Abstract
Surgical resection is the mainstay of treatment for solid tumors, but the postoperative period is uniquely inclined to the formation of metastases, largely due to the suppression of natural killer (NK) cells. We found that preoperative influenza vaccination prevents postoperative NK-cell dysfunction, attenuating tumor dissemination in murine models and promoting the activation of NK cells in cancer patients.
Keywords: cancer metastases, influenza vaccine, immunosuppression, natural killer cells, surgical stress
Surgical resection is often required for the treatment of localized solid malignancies. However, even upon complete resection, many patients harbor microscopic residual disease and ultimately die of recurrence.1 Surgeons have long suspected that the physiological response to surgery facilitates cancer growth and metastatic dissemination, and numerous studies have established that the postoperative period provides an ideal setting for the formation of metastases.2-5 Nevertheless, the perioperative period may provide a therapeutic window that has largely been ignored, and to date there are no cancer therapies specifically targeting this period.4,6
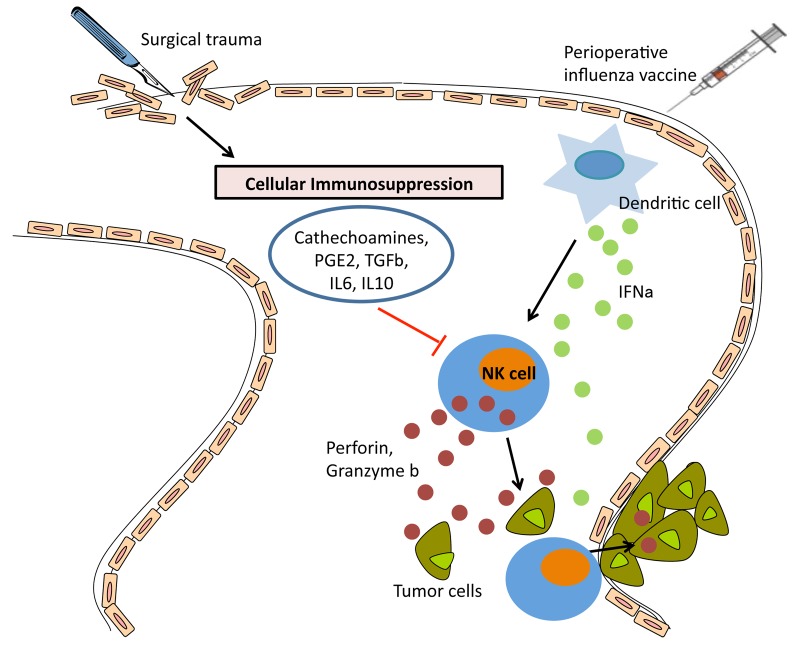
Natural killer (NK) cells play a critical role in tumor clearance in vivo, but their functions are markedly impaired upon surgery.6 Postoperative NK-cell suppression correlates with increased metastatic burden in animal models, while in cancer patients reduced NK-cell activity during the postoperative period has been associated with a high rate of disease recurrence and mortality.4 Several mechanisms are thought to be responsible for the postoperative dysfunction of NK cells, including the secretion of catecholamines, prostaglandins, and immunosuppressive cytokines such as transforming growth factor β (TGFβ), interleukin (IL)-6, and IL-10, but mechanistic details on this process are lacking6-8 (Fig. 1). We have previously demonstrated that surgery causes a global dysfunction in NK cells.4 Based on these findings, we hypothesized that non-specific stimulation of the immune system, such as that obtained with an inactivated prophylactic vaccine against an infectious pathogen, could prevent postoperative NK-cell dysfunction and attenuate the metastatic dissemination of malignant cells if administered before surgery.
Figure 1. Perioperative influenza vaccine activates NK cells and protects against postoperative metastases. Surgical trauma results in a variety of physiologic changes in the host, including profound immunosuppression. This state is characterized by the secretion of catecholamines, prostaglandins (PGs), transforming growth factor β (TGFβ), interleukin (IL)-6, and IL-10, resulting in natural killer (NK) cell dysfunction following surgery. Dysfunctional NK cells are unable to clear malignant cells and micrometastases that are disseminated or become established in the postoperative period. The preoperative administration of an influenza vaccine results in increased circulating levels of interferon α (IFNα), most likely secreted by dendritic cells, which activates cytotoxicity and cytokine secretion by NK cells. Thus, preoperative influenza vaccination prevents surgery-induced NK-cell dysfunction, hence stimulating NK cells to attack cancer cells and micrometastases in the postoperative period.
And the Winner is…Influenza Vaccine
To explore this hypothesis, we assessed a panel of routinely used prophylactic vaccines, including preparations against influenza, meningitis, measles/mumps/rubella, diphtheria/tetanus/pertussis/polio, pneumonia, and influenza for their ability to activate NK cells, (measured by CD69 expression) and enhance their function (measured by cytotoxicity assay and interferon IFNγ secretion). The influenza vaccine turned out to be the most potent activator of NK cells among the prophylactic vaccines tested, although, not unexpectedly, inoculating mice with live replicating viruses (such as vaccinia virus) induced even higher levels of NK-cell cytotoxicity. Using murine models of experimental (B16 melanoma), or spontaneous (4T1 breast carcinoma) metastasis, and surgical stress (laparotomy and nephrectomy), we demonstrated that the preoperative delivery of a single dose of influenza vaccine resulted in a dramatic reduction in the metastatic dissemination of cancer cells to the lungs.9
Influenza Vaccine Prevents Postoperative Metastases by Enhancing NK-cell Function Through IFNα
In order to confirm that NK cells play a critical role in preventing postoperative metastases upon the preoperative administration of an influenza vaccine, we pharmacologically depleted NK cells and observed a complete abrogation of the therapeutic effect of influenza vaccination. By evaluating a panel of cytokines that are known to directly or indirectly activate NK cells, we observed that IFNα levels underwent the most dramatic increase upon vaccination. We also observed that low dose preoperative IFNα was able to rescue surgery-induced NK-cell dysfunction and control postoperative metastatic dissemination to the same degree as influenza vaccination. The central role for IFNα was further confirmed by that fact the influenza vaccination was not able to increase postoperative NK-cell activity or attenuate postoperative metastases in IFNα receptor-deficient mice. Moreover, a Type I IFN-blocking antibody prevented the influenza vaccine from activating NK cells among peripheral blood mononuclear cells (PBMCs) isolated from healthy people.9 While our study did not explore the role of dendritic cells in the production of IFNα upon influenza vaccination, it is very likely that these cells represent the primary source of IFNα, resulting in secondary NK-cell stimulation (Fig. 1).
Timing is Everything
We hypothesized that, in order to exhibit maximal efficacy against postoperative metastases, the influenza vaccine had to be delivered so that the stimulation of NK cells would be maximal during the immediate postoperative period, when NK-cell suppression is most pronounced. This was indeed the case. NK-cell activation by preoperative influenza vaccination was maximal 1 d after administration, decreasing to baseline levels over 3–5 d. When the influenza vaccine was administered 5 d prior to surgery, we observed a significant reduction in its ability to prevent postoperative metastases. Moreover, despite the fact that repeated dosing of the influenza vaccine could re-activate NK cells to the same degree as the initial dose, adding multiple postoperative courses of vaccination did not further reduce the number of metastases. Data from cancer patients who underwent surgery also confirm that the timing of vaccination is critical for its antineoplastic effects. In 4/4 patients, NK cells isolated prior to surgical resection exhibited increased activity upon exposure to the influenza vaccine ex vivo. Conversely, only in 1/4 patients a similar activation could be documented on NK cells that were isolated 1 d after surgery. This suggests that the surgery-induced dysfunction of NK cells can be prevented but not reversed by influenza vaccines. In individuals receiving a flu shot as part of a vaccination campaign, NK-cell activation peaked 1–2 d after immunization. Taken together, these results suggest that influenza vaccination should be delivered one day before surgery, allowing sufficient time for the optimal activation of NK cells prior to surgical stress.9
Influenza: Dirty but Safe
There is an argument to be made for administering IFNα prior to cancer surgery, since our results strongly indicate that it is responsible for the beneficial effects of preoperative influenza vaccination. IFNα might generate well-defined conditions to achieve NK-cell stimulation and overcome the individual variability in the responses to influenza vaccination. This strategy has previously been explored in a handful of clinical studies, demonstrating increased NK-cell activity with acceptable toxicity, but these trials were not powered to explore clinical outcomes.10 This said, the safety profile of influenza vaccination is unparalleled. This prophylactic intervention has been used in national vaccination campaigns, is widely available, cost effective and acceptable to patients. After all, it’s only a flu shot.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- IFN
interferon α
- IFNγ
interferon γ
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
Citation: Tai L, Zhang J, Auer RC. Preventing surgery-induced NK cell dysfunction and cancer metastases with influenza vaccination. OncoImmunology 2013; 2:e26618; 10.4161/onci.26618
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26618
References
- 1.Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–8. doi: 10.1016/S1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 2.Colacchio TA, Yeager MP, Hildebrandt LW. Perioperative immunomodulation in cancer surgery. Am J Surg. 1994;167:174–9. doi: 10.1016/0002-9610(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 3.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253:798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 4.Tai LH, de Souza CT, Bélanger S, Ly L, Alkayyal AA, Zhang J, Rintoul JL, Ananth AA, Lam T, Breitbach CJ, et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013;73:97–107. doi: 10.1158/0008-5472.CAN-12-1993. [DOI] [PubMed] [Google Scholar]
- 5.Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–84. doi: 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 6.Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10:972–92. doi: 10.1245/ASO.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–8. doi: 10.1002/(SICI)1097-0215(19990315)80:6<880::AID-IJC14>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Hensler T, Hecker H, Heeg K, Heidecke CD, Bartels H, Barthlen W, Wagner H, Siewert JR, Holzmann B. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65:2283–91. doi: 10.1128/iai.65.6.2283-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai LH, Zhang J, Scott KJ, de Souza CT, Alkayyal AA, Ananth AA, Sahi S, Adair RA, Mahmoud AB, Sad S, et al. Perioperative Influenza Vaccination Reduces Postoperative Metastatic Disease by Reversing Surgery-Induced Dysfunction in Natural Killer Cells. Clin Cancer Res. 2013;19:5104–15. doi: 10.1158/1078-0432.CCR-13-0246. [DOI] [PubMed] [Google Scholar]
- 10.Oosterling SJ, van der Bij GJ, Mels AK, Beelen RH, Meijer S, van Egmond M, van Leeuwen PA. Perioperative IFN-alpha to avoid surgically induced immune suppression in colorectal cancer patients. Histol Histopathol. 2006;21:753–60. doi: 10.14670/HH-21.753. [DOI] [PubMed] [Google Scholar]