Abstract
The present study aimed to evaluate whether circulating C-reactive protein (CRP) levels are a biomarker of systemic inflammation and a significant predictor of future chronic obstructive pulmonary disease (COPD) outcome. During the study, 116 patients with stable COPD and 35 age- and gender-matched healthy subjects with normal pulmonary function were observed. Patient follow-up was also performed to evaluate the strength of the associations between CRP levels and future outcomes. The observations from the present study showed that serum CRP levels were significantly higher in stable COPD patients than in control subjects (4.48±0.83 vs. 1.01±0.27 mg/l, respectively; P<0.05). In addition, it was identified that a serum CRP concentration of >3 mg/l is a poor prognostic variable of COPD compared with a CRP concentration of ≤3 mg/l [hazard ratio (HR), 2.71; 95% confidence interval (CI), 1.05–6.99; P<0.05]. A quantitative synthesis of four studies including 1,750 COPD patients was performed and statistically similar results were obtained (HR, 1.54; 95% CI, 1.14–2.07; P<0.01). The present study showed that circulating CRP levels are higher in stable COPD patients and, therefore, may be used as a long-term predictor of future outcomes. These observations highlight the importance of high sensitivity CRP assays in patients with stable COPD.
Keywords: C-reactive protein, chronic obstructive pulmonary disease, survival
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality and is predicted to be the third leading cause of mortality worldwide by the year 2020 (1). The most significant risk factor for COPD is cigarette smoking, and for a long time COPD was considered as a consequence of local damage to small airways. However, epidemiological studies have shown that the levels of a serum marker of inflammation, C-reactive protein (CRP), are higher in patients with stable COPD than in healthy controls (2,3). In addition, CRP has been reported as a strong and independent predictor of future outcomes in individuals with COPD (4). Although the hallmark feature of COPD is airflow obstruction, it is poorly predicted by forced expiratory volume in the first second (FEV1) only (5,6). As CRP assays are inexpensive and convenient, CRP levels may be one of the most valuable predictors of outcomes in stable COPD patients. However, currently, CRP levels are usually only determined if an exacerbation of COPD is suspected. The patients included in other specific studies were in the acute exacerbations phase of COPD (7–9). In addition, the association between CRP and mortality in COPD patients remains conflicting rather than conclusive (10–13). It was on this background that the present study was conducted, which aimed to examine whether CRP levels in patients with stable COPD are a significant predictor of prognosis following adjustment for specific prognostic factors.
Materials and methods
Patients
The prospective cohort study included a total of 116 patients that had been diagnosed with COPD ≥6 months previously and had been under treatment for ≥6 months. A diagnosis of COPD was based on medical history, current symptoms and available pulmonary function tests following Global Initiative for Chronic Obstructive Lung Disease guidelines (14). To exclude patients with asthma, subjects with a history of allergic rhinitis or an improvement in FEV1 of >12% from the predicted values following inhalation of a bronchodilator, were not included. Patients with evidence of extensive pulmonary tuberculosis, malignancy or who were suffering from psychosis were excluded from the study. All patients with COPD were clinically stable and none had a history of respiratory infection for at least a 4-week period preceding the study. Approval for this study was obtained from the Institutional Review Board for Human Studies of Affiliated Hospital, School of Medicine, Ningbo University (Ningbo, China) and informed consent was obtained from all participating subjects.
CRP measurement
Fasting blood samples were obtained from the patients whilst at rest, prior to any other test being performed. Serum CRP levels were measured by high sensitivity immunoturbidimetry (Beckman Coulter, Inc., Miami, FL, USA). The results were given in units of mg/l and the analytical sensitivity of this analysis was 0.1 mg/l. The cutoff point for the CRP concentration was 3 mg/l, as indicated in previous studies (10,11,15).
Follow-up
The study was conducted between August 2009 and April 2012, with a follow-up of 32 months or until patient mortality. The follow-up was carried out by telephoning the patients or their next of kin and/or checking hospital records. Critical events were recorded by the physicians in charge of the follow-ups. Subjects who were not located at follow-up and were not known to have succumbed to their illness were considered as censored at the end of the study period.
Statistical Analysis
The continuous variables are presented as mean ± SD and the categorical variables are presented as absolute numbers and percentages. Cox regression analysis was used to examine time to COPD mortality using hazard ratios (HR) and 95% confidence intervals (CIs). Risk measures were adjusted for age, gender, FEV1%pred, smoking and presence of disease. Kaplan-Meier mortality curves were created to exhibit differences in mortality by selected risk factors. Quantitative synthesis of all relevant studies was performed and the methods used have been described in detail in previous studies (16,17). The HRs of time-to-event data were directly extracted from the original study or were read off survival curves to estimate the logHR and its variance, as suggested by Parmar et al(18). The statistical analyses were performed using SPSS, version 13.0 (SPSS, Inc., Chicago, IL, USA) and Review Manager 5.0.17 (Cochrane Library Software, Oxford, UK). Two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
A total of 116 consecutive COPD patients (including 75 males) were recruited into the study, as well as 35 healthy subjects (including 18 males) aged over 50 years, with no evidence of COPD. The healthy subjects were randomly selected from a population sample of subjects living in the same area as the patients.
The characteristics of the COPD patients are summarized in Table I. The majority of patients were elderly with a mean age of 71 years (range, 47–91 years) and over half were male (65%). A number of the patients had comorbid illnesses, including hypertension, diabetes mellitus, chronic gastritis, cataract and coronary heart disease. The healthy controls were matched to the COPD patients with respect to age, gender, body mass index (BMI) and smoking status. No significant differences were observed in these parameters between the healthy controls and COPD patients. However, serum CRP levels were significantly higher in the stable COPD patients than in the control subjects (4.48±0.83 vs. 1.01±0.27 mg/l, respectively; P<0.05).
Table I.
Baseline characteristics of the study participants.
| Characteristics | COPD patients n=116 | Healthy controls n=35 | P-value |
|---|---|---|---|
| Gender | 0.16 | ||
| Male | 75 | 18 | |
| Female | 41 | 17 | |
| Age, years | 71.0±9.0 | 68.9±5.1 | 0.09 |
| BMI, kg/m2 | 24.6±3.2 | 25.8±5.7 | 0.12 |
| Smoking status | 0.13 | ||
| Current smoker | 46 | 14 | |
| Ex-smoker | 34 | 5 | |
| Never | 36 | 16 | |
| FEV1, % predicted | 44.7±12.1 | 90.4±6.8 | <0.001 |
| Inhaled steroid, % | 61.2 | ||
| β2-agonist, % | 61.2 | ||
| Inhaled ipratropium, % | 53.4 | ||
| Theophylline, % | 36.2 | ||
| Comorbid illnesses, n | |||
| 0 | 36 | ||
| 1 | 52 | ||
| 2 | 15 | ||
| 3 | 10 | ||
| 4 | 3 | ||
| CRP, mg/l | 4.48±0.83 | 1.01±0.27 | 0.025 |
Data are presented as mean ± SD, n or %, unless otherwise indicated. COPD, chronic obstructive pulmonary disease; BMI, body mass index; FEV1, forced expiratory volume in 1 sec; CRP, C-reactive protein.
At the end of follow-up, 21 patients had succumbed (18%). However, information concerning the cause of mortality of four patients was not available since contact details had been changed or through lack of cooperation from the patients’ families. When CRP ≤3 mg/l was used as the reference category, values >3 mg/l were associated with increased mortality (HR, 2.71; 95% CI, 1.05–6.99; P<0.05). Kaplan-Meier survival curves for all-cause mortality, according to CRP categories, are shown in Fig. 1. The clinical parameters between survivors and nonsurvivors were also compared. Compared with survivors, non-survivors had a high degree of airflow obstruction (FEV1% pred, 40.5±17.9 vs. 54.6±18.0, respectively; P<0.05) and CRP concentration (7.56±5.18 vs. 3.48±6.55 mg/l, respectively; P<0.05). However, no significant difference in age, gender and BMI was observed between the two groups.
Figure 1.
Survival curves for mortality in patients with chronic obstructive pulmonary disease according to a baseline serum CRP level of >3 mg/l or ≤3 mg/l. CRP, C-reactive protein.
In addition, a quantitative synthesis of four studies, comprising the current study and three published studies, was performed (10,11,15). Among these studies, the median duration of the follow-ups ranged between 3 and 10 years. Risk measures were frequently adjusted for age, gender, FEV1%pred and smoking. As shown in Fig. 2, the HR of mortality in patients with a CRP >3 mg/l was 1.54 (95% CI, 1.14–2.07) compared with those with CRP ≤3 mg/l.
Figure 2.
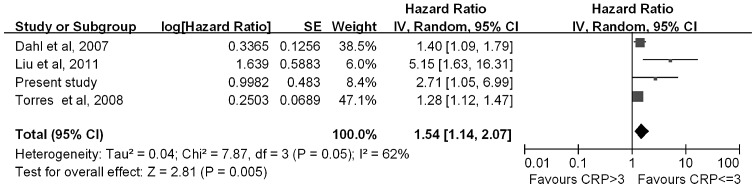
Hazard ratio of mortality with C-reactive protein level (>3 mg/l or ≤3 mg/l.) among patients with chronic obstructive pulmonary disease.
Discussion
The present study was performed to evaluate whether circulating CRP levels are a biomarker of systemic inflammation and a significant predictor of future COPD outcomes. In this study, serum CRP levels were found to be significantly higher in stable COPD patients than in well-matched healthy control subjects. The results obtained are consistent with previous studies, indicating the presence of systemic inflammation in patients with stable COPD (2,3).
A number of independent predictors of future COPD outcomes have been identified previously, including exercise capacity (19), biomarkers of systemic inflammation (20), BMI (21,22), smoking status (23), severity of dyspnea (24), FEV1(5) and PaO2(23). Among these, the best studied and most convenient to evaluate is serum CRP levels.
The present study showed that increased serum CRP levels are a strong predictor of COPD mortality. Liu et al determined that a serum CRP concentration of >3 mg/l was a poorer prognostic variable of COPD compared with a CRP concentration ≤3 mg/l (11). In the study by Dahl et al, the HR of mortality due to COPD was 2.2-fold higher in patients with a high CRP level than in those with a low CRP level (15). The observations in the present study are in agreement with these studies. However, de Torres et al reported that CRP levels are not associated with survival status (10).
Considering the inconsistent results between previous studies, a quantitative synthesis of the evidence, using rigorous methods, was performed. Meta-analysis was conducted on four studies with 1,750 subjects to evaluate the association between serum CRP levels and mortality in patients with COPD. This meta-analysis indicated that a high level of serum CRP is associated with an increased risk of mortality in COPD patients.
At present, CRP levels are only determined if an exacerbation of COPD is suspected. The results of the present study have several implications, showing that stable COPD patients had a higher level of CRP than healthy controls, indicating the presence of systemic inflammation in COPD. As high sensitivity CRP assays are inexpensive and convenient, it is important for clinicians to use CRP values in stable COPD patients. The study also found that a high level of serum CRP is associated with an increased risk of mortality in COPD patients. These results indicate that selection of serum CRP concentration as a prognostic biomarker in stable COPD patients may be useful for physicians.
However, there were limitations in this study that should be acknowledged. Firstly, although the patients were clinically stable, serum CRP concentration may fluctuate slightly over time, which may affect the validity of CRP levels as a predictor marker. Secondly, various drugs and treatments among patients appear to have an unpredictable effect on serum CRP concentration.
In conclusion, the present study confirms that circulating CRP levels are higher in stable COPD patients than in healthy individuals and are a significant long-term predictor of future COPD outcomes in individuals with airway obstruction. These observations highlight the significance of high sensitivity CRP assays in patients with stable COPD.
Acknowledgements
This study was partly supported by grants from the Social Development Science and Technology Project (no. 2011C50025), Natural Science Foundation (no. 2012A610257) and the Social Development Science and Technology Project of Ningbo (no. 2011C50073).
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Karadag F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med. 2008;19:104–108. doi: 10.1016/j.ejim.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 3.He Z, Chen Y, Chen P, Wu G, Cai S. Local inflammation occurs before systemic inflammation in patients with COPD. Respirology. 2010;15:478–484. doi: 10.1111/j.1440-1843.2010.01709.x. [DOI] [PubMed] [Google Scholar]
- 4.Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with chronic obstructive pulmonary disease: a prospective cohort study. Chest. 2011;140:331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 6.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 7.Stolz D, Christ-Crain M, Morgenthaler NG, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131:1058–1067. doi: 10.1378/chest.06-2336. [DOI] [PubMed] [Google Scholar]
- 8.Murphy SA, Haja Mydin H, Fatah S, Antunes G. Predicting end-of-life in patients with an exacerbation of COPD by routine clinical assessment. Respir Med. 2010;104:1668–1674. doi: 10.1016/j.rmed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Lacoma A, Prat C, Andreo F, et al. Value of procalcitonin, C-reactive protein, and neopterin in exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:157–169. doi: 10.2147/COPD.S16070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Torres JP, Pinto-Plata V, Casanova C, et al. C-reactive protein levels and survival in patients with moderate to very severe COPD. Chest. 2008;133:1336–1343. doi: 10.1378/chest.07-2433. [DOI] [PubMed] [Google Scholar]
- 11.Liu SF, Wang CC, Chin CH, Chen YC, Lin MC. High value of combined serum C-reactive protein and BODE score for mortality prediction in patients with stable COPD. Arch Bronconeumol. 2011;47:427–432. doi: 10.1016/j.arbres.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Miniati M, Monti S, Bottai M, Cocci F, Fornai E, Lubrano V. Prognostic value of C-reactive protein in chronic obstructive pulmonary disease. Intern Emerg Med. 2011;6:423–430. doi: 10.1007/s11739-011-0520-z. [DOI] [PubMed] [Google Scholar]
- 13.Man SF, Xing L, Connett JE, et al. Circulating fibronectin to C-reactive protein ratio and mortality: a biomarker in COPD? Eur Respir J. 2008;32:1451–1457. doi: 10.1183/09031936.00153207. [DOI] [PubMed] [Google Scholar]
- 14.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 15.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–255. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 16.Cao C, Fang JJ, Ying T, et al. Vascular endothelial growth factor +936C/T and +405G/C polymorphisms and cancer risk: a meta-analysis. Arch Med Res. 2010;41:548–557. doi: 10.1016/j.arcmed.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Cao C, Ying T, Fang JJ, et al. Polymorphism of vascular endothelial growth factor -2578C/A with cancer risk: evidence from 11263 subjects. Med Oncol. 2011;28:1169–1175. doi: 10.1007/s12032-010-9613-1. [DOI] [PubMed] [Google Scholar]
- 18.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20:539–544. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 20.Schembri S, Anderson W, Morant S, et al. A predictive model of hospitalization and death from chronic obstructive pulmonary disease. Respir Med. 2009;103:1461–1467. doi: 10.1016/j.rmed.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med. 2004;170:748–752. doi: 10.1164/rccm.200405-651OC. [DOI] [PubMed] [Google Scholar]
- 22.Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One. 2012;7:e43892. doi: 10.1371/journal.pone.0043892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nizet TA, van den Elshout FJ, Heijdra YF, van de Ven MJ, Mulder PG, Folgering HT. Survival of chronic hypercapnic COPD patients is predicted by smoking habits, comorbidity, and hypoxemia. Chest. 2005;127:1904–1910. doi: 10.1378/chest.127.6.1904. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]



