Abstract
Background
The cause of historically higher rates of invasive pneumococcal disease among blacks than whites has remained unknown. We tested the hypothesis that sickle cell trait or hemoglobin C trait is an independent risk factor for invasive pneumococcal disease.
Methods
Eligible children were born in Tennessee (1996–2003), had a newborn screen, enrolled in TennCare aged <1 year, and resided in a Tennessee county with laboratory-confirmed, pneumococcal surveillance. Race/ethnicity was ascertained from birth certificates. Children were followed through 2005 until loss of enrollment, pneumococcal disease episode, 5th birthday or death. We calculated incidence rates by race/ethnicity and hemoglobin type before and after pneumococcal conjugate vaccine (PCV7) introduction. Poisson regression analyses compared IPD rates among blacks with sickle cell trait or hemoglobin C trait to whites and blacks with normal hemoglobin, controlling for age, gender, time (pre-PCV7, transition year or post-PCV7) and high-risk conditions (i.e. heart disease).
Results
Over 10 years, 415 invasive pneumococcal disease episodes occurred during 451,594 observed child-years. Before PCV7 introduction, disease rates/100,000 child-years were 2941 for blacks with sickle cell disease, 258 for blacks with sickle cell trait or hemoglobin C trait and 188, 172, and 125 for blacks, whites, and Hispanics with normal hemoglobin. Post-PCV7, rates declined for all groups. Blacks with sickle cell trait or hemoglobin C trait had 77% (95% CI 22%–155%) and 42% (95% CI 1%–100%) higher rates than whites and blacks with normal hemoglobin.
Conclusion
Black children with sickle cell trait or hemoglobin C trait have an increased risk of invasive pneumococcal disease.
In 2000, heptavalent pneumococcal conjugate vaccine (PCV7) was recommended for all U.S. children at 2, 4, 6 and 12–15 months. Since that time, invasive pneumococcal disease has declined and racial differences in blacks and whites have narrowed significantly in infants, children and adults.1–3 Yet, non-PCV7 pneumococcal serotypes, such as 19A, have emerged as important pathogens.4,5
Because children with sickle cell disease (hemoglobin SS and SC diseases) have an increased risk of pneumococcal diseases,6 pneumococcal vaccination (PCV7 during infancy and 23-valent pneumococcal polysaccharide vaccine at ≥2 years) and penicillin prophylaxis during the first 5 years of life are recommended by the Center for Disease Control Advisory Committee on Immunization Practices.7 Hypothesized reasons for this increased susceptibility include inadequate splenic functioning and decreased serum opsonization of pneumococci.8 PCV7 has been associated with protection among all children, including those with sickle cell disease.3,6,9,10 Although the exact mechanism for this protection is not known, serum-opsonization activity in children with sickle cell disease increased after PCV7 vaccination, and is correlated with antibody levels.11 In contrast, sickle cell trait has been generally considered benign and not associated with increased risk of bacterial infections in childhood.
Homozygous hemoglobin C disease arose in West Africa on both β-globin genes at the same amino acid as sickle cell disease. It is characterized by bizarre red blood cell shapes, risk of splenomegaly and mild hemolytic anemia.12 Both homozygous hemoglobin C disease and trait are considered benign without reports of increased risk of bacterial infection.
We linked four population-based databases: Tennessee Active Bacterial Core Surveillance (supported by the CDC), TennCare (Tennessee’s Medicaid Program), Tennessee birth certificate registry and Tennessee newborn screens. The goals of this study were (1) to compare rates of invasive pneumococcal disease among blacks with sickle cell disease, sickle cell trait or hemoglobin C trait to those among blacks, whites and Hispanics with normal hemoglobin, and (2) to determine if either sickle cell trait or hemoglobin C trait explains the historically higher rates of invasive pneumococcal disease among blacks than whites.
Methods
Ascertainment of episodes of invasive pneumococcal disease
The Core Surveillance program has conducted active, laboratory-based surveillance for pneumococcus since 1995 in five urban counties encompassing four major metropolitan cities in Tennessee.13 In August 1999, the program network added six counties, which increased the surveillance population to more than 2.8 million persons.14 Invasive pneumococcal disease was defined as S. pneumoniae isolated from a normally sterile site (i.e. blood).3,13
TennCare database
In 1994, TennCare replaced the federal Medicaid program as a state-based, capitated, managed-health-care program, covering Medicaid-eligible, uninsured, and uninsurable state residents.15 The following administrative data from TennCare were computerized: an enrollment file, a pharmacy file that captures filled outpatient prescriptions, an inpatient file, and an outpatient file that includes encounter records for emergency department, hospital outpatient, and physician visits. Diagnoses were coded using the International Classification of Diseases, 9th edition (ICD-9).16
Using this TennCare database, we identified all children younger than 5 years of age who were enrolled within the first year of life and resided in a surveillance county. High-risk co-morbid conditions included: (1) 1 or more hospital or emergency department discharges with a condition-specific diagnostic code, (2) 2 or more outpatient visits with a condition-specific diagnostic code, or (3) filled prescription for condition-specific medications, as previously described.17 High-risk conditions included HIV infection, sickle cell disease, congenital heart disease, chronic lung disease, diabetes mellitus, hepatic disease, renal disease, cancer or immunosuppression. The first three conditions were considered present since birth whereas all others began on the date the child first met the high-risk definition.
Because asthma is associated with an increased risk of invasive pneumococcal disease,17 we determined if children without the above-mentioned high-risk conditions had asthma. The association between asthma and risk of invasive pneumococcal disease has been identified more recently than the other high-risk conditions and thus we evaluated it separately. Asthma was defined as (1) 1 or more hospitalization or emergency department visit with discharge ICD-9 code of 493.XX, (2) 2 or more outpatient visits with 493.XX, (3) 2 or more filled prescriptions for short-acting β-agonists, or (4) any prescription for inhaled corticosteroids, long-acting β-agonists, nedocromil, cromolyn or leukotriene antagonists.
Race/ethnicity
We determined the race of each child using the state’s classification from the birth certificate. In Tennessee, the mother was instructed to report the race of herself and the father (white, black, American Indian, Chinese, Japanese, Hawaiian, Filipino, other Asian, other or unknown) on the child’s birth certificate. Children were considered black if either parent was black. Children were considered white if both parents were white or if the mother was white and the race of the father was unknown. Hispanic origin of the parents, which was completed for 85% of all birth certificates, was used to determine ethnicity. Children were considered Hispanic if the either parent was Hispanic. We categorized children as black non-Hispanic, white non-Hispanic or white Hispanic. Children of unknown and other races were excluded.
Tennessee Newborn Screen
Tennessee has screened newborns during the birth hospitalization for hemoglobinopathies since 1988. Sickle cell disease is the most prevalent hemoglobinopathy. Sickle cell trait occurs in approximately 1 in 14 blacks; hemoglobin C trait occurs in approximately 1 in 48 blacks.18 The newborn screen for hemoglobinopathies is performed by isoelectric focusing from filter-paper dried-blood spots, and results are reported in this database. If the hemoglobin screen is abnormal, the physician is contacted for a confirmatory blood test using high-performance liquid chromatography or cellulose-acetate electrophoresis, which was not entered into this database.
The Tennessee Newborn Screen database included 701,808 observations among infants born 1995–2003. Hemoglobin profile is reported in the order of prevalence of hemoglobin types, which include fetal hemoglobin (the most prevalent hemoglobin in newborns), adult hemoglobin, sickle hemoglobin, hemoglobin C and additional hemoglobin variants. We included three hemoglobin profiles: sickle cell disease ([1] fetal and sickle hemoglobins; [2] fetal hemoglobin, sickle hemoglobin and hemoglobin C; and [3] fetal hemoglobin, hemoglobin C and sickle hemoglobin), sickle cell trait (fetal, adult and sickle hemoglobins), hemoglobin C trait (fetal hemoglobin, adult hemoglobin, and hemoglobin C) and normal fetal and adult hemoglobins. Observations for 31 other hemoglobin profiles (n = 17,208) were excluded. We also excluded hemoglobin profiles from 1995, because these were incomplete (only 6% of the expected observations based on later years).
Children may have duplicate newborn screens if the initial screen was obtained before 24 hours of life, or before lactose feeds were initiated, or if the provider repeated the measure as part of an abnormal screen evaluation. To confirm the accuracy of the Tennessee Newborn Screen database, we compared the first and last newborn screen result among 4279 newborns with duplicate isoelectric focusing screens; concordance was 99%.
Linkage of databases
Tennessee Newborn Screen data, birth certificate files and Core surveillance data for invasive pneumococcal disease were linked to the TennCare enrollment file by patient-specific identifiers. After linkage, a de-identified data set was created for analysis.
Study Approvals
This study was approved by the institutional review boards of Vanderbilt University and Wake Forest University and was reviewed and approved by Tennessee Department of Health and the Bureau of TennCare.
Analysis
Children entered the study at the time of enrollment in TennCare, either at birth (87%) or during their first year of life (13%) and were followed until the first of the following: end of study (31 December 2005), day of first laboratory-confirmed invasive pneumococcal disease, loss of enrollment in TennCare for >30 days, move out of surveillance county, or 5th birthday. Annual invasive pneumococcal disease rates in children by race/ethnicity and newborn screen hemoglobin profile were determined by the number of first episodes of invasive pneumococcal disease per year divided by the total number of eligible child-years, and multiplied by 100,000. Average invasive pneumococcal disease rates in children by race/ethnicity and hemoglobin profile were calculated by demographic characteristics before the vaccine was available (January 1996–June 2000), during the transition (July 2000–June 2001) and after PCV7 introduction (July 2001–December 2005). Confidence intervals (CIs) were obtained from univariate Poisson regression, limited to the two groups being compared for each test.
We computed invasive pneumococcal disease rates for children with sickle cell disease; univariate and multivariate Poisson regression analyses were limited to children with normal hemoglobin, sickle cell trait or hemoglobin C trait to determine other characteristics associated with increased invasive pneumococcal disease risk. Race/ethnicity and sickle cell trait or hemoglobin C trait status were coded together as one categorical variable to test the a priori hypothesis that either trait mutation would be associated with increased risk of invasive pneumococcal disease. Secondary analyses evaluated black children with sickle cell trait separately from those with hemoglobin C trait. Contrasts were computed to test for an interaction of age group by time (P = 0.96) and by race/ethnicity/hemoglobin type by high-risk conditions, combining whites and Hispanics due to low child-years in Hispanics (P = 0.49 for interaction). Both interaction terms were non significant in a priori secondary analyses, and we dropped these from the final model. Statistical analyses were performed using SAS for Windows 9.1 (SAS Institute Inc., Cary, NC).
Results
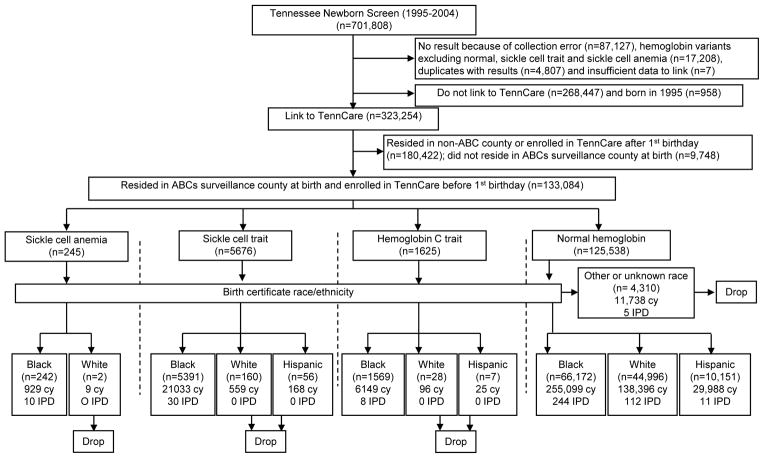
Of 593,866 screened Tennessee newborns (701,808 screens), 592,659 (99%) infants had one of the major hemoglobin types (sickle cell disease, sickle cell trait, hemoglobin C trait or normal hemoglobin) and sufficient information to link to TennCare enrollment files (Figure 1). We limited the observations to non-Hispanic black, non-Hispanic white or white Hispanic children, excluding 4310 (3%) children with unknown or other race/ethnicity. Two non-Hispanic white children with sickle cell disease and 251 white non-Hispanic or white Hispanic children with sickle cell trait or hemoglobin C trait were excluded because of small numbers and the absence of invasive pneumococcal disease episodes to make meaningful comparisons. The final study population comprised 128,521 children.
Figure 1.
Study population. cy indicates child-years of observation; IPD, number of first episodes of laboratory-confirmed, invasive pneumococcal disease in Tennessee’s Active Bacterial Core surveillance.
A total of 415 first episodes of invasive pneumococcal disease were recorded during 451,594 child-years of observation, yielding 92 episodes per 100,000 child-years (Table). Of 415 children with invasive pneumococcal disease, 10 (2.4%) had sickle cell disease, 30 (7.2%) had sickle cell trait, 8 (1.9%) had hemoglobin C trait, and 367 (88.4%) had normal hemoglobin. Children with sickle cell disease had a higher rate of invasive pneumococcal disease per 100,000 child-years (1076 [95% CI = 517–1971]) than children with sickle cell trait or hemoglobin C trait (140 [99–192]) or those with normal hemoglobin (81 [67–97]). The remaining analyses focus on the comparison of interest, children with sickle cell trait or hemoglobin C trait and those with normal hemoglobin.
Table.
Rate of invasive pneumococcal disease per 100,000 child-years and univariate and multivariate Poisson regression analysis for risk factors of IPD
| Study Period | Disease Rate (No. cases) | Univariate analysis | Multivariate analysisa | ||
|---|---|---|---|---|---|
| Cumulative (Jan 1996–Dec 2005) | Pre-PCV7 (Jan 1996–Jun 2000) | Post-PCV7 (Jul 2001–Dec 2005) | RR (95%CI) | RR (95%CI) | |
| Years | |||||
| January 1996–June 2000b | 188.3 (228) | 188.3 (228) | -- | 1.00 | 1.00 |
| July 2000–June 2001 | 146.3 (89) | -- | -- | 0.78 (0.61–0.99) | 1.03 (0.80–1.32) |
| July 2001–December 2005 | 32.7 (88) | -- | 32.7 (88) | 0.17 (0.14–0.22) | 0.26 (0.20–0.34) |
| Age (months) | |||||
| 0–23b | 151.3 (339) | 236.5 (207) | 57.8 (62) | 1.00 | 1.00 |
| 24–59 | 41.9 (39) | 77.0 (17) | 20.3 (12) | 0.28 (0.20–0.39) | 0.28 (0.20–0.40) |
| 36–59 | 20.2 (27) | 34.8 (4) | 13.7 (14) | 0.13 (0.09–0.20) | 0.16 (0.11–0.24) |
| Sex | |||||
| Boyb | 110.8 (254) | 223.7 (138) | 41.8 (57) | 1.00 | 1.00 |
| Girl | 68.2 (151) | 151.5 (90) | 23.4 (31) | 0.61 (0.50–0.75) | 0.66 (0.54–0.80) |
| High-risk conditions | |||||
| Yesc | 201.1 (80) | 456.7 (39) | 103.4 (27) | 2.80 (2.18–3.61) | 4.01 (3.10–5.17) |
| Asthma only | 110.4 (87) | 282.8 (47) | 34.8 (18) | 1.54 (1.20–1.97) | 2.09 (1.63–2.69) |
| Nob | 71.7 (238) | 148.0 (142) | 22.5 (43) | 1.00 | 1.00 |
| Race/ethnicity and hemoglobin type | |||||
| White normal hemoglobinb | 80.9 (112) | 174.4 (64) | 30.1 (25) | 1.00 | 1.00 |
| Black hemoglobin S or C trait | 139.8 (38) | 260.8 (21) | 46.0 (7) | 1.73 (1.20–2.50) | 1.77 (1.22–2.55) |
| Hemoglobin S traitd | 142.6 (30) | 300.9 (19) | 25.6 (3) | −1.76 (1.18–2.64) | −1.80 (1.20–2.69) |
| Hemoglobin C traitd | 130.1 (8) | 115.0 (2) | 113.7 (4) | −1.61 (0.78–3.29) | −1.66 (0.81–3.39) |
| Black normal hemoglobin | 95.6 (244) | 190.6 (138) | 34.6 (51) | 1.18 (0.95–1.48) | 1.25 (1.00–1.56) |
| Hispanic normal hemoglobin | 36.7 (11) | 126.2 (5) | 21.7 (5) | 0.45 (0.24–0.84) | 0.61 (0.33–1.14) |
The original multivariate model reported here includes variables (categorical) for study years, ages, gender, high risk conditions and four race-ethnicity hemoglobin groups: one combined risk group for hemoglobin S or C trait (blacks only) and three separate groups for normal hemoglobin (blacks, Hispanics and whites as reference).
Reference category.
High-risk conditions include HIV, cancer or immunosuppression, sickle cell disease, congenital heart disease, diabetes mellitus, renal disease, hepatic disease and chronic lung disease.
The subgroup-specific risk ratios for hemoglobin S trait and hemoglobin C trait are reported from an identical (secondary) multivariate model, except without trait risk groups S and C combined.
The invasive pneumococcal disease rate among children with sickle cell trait or hemoglobin C trait and normal hemoglobin decreased 83% (RR = 0.17 [95%CI = 0.14–0.22]) from before PCV7 introduction to after introduction (188/100,000 versus 33/100,000). Overall, higher rates were observed for younger children, boys and children with high-risk conditions including asthma (Table). Invasive pneumococcal disease rates among black children with sickle cell trait or hemoglobin C trait were 37% higher (RR = 1.37 [95% CI = 0.86–2.17]) than those with normal hemoglobin before PCV7 introduction; after vaccine introduction, rates were 33% higher (RR = 1.33 [0.60–2.93]) (Figure 2). Among children with normal hemoglobin, rates among blacks were 9% higher (1.09 [0.81–1.47]) and 15% (1.15 [0.71–1.85]) higher than among whites before and after PCV7, respectively.
Figure 2.
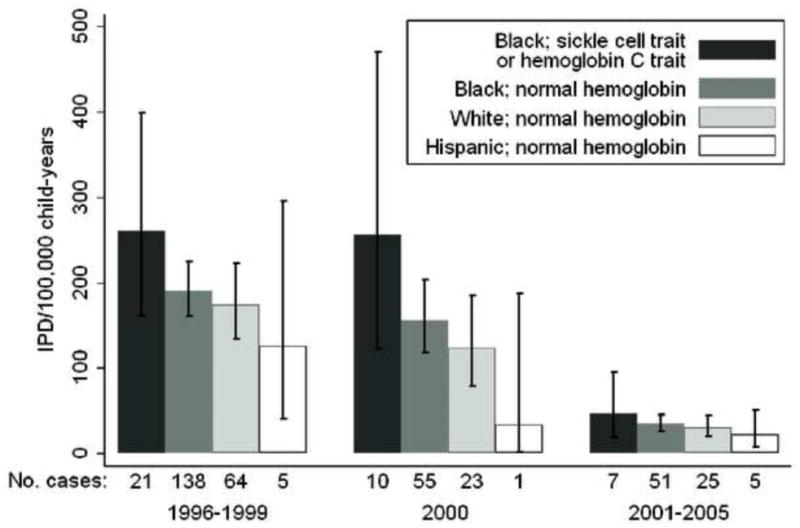
Incident rates of laboratory-confirmed invasive pneumococcal disease per 100,000 child-years, with 95% confidence intervals, by study period and by race and hemoglobin type per newborn screen.
In univariate and multivariate Poisson regression analyses, younger age, male sex, years before PCV introduction, traditional high-risk factors and asthma were all associated with increased risk of invasive pneumococcal disease. Black children with either sickle cell trait or hemoglobin C trait had increased risk, controlling for age, sex, time and high-risk conditions, compared with white (aRR = 1.77 [1.22–2.55]), black (1.42 [1.01–2.00]), and Hispanic (2.88 [1.47–5.66]) children with normal hemoglobin. Risk of invasive pneumococcal disease among black children with normal hemoglobin was higher than among white children with normal hemoglobin (1.25 [1.00–1.56]), whereas the risk among white Hispanic children was lower (0.61 [0.33–1.14]).
When this multivariate analysis was limited to the post-PCV7 years, similar risks of invasive pneumococcal disease were observed, albeit with wider confidence intervals. The disease risk for black children with sickle cell trait or hemoglobin C trait was aRR = 1.80 (0.78–4.17), compared with white children with normal hemoglobin. For black children with normal hemoglobin the risk was aRR = 1.33 (0.82–2.15) compared with white children with normal hemoglobin.
In secondary analyses, the risk of invasive pneumococcal disease for blacks with hemoglobin C trait was similar to that of blacks with sickle cell trait (aRR = 0.92 [0.42–2.01]). Blacks with sickle cell trait had higher risk of disease compared with Hispanics (2.93 [1.47–5.87]) and whites (1.80 [1.20–2.69]) with normal hemoglobin. The difference for blacks with normal hemoglobin was in the same direction (1.44 [0.99–2.11]), compared with whites with normal hemoglobin.
Discussion
Black children with sickle cell trait or hemoglobin C trait had higher rates of invasive pneumococcal disease than black and white children with normal hemoglobin. Having one of two different genetic mutations of the β-globin gene (sickle cell trait [glutamine to valine] or hemoglobin C trait [glutamine to lysine]) is an independent risk factor for invasive pneumococcal disease among black children, and may partially explain the historically-higher rates among black children. To our knowledge, this study is the first to find that sickle cell trait or hemoglobin C trait is associated with increased risk of an infectious disease in children. This finding contrasts with the common teaching that sickle cell trait and hemoglobin C trait are benign under physiologic conditions.19
The reason that blacks have had historically higher invasive pneumococcal disease rates than whites remains unclear. Previous studies controlled for high-risk conditions predisposing to invasive pneumococcal disease and for socioeconomic factors, but none of these factors completely explains this observed difference.20–22 However, one study reported that one (2%) of 42 children with pneumococcal bone and joint infections had sickle cell trait.23
The availability of newborn screening data enabled testing the hypothesis that rates of invasive pneumococcal disease could differ by hemoglobin type. Data identifying children with sickle cell trait or hemoglobin C trait have not been historically available, since these children do not have distinct clinical characteristics like those with sickle cell disease. Notably, regions of Africa with high prevalence of sickle cell or hemoglobin C traits overlap the meningitis belt. This raises the possibility that sickle cell or hemoglobin C traits may confer some risk of infection with encapsulated organisms. Although these two traits explain part of the observed racial difference in invasive pneumococcal disease rates, black children with normal hemoglobin had 25% increased risk compared with white children who had normal hemoglobin in multivariate analysis. Because the modest increase associated with sickle cell trait or hemoglobin C trait did not markedly increase the rates in all black children combined, factors other than sickle cell or hemoglobin C trait likely contribute to this difference. For example, race/ethnicity could be a surrogate marker of unmeasured factors such as access to or location of care.
The biologic mechanism by which sickle cell trait or hemoglobin C trait could cause an increased risk of invasive pneumococcal disease is unknown. Both sickle cell and hemoglobin C traits are associated with modest decreases in the red-blood-cell lifespan, with levels of hemoglobin within the lower limits of normal.24,25 Previous studies indicate that persons with sickle cell trait as compared to persons with normal hemoglobin are protected against severe Plasmodium falciparum infection.26–31 Similarly hemoglobin C trait has been associated with protection against severe P. falciparum infection in many, but not all, studies.30–37 Sickle cell trait and hemoglobin C trait have been reportedly associated with hematuria, retinopathy and priapism.38–45 Recently, sickle cell trait was associated with a 2-fold increased risk of vasothrombotic embolism; a similar risk was observed among an underpowered sample size with hemoglobin C trait.46 Other conditions reported to be associated with sickle cell trait have been splenic infarction at high altitudes, sudden death after physical exertion, bacteruria, urinary tract infections during pregnancy, hyposthenuria, renal papillary necrosis and renal medullary carcinoma.19,47–53 Most of these events are rare, and the evidence base for some of these associations is weak, which is consistent with the finding that sickle cell trait has little or no detectable increase risk in overall mortality, morbidity or hospitalizations.19
Differences in black and white populations persist for a variety of acute and chronic conditions. Hence, understanding the mechanism by which sickle cell trait or hemoglobin C trait could confer higher risk of invasive pneumococcal disease might add to our understanding of disease pathogenesis. Studies are warranted to determine if sickle cell trait or hemoglobin C trait could contribute to racial differences in other health outcomes such as other infectious diseases, asthma, hypertension, renal failure or cardiovascular disease.
These results have some limitations. Study children resided in Tennessee and were enrolled in TennCare, and hence the generalizability is not known. History of pneumococcal vaccination was not obtained in this study. However, PCV7 vaccination in Tennessee has followed the national trend according to the National Immunization Survey.54 It is unlikely that PCV7 coverage would systematically differ between black children with sickle cell trait and black children with normal hemoglobin, even if small systematic differences in PCV7 coverage occurred between black and white children. Furthermore, results were consistent in pre-PCV7 and post-PCV7 years—although with 88 cases of invasive pneumococcal disease in post-PCV7 years our power to detect a difference in this subanalysis was limited. Hemoglobin type was determined by results of isoelectric focusing from newborn screens. Random misclassification is possible but is likely small, and would reduce the power to detect differences. We found high concordance (>99%) between first and last screens among infants with duplicate screens; another study comparing newborn screen results by isoelectric focusing and high-performance liquid chromatography also showed high concordance.55 Among our black study population, the frequency of sickle cell trait (1 in 14) and hemoglobin C trait (1 in 48) was similar to that reported elsewhere.18 It is possible that linkage disequilibrium between the β-globin genes and other genes may be important in determining risk of invasive pneumococcal disease. We determined race as that reported on birth certificates. We lacked the sample size to analyze sickle cell trait and hemoglobin C among children of other race/ethnicity. Random misclassification of race is possible but would unlikely relate to invasive pneumococcal disease incidence and thus reduce the power to detect differences. Birth certificates during the study period did not permit reporting more than one race for a parent. Laboratory-based surveillance may underestimate the rate of invasive pneumococcal disease if cultures are not obtained, or obtained after antibiotics are administered, or if infants die from invasive pneumococcal disease before receiving medical care. Such laboratory testing may differ by race/ethnicity depending on the site of care; however, it is unlikely to differ by hemoglobin type.
In conclusion, we found that among black children either sickle cell trait or hemoglobin C trait independently predicted invasive pneumococcal disease. The absolute risk for individuals is quite small, especially since the introduction of PCV7 for all infants. Further studies are needed to replicate this finding, uncover the biologic mechanism for this increased risk of invasive pneumococcal disease, determine if this risk persists in later childhood or adulthood, and ascertain if this risk is relevant to other organisms.
Acknowledgments
Financial support: Supported in part by research grant number 6-FY07-284 from the March of Dimes Birth Defects Foundation and an Emerging Infections Cooperative Program Agreement (U50/CCU416123) through the Centers for Disease Control and Prevention. Dr. Poehling received support from the Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Program, National Institutes of Health (K23 AI065805) and Wachovia Research Fund. Dr. Griffin received research support from Wyeth. Dr. Halasa received support from Medimmune and Sanofi-Pasteur and served as a consultant for Novaris. Dr. Schaffner was a consultant for Wyeth and served on a Data Safety Monitoring Board for experimental vaccines for Merck.
We greatly appreciate all the reviewers whose comments have led to important improvements in this manuscript.
References
- 1.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668–1674. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 2.Flannery B, Schrag S, Bennett NM, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004;291:2197–2203. doi: 10.1001/jama.291.18.2197. [DOI] [PubMed] [Google Scholar]
- 3.Talbot TR, Poehling KA, Hartert TV, et al. Reduction in high rates of antibiotic-nonsusceptible invasive pneumococcal disease in Tennessee after introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2004;39:641–648. doi: 10.1086/422653. [DOI] [PubMed] [Google Scholar]
- 4.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–1792. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 5.Moore MR, Gertz RE, Jr, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016–1027. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 6.Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008;121:562–569. doi: 10.1542/peds.2007-0018. [DOI] [PubMed] [Google Scholar]
- 7.Preventing pneumococcal disease among infants and young children recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49(RR-09):1–35. [PubMed] [Google Scholar]
- 8.Pearson HA. Prevention of pneumococcal disease in sickle cell anemia. J Pediatr. 1996;129:788–789. doi: 10.1016/s0022-3476(96)70019-7. [DOI] [PubMed] [Google Scholar]
- 9.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 10.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1428–1433. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 11.Nowak-Wegrzyn A, Winkelstein JA, Swift AJ, Lederman HM. Serum opsonic activity in infants with sickle-cell disease immunized with pneumococcal polysaccharide protein conjugate vaccine. The Pneumococcal Conjugate Vaccine Study Group. Clin Diagn Lab Immunol. 2000;7:788–793. doi: 10.1128/cdli.7.5.788-793.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairhurst RM, Casella JF. Images in clinical medicine. Homozygous hemoglobin C disease. N Engl J Med. 2004;350:e24. doi: 10.1056/NEJMicm030486. [DOI] [PubMed] [Google Scholar]
- 13.Schuchat A, Hilger T, Zell E, et al. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis. 2001;7:92–99. doi: 10.3201/eid0701.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Census. U S Census Bureau; [Accessed August 31, 2009.]. Available at: www.factfinder.census.gov. [Google Scholar]
- 15.Mirvis DM, Chang CF, Hall CJ, Zaar GT, Applegate WB. TennCare--health system reform for Tennessee. JAMA. 1995;274:1235–1241. doi: 10.1001/jama.274.15.1235. [DOI] [PubMed] [Google Scholar]
- 16.International classification of diseases. 6th ed., 9th rev. clinical modification: ICD-9-CM professional for hospitals. 6. Salt Lake City, UT: Ingenix/St.Anthony Publishing; 2002. [Google Scholar]
- 17.Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352:2082–2090. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 18.Lorey FW, Arnopp J, Cunningham GC. Distribution of hemoglobinopathy variants by ethnicity in a multiethnic state. Genet Epidemiol. 1996;13:501–512. doi: 10.1002/(SICI)1098-2272(1996)13:5<501::AID-GEPI6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Sears DA. The morbidity of sickle cell trait: a review of the literature. Am J Med. 1978;64:1021–1036. doi: 10.1016/0002-9343(78)90458-8. [DOI] [PubMed] [Google Scholar]
- 20.Robinson KA, Baughman W, Rothrock G, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–1735. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 21.Breiman RF, Spika JS, Navarro VJ, Darden PM, Darby CP. Pneumococcal bacteremia in Charleston County, South Carolina. A decade later. Arch Intern Med. 1990;150:1401–1405. [PubMed] [Google Scholar]
- 22.Schlech WF, III, Ward JI, Band JD, Hightower A, Fraser DW, Broome CV. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA. 1985;253:1749–1754. [PubMed] [Google Scholar]
- 23.Bradley JS, Kaplan SL, Tan TQ, et al. Pediatric pneumococcal bone and joint infections. The Pediatric Multicenter Pneumococcal Surveillance Study Group (PMPSSG) Pediatrics. 1998;102:1376–1382. doi: 10.1542/peds.102.6.1376. [DOI] [PubMed] [Google Scholar]
- 24.McCurdy PR. 32-DFP and 51-Cr for measurement of red cell life span in abnormal hemoglobin syndromes. Blood. 1969;33:214–224. [PubMed] [Google Scholar]
- 25.Prindle KH, Jr, McCurdy PR. Red cell lifespan in hemoglobin C disorders (with special reference to hemoglobin C trait) Blood. 1970;36:14–19. [PubMed] [Google Scholar]
- 26.Abu-Zeid YA, Theander TG, Abdulhadi NH, et al. Modulation of the cellular immune response during Plasmodium falciparum infections in sickle cell trait individuals. Clin Exp Immunol. 1992;88:112–118. doi: 10.1111/j.1365-2249.1992.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ntoumi F, Mercereau-Puijalon O, Ossari S, et al. Plasmodium falciparum: sickle-cell trait is associated with higher prevalence of multiple infections in Gabonese children with asymptomatic infections. Exp Parasitol. 1997;87:39–46. doi: 10.1006/expr.1997.4173. [DOI] [PubMed] [Google Scholar]
- 28.Le Hesran JY, Personne I, Personne P, et al. Longitudinal study of Plasmodium falciparum infection and immune responses in infants with or without the sickle cell trait. Int J Epidemiol. 1999;28:793–798. doi: 10.1093/ije/28.4.793. [DOI] [PubMed] [Google Scholar]
- 29.Williams TN, Mwangi TW, Roberts DJ, et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verra F, Simpore J, Warimwe GM, et al. Haemoglobin C and S role in acquired immunity against Plasmodium falciparum malaria. PLoS ONE. 2007;2:e978. doi: 10.1371/journal.pone.0000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cholera R, Brittain NJ, Gillrie MR, et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A. 2008;105:991–996. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson GR. Malaria and stress in relation to haemoglobins S and C. Br Med J. 1963;2:976–978. doi: 10.1136/bmj.2.5363.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A, Guindo A, Cissoko Y, et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000;96:2358–2363. [PubMed] [Google Scholar]
- 34.May J, Evans JA, Timmann C, et al. Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA. 2007;297:2220–2226. doi: 10.1001/jama.297.20.2220. [DOI] [PubMed] [Google Scholar]
- 35.Rihet P, Flori L, Tall F, Traore AS, Fumoux F. Hemoglobin C is associated with reduced Plasmodium falciparum parasitemia and low risk of mild malaria attack. Hum Mol Genet. 2004;13:1–6. doi: 10.1093/hmg/ddh002. [DOI] [PubMed] [Google Scholar]
- 36.Guinet F, Diallo DA, Minta D, et al. A comparison of the incidence of severe malaria in Malian children with normal and C-trait hemoglobin profiles. Acta Trop. 1997;68:175–182. doi: 10.1016/s0001-706x(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 37.Modiano D, Luoni G, Sirima BS, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 38.Heller P, Best WR, Nelson RB, Becktel J. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. N Engl J Med. 1979;300:1001–1005. doi: 10.1056/NEJM197905033001801. [DOI] [PubMed] [Google Scholar]
- 39.Robertson MG. Priapism and painless hematuria in hemoglobin C trait. JAMA. 1971;216:677. [PubMed] [Google Scholar]
- 40.Oksenhendler E, Bourbigot B, Desbazeille F, et al. Recurrent hematuria in 4 white patients with sickle cell trait. J Urol. 1984;132:1201–1203. doi: 10.1016/s0022-5347(17)50097-x. [DOI] [PubMed] [Google Scholar]
- 41.Sakarcan A, Stallworth J. Urea resolves gross hematuria in a 15 year old with hemoglobin C trait. Pediatr Nephrol. 2001;16:145–147. doi: 10.1007/s004670000532. [DOI] [PubMed] [Google Scholar]
- 42.Hingorani M, Bentley CR, Jackson H, et al. Retinopathy in haemoglobin C trait. Eye. 1996;10:338–342. doi: 10.1038/eye.1996.70. [DOI] [PubMed] [Google Scholar]
- 43.Nagpal KC, Asdourian GK, Patrianakos D, et al. Proliferative retinopathy in sickle cell trait. Report of seven cases. Arch Intern Med. 1977;137:325–328. [PubMed] [Google Scholar]
- 44.Gibson BR, Peterson AC, Costabile RA. Priapism associated with hemoglobin C trait. J Urol. 2002;168:2122. doi: 10.1016/S0022-5347(05)64314-5. [DOI] [PubMed] [Google Scholar]
- 45.Birnbaum BF, Pinzone JJ. Sickle cell trait and priapism: a case report and review of the literature. Cases J. 2008;1:429. doi: 10.1186/1757-1626-1-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Austin H, Key NS, Benson JM, et al. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110:908–912. doi: 10.1182/blood-2006-11-057604. [DOI] [PubMed] [Google Scholar]
- 47.Ashcroft MT, Miall WE, Milner PF. A comparison between the characteristics of Jamaican adults with normal hemoglobin and those with sickle cell trait. Am J Epidemiol. 1969;90:236–243. doi: 10.1093/oxfordjournals.aje.a121066. [DOI] [PubMed] [Google Scholar]
- 48.Baill IC, Witter FR. Sickle trait and its association with birthweight and urinary tract infections in pregnancy. Int J Gynaecol Obstet. 1990;33:19–21. doi: 10.1016/0020-7292(90)90649-6. [DOI] [PubMed] [Google Scholar]
- 49.Miller JM., Jr Sickle cell trait in pregnancy. South Med J. 1983;76:962–3. 965. doi: 10.1097/00007611-198308000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Tuck SM, Studd JW, White JM. Pregnancy in women with sickle cell trait. Br J Obstet Gynaecol. 1983;90:108–111. doi: 10.1111/j.1471-0528.1983.tb08892.x. [DOI] [PubMed] [Google Scholar]
- 51.Wirthwein DP, Spotswood SD, Barnard JJ, Prahlow JA. Death due to microvascular occlusion in sickle-cell trait following physical exertion. J Forensic Sci. 2001;46:399–401. [PubMed] [Google Scholar]
- 52.Warren KE, Gidvani-Diaz V, Duval-Arnould B. Renal medullary carcinoma in an adolescent with sickle cell trait. Pediatrics. 1999;103:e22. doi: 10.1542/peds.103.2.e22. [DOI] [PubMed] [Google Scholar]
- 53.Tsaras G, Owusu-Ansah A, Boateng FO, Moateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. 2009;122:507–512. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Department of Health and Human Services. National Center for Health Statistics. Vaccines and Immunizations. Centers for Disease Control and Prevention; Aug 6, 2007. [Accessed on August 31, 2009]. 2005 National Immunization Surveys. Available at: http://www.cdc.gov/vaccines/stats-surv/nis/data/tables_2005.htm. [Google Scholar]
- 55.Campbell M, Henthorn JS, Davies SC. Evaluation of cation-exchange HPLC compared with isoelectric focusing for neonatal hemoglobinopathy screening. Clin Chem. 1999;45:969–975. [PubMed] [Google Scholar]