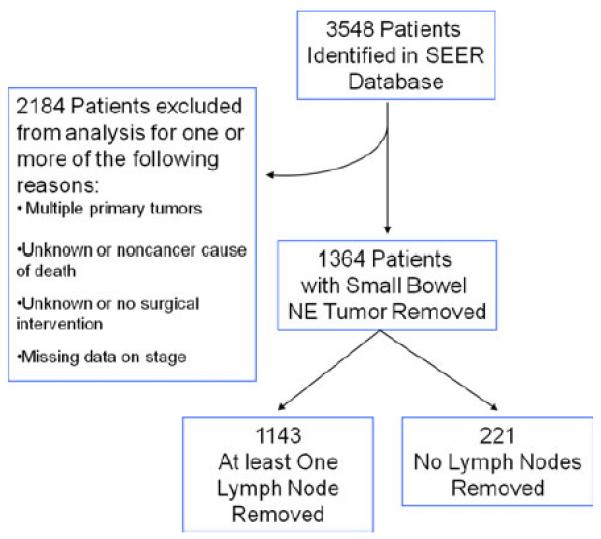
Description of Surveillance Epidemiology and End Results (SEER) database utilized for analysis. NE neuroendocrine
Clinicopathologic characteristics were compared among patients according to whether LNs were removed at operation. The χ2 test and Fisher’s exact test were used to evaluate the association between categoric variables. Wilcoxon’s rank-sum test or t test and the Kruskal–Wallis test or analysis of variance (ANOVA) were used to compare the distributions of continuous variables. The distribution of overall survival was estimated using the Kaplan–Meier method, and differences in survival between groups were performed using log-rank tests. Regression analyses of survival data, based on the Cox proportional hazards model, were conducted on overall survival to determine the effects of clinicopathologic factors. Classification and regression tree (CART) analysis was used to determine binary cutoff points of the number of LNs removed during mesenteric lymphadenectomy to determine which patients achieved improved cancer-specific survival. Statistical analyses were performed using SAS for Windows (release 9.1; SAS Institute, Cary, NC, USA) and S-Plus version (Version 8; Insightful, Seattle, WA, USA).
Results
A search of the SEER database identified 3,548 patients with jejunal or ileal neuroendocrine tumors. Patients were excluded from further analysis if they had one or more of the following characteristics: more than one primary tumor (n = 1,140), died of non-cancer-related or unknown causes (n = 856), underwent no surgical intervention (n = 237), the type of operation was unknown (n = 64), or there was inadequate information available to stage the patients (n = 505). After excluding the above patients, 1,364 were left for analysis (Fig. 1). Among this group, 704 (52 %) were male. In all, 1,199 (88 %) had primary tumors in the ileum. The median tumor size was 2 cm, the median age at diagnosis was 60 years, and the mean and median follow-ups for all 1,364 patients were 59 and 39 months, respectively. The overall survival of the patients included in this data set was significantly different when stratified by stage (p < 0.0001 (Fig. 2)).
Fig. 2.
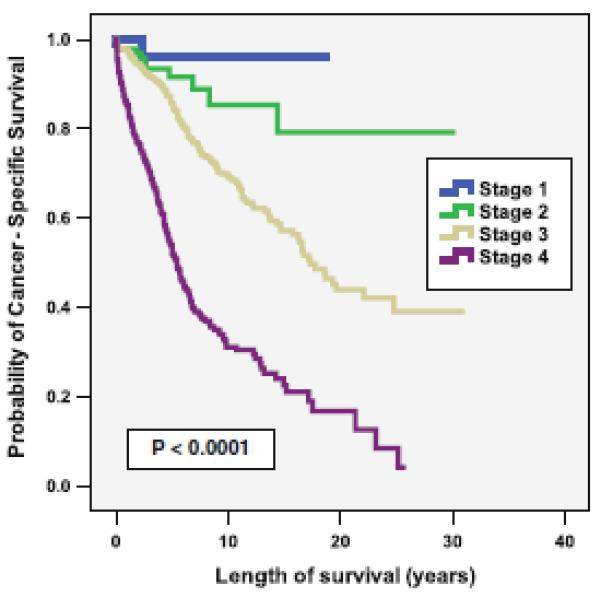
Cancer-specific survival according to stage (n = 1,364)
The results of the statistical comparison between patients in whom no lymph nodes were examined versus those in whom at least one lymph node was removed are displayed in Table 1. Patients were more likely to undergo removal of at least one lymph node if they were young (p = 0.0023), had a large primary tumor (p = 0.021), had no evidence of distant metastatic disease (p < 0.0001), or had a primary tumor of ileal origin (p = 0.0057). Removal of at least one LN was associated with improved survival when compared to patients with no LNs removed on univariate analysis [p = 0.0027; hazard ratio (HR) 0.64, 95 % confidence interval (CI) 0.48–0.86], but not multivariate analysis when adjusted for age, histology, tumor size, and overall stage (p = 0.14; HR 0.93, 95 % CI 0.62–1.38).
Table 1.
Clinical characteristics according to whether patients underwent LN removal at operation (n = 1,364)
| Clinical characteristic |
LNs removed at operation |
No LNs removed at operation |
No. of missing variables |
p |
|---|---|---|---|---|
| Median age | 0 | 0.0023 | ||
| ≤60 years | 609 (87 %) | 93 (13 %) | ||
| >60 years | 534 (81 %) | 128 (19 %) | ||
| Sex | 0 | 0.24 | ||
| Female | 561 (85 %) | 99 (15 %) | ||
| Male | 582 (83 %) | 122 (17 %) | ||
| Race | 4 | 0.57 | ||
| White | 1,016 (84 %) | 200 (16 %) | ||
| Other | 123 (85 %) | 21 (15 %) | ||
| Location | 0 | 0.0057 | ||
| Jejunum | 126 (76 %) | 39 (24 %) | ||
| Ileum | 1,017 (85 %) | 182 (15 %) | ||
| Tumor size (median) | 207 | 0.021 | ||
| ≤2 cm | 585 (8 4 %) | 113 (16 %) | ||
| >2 cm | 407 (89 %) | 52 (11 %) | ||
| LN metastasis | 80 | <0.0001 | ||
| No | 206 (60 %) | 135 (40 %) | ||
| Yes | 922 (98 %) | 21 (2 %) | ||
| Distant metastasis | 0 | <0.0001 | ||
| No | 788 (88 %) | 109 (12 %) | ||
| Yes | 355 (76 %) | 112 (24 %) | ||
LN lymph node
Further analysis was performed on patients who had at least one LN removed (n = 1,143) (Fig. 1). Among this group, a median of eight LNs were identified in resection specimens with a median lymph node ratio (LNR) of 0.29 (range 0–1). Within this patient group, longer overall survival was associated with those who were younger in age (p < 0.001), had smaller primary tumors (p < 0.001), had primary tumors of ileal origin (p = 0.042), or who had no evidence of metastases to regional LNs (p = 0.05) or distant sites (p < 0.0001). Univariate and multivariate analysis was performed on the median number of LNs removed (=8) and the median LNR (=0.29). Importantly, improved overall survival was also associated with removal of [8 LNs (p = 0.05) or a LNR of <0.29 (p = 0.0019) (Table 2). Examination of more than eight lymph nodes (p = 0.0231, HR 0.66, 95 % CI 0.46–0.94) or an LNR of <0.29 (p = 0.029, HR 1.5, CI 1.04–2.15) were both significantly associated with overall survival by multivariate analysis (Table 3).
Table 2.
Overall survival among patients with ≥1 LN removed (n = 1,143)
| Clinical characteristic | No. | No. of variables | p | HR | 95 % CI |
|---|---|---|---|---|---|
| Age at diagnosis (median) | 1,143 | 0 | <0.0001 | 2.96 | 2.18–4.01 |
| ≤59 years | |||||
| >59 years | |||||
| Sex | 0 | 0.68 | 1.06 | 0.8–1.41 | |
| Female | 561 (49 %) | ||||
| Male | 582 (51 %) | ||||
| Race | 4 | 0.97 | 1.01 | 0.64–1.59 | |
| Black | 104 (9 %) | ||||
| White | 1,016 (89 %) | ||||
| Other | 19 (2 %) | ||||
| Location | 0 | 0.042 | 0.66 | 0.44–0.99 | |
| Jejunum | 126 (11 %) | ||||
| Ileum | 1,017 (89 %) | ||||
| Tumor size (cm)a | 0 | <0.0001 | 1.73 | 1.44–2.09 | |
| Mean | 2.3 | ||||
| Median | 2 | ||||
| Range | 0.3–17.0 | ||||
| No. of LNs removed (median) (>8 vs. ≤8) | 1,035 | 108 | 0.05 | 0.73 | 0.53–1.00 |
| LN metastasis | 15 | 0.05 | 1.5 | 1.00–2.24 | |
| No | 206 (18 %) | ||||
| Yes | 922 (82 %) | ||||
| Distant metastasis | 0 | <0.0001 | 2.98 | 2.25–3.96 | |
| No | 788 (69 %) | ||||
| Yes | 355 (31 %) | ||||
| LN ratio (median) (>0.29 vs. ≤0.29) | 1,034 | 109 | 0.0019 | 1.65 | 1.20–2.27 |
LN lymph node, HR hazard ratio, CI confidence interval
Continuous variable
Table 3.
Effect of the LN ratio and the total number of LNs removed on overall survival adjusting for age and tumor size (n = 1,143)
| Variable | p | Hazard ratio |
95 % CI |
|---|---|---|---|
| Effect of no. of LNs removed | |||
| No. of LNs removed: >8 versus ≤8 |
0.0231 | 0.66 | 0.46–0.94 |
| Median age: >59 versus ≤59 years |
<0.0001 | 3.23 | 2.22–4.71 |
| Tumor size: 1-fold increase | <0.0001 | 1.71 | 1.38–2.11 |
| Effect of LN ratio | |||
| Median LN ratio: >0.29 versus ≤0.29 |
0.029 | 1.50 | 1.04–2.15 |
| Median age: >59 versus ≤59 years |
<0.0001 | 3.24 | 2.23–4.71 |
| Tumor size: 1-fold increase | <0.0001 | 1.63 | 1.3–2.04 |
Using CART analysis (adjusting for age and tumor size), patients with [7 LNs removed at operation experienced a better cancer-specific survival over those with B7 LNs removed (median survival not reached vs. 140 months; p = 0.002, HR 0.573, 95 % CI 0.402–0.817). This relation is expressed in the K–M plot in Fig. 3a (p = 0.001). The influence of an LNR of greater or less than 0.29 on overall survival is demonstrated in the K–M plot in Fig. 3b (p = 0.001).
Fig. 3.

Cancer-specific survival. a According to the number of lymph nodes removed. b According to the lymph node ratio
Discussion
Our analysis demonstrated that patients who underwent resection of mesenteric lymph nodes achieved improved cancer-specific survivals. Despite recommendations from the NCCN, roughly 16 % of patients in this study did not have any mesenteric lymph nodes removed for jejunal or ileal neuroendocrine tumors. According to the SEER database, patients who underwent lymph node resection were more likely to be young, have large tumors, and have primary tumors of ileal origin. Also, patients with lower LNRs exhibited better survival.
The extent of regional lymphadenectomy for ileal and jejunal neuroendocrine tumors required to achieve a benefit in cancer-specific survival is unclear, but there are data to suggest that resection of mesenteric lymph nodes improves survival [4]. Neuroendocrine tumors were often thought to be indolent, especially because the primary tumor is usually small. As a result, aggressive surgical resection was not always performed [5, 6]. However, more recent studies have demonstrated that an R0 resection results in better overall survival [4, 7].
Even though the performance of a mesenteric lymph node dissection for patients with jejunal and ileal neuroendocrine tumors has been linked to improved patient outcome, removal is not always possible. Mesenteric metastases frequently occur with these tumors, and they are often much larger than the primary tumor [8]. Local effects of serotonin and other growth factors may cause a fibrotic reaction in the mesentery that can lead to mesenteric encasement of vessels. Because of the concern of compromising the blood supply to large portions of small bowel, mesenteric lymph node dissection is not performed in as many as 33 % of patients with mesenteric lymph node metastases [4, 8].
Unlike the literature for colon adenocarcinoma, there is a lack of published evidence to support the extent of lymph node dissection required to ensure the best outcome for patients with jejunal and ileal neuroendocrine tumors. The purpose of our analysis was to help define a target number of lymph nodes to remove. We found that removal of at least eight lymph nodes predicted a better overall survival on both univariate and multivariate analyses. Even though this information is limited because it was not confirmed with a separate database of patients, it still can be applied to clinical practice. Not only can this information be used by surgeons as a guide at operation, but it can be used to help dictate appropriate follow-up. For instance, patients with a large number of lymph node metastases are at higher risk for developing liver metastasis. By following these patients more frequently with serial imaging, liver metastases may be identified early and treated accordingly. Moreover, as targeted therapies and chemotherapeutic regimens are developed for this disease, the ability to stage these patients appropriately will help determine the need for adjuvant therapy as it does for other malignancies. Clinical trials at the University of Texas M. D. Anderson Cancer Center have already demonstrated antitumor activity in patients receiving everolimus with octreotide long-acting repeatable (LAR) [9, 10].
An increasing LNR confers a poorer prognosis according to both univariate and multivariate analyses. The true significance of this variable as an independent predictor of overall survival remains to be determined. Many of the patients in the SEER database did not undergo adequate lymphadenectomy and may have not been adequately staged. As a result, the calculated ratio may have been affected.
Our analysis has several limitations. First, the study was retrospective and involved a large, population-based database across several states with a large number of missing variables. The SEER database does not provide the reason why mesenteric lymphadenectomy was not performed, and all stages of disease were represented in this group. Some patients likely had unresectable disease, and others may have not undergone lymph node removal because the surgeon was not aware of the pathologic diagnosis at the time of operation. Also, the degree of differentiation (grade) was not included in the analysis because information in the database was limited to a few patients. Likewise, several patients were eliminated from the analysis if they had non-cancer-related causes of death or multiple primary tumors. There was no information on disease-free survival, imaging characteristics, specific details of chemotherapy, or specific details regarding operative intervention. The combination of limited information along with the potential error of multiple contributors from different centers may have affected the results. On the other hand, using data from the SEER database provides the ability to evaluate large numbers of patients with long-term follow-up. The rarity of neuroendocrine tumors of the small bowel makes it difficult to evaluate large numbers of patients at a single institution. Although our data set may not be ideal, the data do show that mesenteric lymphadenectomy improves overall survival. Our results require validation in a large, prospective setting at a tertiary care center.
Conclusions
This review of a large number of surgical patients demonstrates that regional mesenteric lymphadenectomy that includes at least eight lymph nodes in conjunction with resection of the primary tumor is associated with improved survival in patients with small bowel neuroendocrine tumors. Likewise, a higher LNR is associated with a worse cancer-specific survival. Because of the limitations of the SEER database, further evaluation is required in the setting of a prospective clinical trial.
Acknowledgments
This study was supported by the Khalifa Bin Zayed Al Nahyan Foundation, various donors to the Pancreatic Research Fund at MD Anderson Cancer Center, and the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
References
- 1.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous . AJCC cancer staging handbook. 7th American Joint Committee on Cancer; Chicago: 2010. [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Hellman P, Lundstrom T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg. 2002;26:991–997. doi: 10.1007/s00268-002-6630-z. doi:10.1007/s00268-002-6630-z. [DOI] [PubMed] [Google Scholar]
- 5.Martin RG. Management of carcinoid tumors. Cancer. 1970;26:547–551. doi: 10.1002/1097-0142(197009)26:3<547::aid-cncr2820260307>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Morgan JG, Marks C, Hearn D. Carcinoid tumors of the gastrointestinal tract. Ann Surg. 1974;180:720–727. doi: 10.1097/00000658-197411000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landerholm K, Zar N, Andersson RE, et al. Survival and prognostic factors in patients with small bowel carcinoid tumour. Br J Surg. 2011;98:1617–1624. doi: 10.1002/bjs.7649. [DOI] [PubMed] [Google Scholar]
- 8.Kerstrom G, Hellman P, Hessman O. Midgut carcinoid tumours: surgical treatment and prognosis. Best Pract Res Clin Gastroenterol. 2005;19:717–728. doi: 10.1016/j.bpg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]


