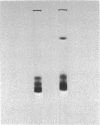
Abstract
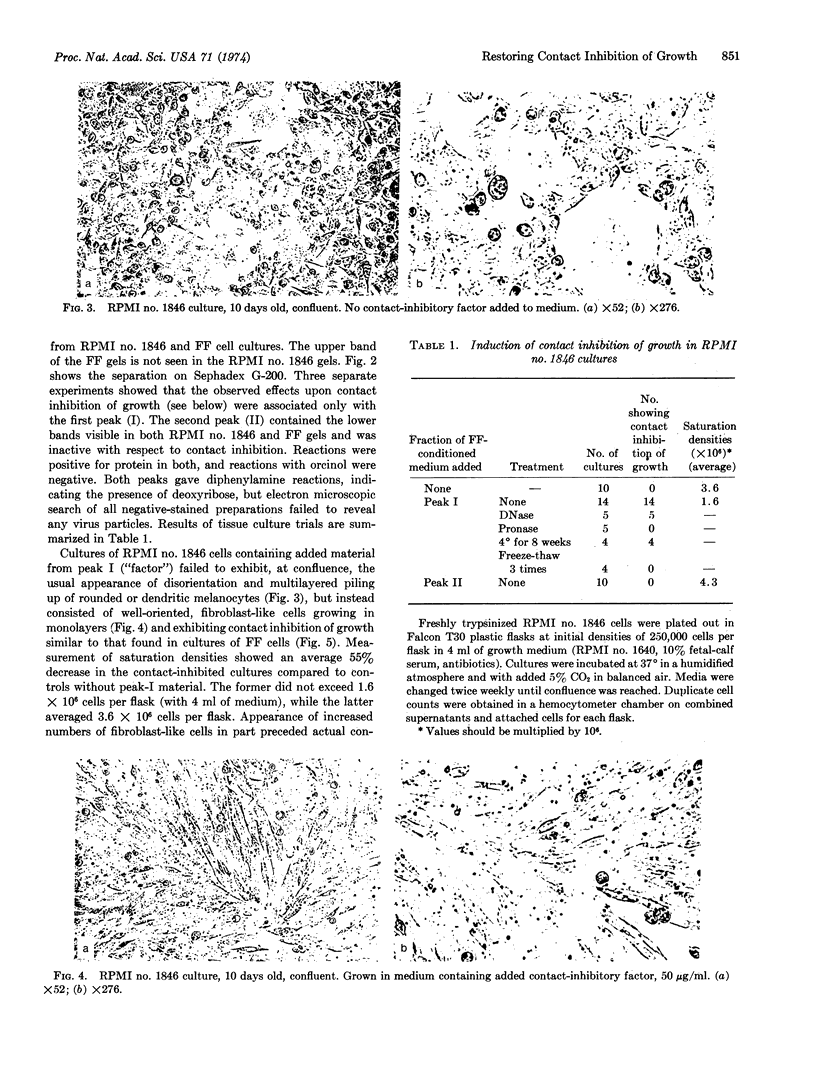
Contact inhibition of growth is the property in vitro whose loss is most closely correlated with tumorigenicity in vivo. A contact-inhibited melanocytic cell line produces a diffusible protein-containing factor capable of restoring contact inhibition of cell division to highly malignant hamster melanocytes. Its addition to subconfluent cultures of the malignant cells is followed by the obligatory acquisition, at confluence, of the contact-inhibited state. Cultures are flat, oriented, and fibroblast-like, and show a 55% decrease in saturation density with no loss of viability. The effect is reversible. Present in conditioned media of cultures of the contact-inhibited melanocytic cell line, isolated by column chromatography on Sephadex G-200, the factor appears to be of high molecular weight. Activity is preserved in aqueous solutions at 4° for at least 8 weeks, but is destroyed by repeated freezethawing or by treatment with Pronase.
This newly recognized contact-inhibitory factor may be a prototype for a more general and fundamental mechanism for regulation of normal cell-cell interactions. It is the first cell-elaborated factor to be isolated that is capable of restoring the capacity for contact inhibition of growth to malignant cells.
Keywords: conditioned medium, orientation, saturation density
Full text
PDF
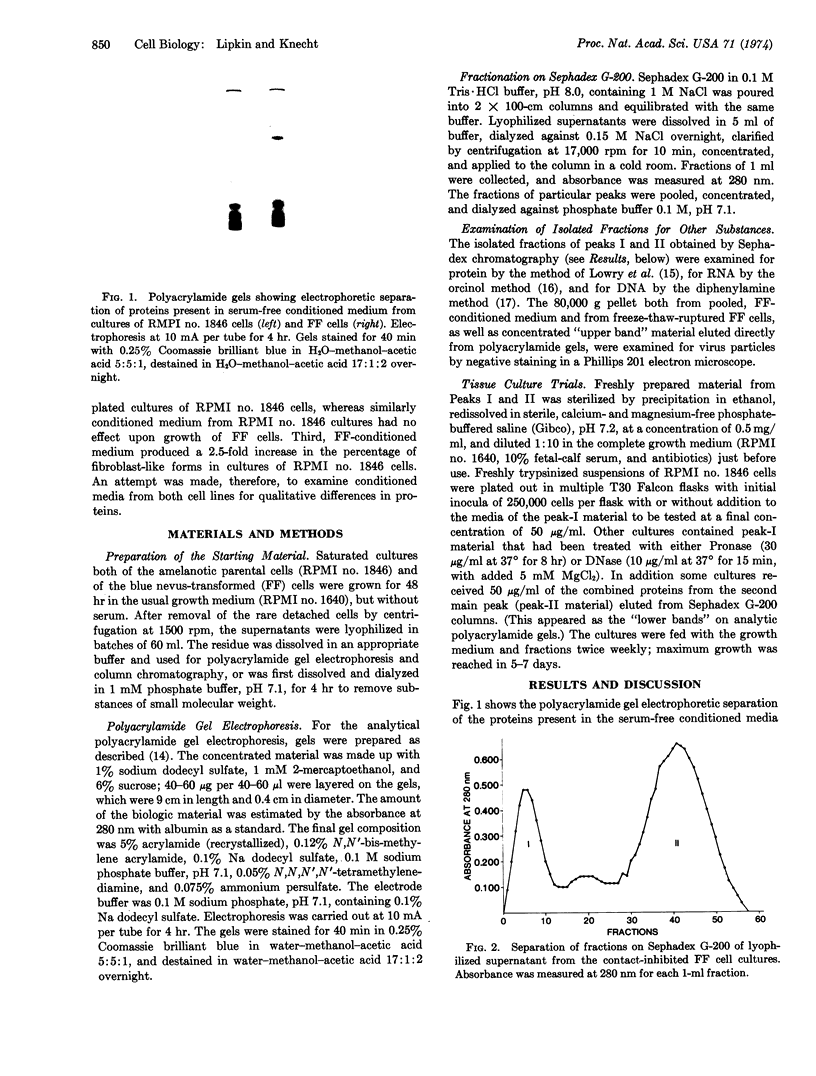


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Baker J. B., Humphreys T. Serum-stimulated release of cell contacts and the initiation of growth in contact-inhibited chick fibroblasts. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2161–2164. doi: 10.1073/pnas.68.9.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G. D., Stoker M. G., Ludlow A., Thornton M. Requirement of serum for DNA synthesis in BHK 21 cells: effects of density, suspension and virus transformation. Nature. 1970 Aug 22;227(5260):798–801. doi: 10.1038/227798a0. [DOI] [PubMed] [Google Scholar]
- Cox R. P., Krauss M. R., Balis M. E., Dancis J. Evidence for transfer of enzyme product as the basis of metabolic cooperation between tissue culture fibroblasts of Lesch-Nyhan disease and normal cells. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1573–1579. doi: 10.1073/pnas.67.3.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Stoker M. G. Conditions determining initiation of DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1970 May;66(1):204–210. doi: 10.1073/pnas.66.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Gurney T., Jr Local stimulation of growth in primary cultures of chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1969 Mar;62(3):906–911. doi: 10.1073/pnas.62.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMBERG B. On the in vitro release of cytoplasmic enzymes from ascites tumor cells as compared with strain L cells. Cancer Res. 1961 Nov;21:1386–1393. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht M. E., Busch H. Isolation of an electrophoretically homogeneous nonhistone nucleolar protein. Life Sci II. 1971 Nov 22;10(22):1297–1309. doi: 10.1016/0024-3205(71)90237-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipkin G. Pigment transformation and induction in hamster malignant amelanotic melanocytes. An effect of nucleic acids from hamster benign blue nevus. J Invest Dermatol. 1971 Jul;57(1):49–65. doi: 10.1111/1523-1747.ep12292072. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M. G., Shearer M., O'Neill C. Growth inhibition of polyoma-transformed cells by contact with static normal fibroblasts. J Cell Sci. 1966 Sep;1(3):297–310. doi: 10.1242/jcs.1.3.297. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Yeh J., Fisher H. W. A diffusible factor which sustains contact inhibition of replication. J Cell Biol. 1969 Feb;40(2):382–388. doi: 10.1083/jcb.40.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]