Abstract
Radiotherapy can convert malignant cells into an in situ anticancer vaccine, but is often inadequate at generating sufficient pro-inflammatory signals to optimally activate innate and adaptive immune responses. Topical imiquimod is a powerful pro-inflammatory agent with clinical activity against superficial skin cancers. These two modalities appear to complement each other, hence achieving local and systemic tumor control.
Keywords: abscopal effect, breast cancer, cutaneous metastases, cyclophosphamide, immunological adjuvant, radiotherapy, synergy, T cells, Toll-like receptor 7, tumor rejection
Advanced breast carcinoma can disseminate and generate metastases in multiple organs. While cutaneous metastases are not themselves responsible for the death of breast carcinoma patients, they can profoundly affect their quality of life and remain a therapeutic challenge. Because of their accessibility, cutaneous metastasis provide a unique opportunity to straightforwardly monitor the activity of local antineoplastic agents.1
A local treatment commonly employed against cutaneous metastases of breast carcinoma is radiotherapy. The widespread use of ionizing radiation in oncology has been driven by its ability to damage the DNA causing death of rapidly proliferating malignant cells. However, accumulating evidence indicates that radiotherapy exerts multiple immunostimulatory effects, supporting a paradigm change according to which the main goal of radiotherapy would be to convert neoplastic cells into an in situ anticancer vaccine.2 By inducing immunogenic cell death, radiation stimulates dendritic cells (DCs) to cross-present tumor-associated antigens to tumor-specific CD8+ T lymphocytes, hence priming an adaptive immune response.3 These T cells, which are recruited to irradiated tumors by chemokines,4 can easily recognize and reject residual cancer cells (i.e., those that did not die in response to radiotherapy) as they express increased levels of MHC class I molecules, intercellular adhesion molecule 1 (ICAM1) and stress-inducible ligands for killer cell lectin-like receptor subfamily K, member 1 (KLRK1, best known as NKG2D).5,6 The precise degree to which radiation-elicited tumor-specific T cells contribute to disease control by radiotherapy in cancer patients remains to be determined. However, like most other anticancer vaccines, radiotherapy is rarely sufficient by itself to elicit an antitumor immune response strong enough to achieve systemic tumor control.
The most effective way to improve the immune response induced by vaccination is to identify an effective immune adjuvant. Toll-like receptors (TLRs) are danger sensors that activate innate immunity and initiate adaptive immune responses upon stimulation by a variety of pathogen-derived and endogenous ligands.7 Therefore, synthetic TLR agonists are under intensive investigation as immune adjuvants. The TLR7 agonist imiquimod is commercially available for topical use in patients affected by a bunch of skin neoplasms. Indeed, imiquimod provides a convenient modality to treat the often extensive surface of skin involved by the recurrences of breast carcinoma that involve the chest wall. We have previously shown that topical imiquimod enhances immune response to concomitantly administered tumor-associated antigens.8 More recently, we have reported that imiquimod, employed as a standalone therapeutic intervention, mediates antineoplastic effects as it alters the immunological profile of the tumor microenvironment, inducing a partial response in 20% of patients bearing cutaneous metastases of breast carcinoma.1
We have recently demonstrated that the TLR7 agonist imiquimod mediates adjuvant activity when combined with local radiotherapy in a mouse model of cutaneous breast carcinoma.9 Similarly to what observed in clinical settings, topical imiquimod applied to TSA mouse mammary carcinomas had a partial antineoplastic effect, reducing to some extent tumor growth as compared with placebo. Imiquimod-treated murine tumors exhibited increased infiltration by T cells, similar to the neoplastic lesions of patients responding to this immunotherapeutic, as well as by dendritic cells. When imiquimod was applied topically to neoplastic lesions subjected to local radiotherapy, complete tumor regression was observed in a majority of animals. Conversely, radiotherapy alone delayed tumor growth but did not induce complete disease regression. Tumor-specific effector CD8+ T cells that produced interferon γ in response to antigenic stimulation were detected in the tumor-draining lymph nodes of animals subjected to this combinatorial immunotherapeutic regimen. Thus, imiquimod and local radiotherapy exerted synergistic antineoplastic effects, hence mediating local disease control.
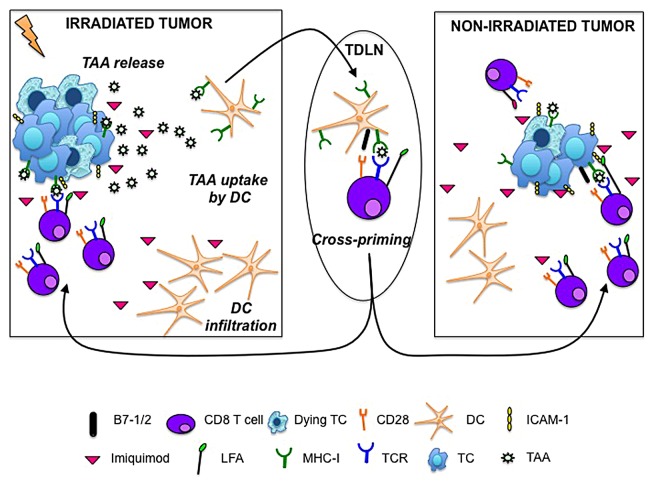
Skin metastases, which frequently manifest after mastectomy and typically present as multiple nodules along the mastectomy scar, can progress to diffusely infiltrating lesions across the chest, back, abdomen and ipsilateral arm. To determine if the delivery of irradiation and imiquimod to one neoplastic lesion, the “vaccination site,” could induce antitumor responses that would also be effective against non-irradiated tumors (abscopal effect), mice were injected with TSA cells at two separate sites and—once tumors became palpable—only one of them was treated with imiquimod plus radiotherapy. Indeed, we observed an abscopal effect that was markedly enhanced by the topical application of imiquimod to the non-irradiated tumor. Imiquimod improved the expression of MHC class I molecules and ICAM1 on the surface of tumor cells, suggesting that some of the effects of radiotherapy that promote the effector phase of anticancer immunity can also be elicited by imiquimod, and are actually required for the efficient rejection of non-irradiated tumors (Fig. 1).
Figure 1. Ionizing radiation and the Toll-like receptor 7 agonist imiquimod cooperate in converting malignant cells into an in situ anticancer vaccine. Radiation-induced immunogenic cell death allow for the release of tumor-associated antigens that are taken up by dendritic cells (DCs), which abundantly infiltrate imiquimod-treated tumors. Activated DCs loaded with tumor-associated antigens migrate to tumor-draining lymph nodes (TDLNs), where they activate naïve tumor-specific T cells. Activated tumor-specific T cells traffic to both irradiated and non-irradiated tumors. The cytotoxic activity of T cells is facilitated by the imiquimod- or radiation-induced upregulation of MHC class I molecules and intercellular adhesion molecule 1 (ICAM1) on the surface of transformed cells (TCs).
We are currently testing the translational relevance of these findings in the context of a Phase I/II clinical trial evaluating concurrent radiation and imiquimod in patients with skin metastases of breast carcinoma (NCT01421017; clinicaltrials.gov). In this study, patients apply imiquimod to skin metastasis but only a limited area of the metastatic lesion is irradiated. The feasibility of this combinatorial regimen is being assessed, along with tumor responses within and outside the field of radiation, including distant non-irradiated sites. Moreover, this study aims at identifying immunologic correlates of response not only by analyzing samples from irradiated and non-irradiated, imiquimod-treated skin metastases, but also by characterizing circulating lymphocytes and cytokines.
Of note, in mouse tumor models the powerful pro-inflammatory activity of imiquimod can activate a feedback immunosuppressive loop based on interleukin-10 (IL-10) that significantly reduces the persistence of antitumor T-cell responses.9,10 The use of monoclonal antibodies that block IL-1010 or the administration of a single immunomodulatory dose of cyclophosphamide9 was shown to limit IL-10 levels, promoting sustained anticancer immune responses. The precise role of IL-10 in imiquimod-treated patients remains to be determined, although no consistent increase in the circulating levels of this cytokine has been observed in a previous trial.1 Ongoing clinical studies will provide critical insights into the therapeutic benefits of imiquimod combined with radiotherapy and, possibly, identify additional targets for optimizing this combinatorial regimen. Most importantly, by monitoring the response of distant metastases, we will assess the potential of this immunotherapeutic strategy to induce systemic antitumor immune responses that that not only achieve local disease control but also increase patient survival.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- DC
dendritic cell
- ICAM1
intercellular adhesion molecule 1
- IL-10
interleukin-10
- TLR
Toll-like receptor
Citation: Demaria S, Vanpouille-Box C, Formenti S, Adams S. The TLR7 agonist imiquimod as an adjuvant for radiotherapy-elicited in situ vaccination against breast cancer. OncoImmunology 2013; 2:e25997; 10.4161/onci.25997
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25997
References
- 1.Adams S, Kozhaya L, Martiniuk F, Meng TC, Chiriboga L, Liebes L, Hochman T, Shuman N, Axelrod D, Speyer J, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748–57. doi: 10.1158/1078-0432.CCR-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–30. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1:949–64. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams S, O’Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, Cruz CM, Angiulli A, Angiulli F, Ritter E, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewan MZ, Vanpouille-Box C, Kawashima N, DiNapoli S, Babb JS, Formenti SC, Adams S, Demaria S. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res. 2012;18:6668–78. doi: 10.1158/1078-0432.CCR-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H, Wagner WM, Gad E, Yang Y, Duan H, Amon LM, Van Denend N, Larson ER, Chang A, Tufvesson H, et al. Treatment failure of a TLR-7 agonist occurs due to self-regulation of acute inflammation and can be overcome by IL-10 blockade. J Immunol. 2010;184:5360–7. doi: 10.4049/jimmunol.0902997. [DOI] [PubMed] [Google Scholar]