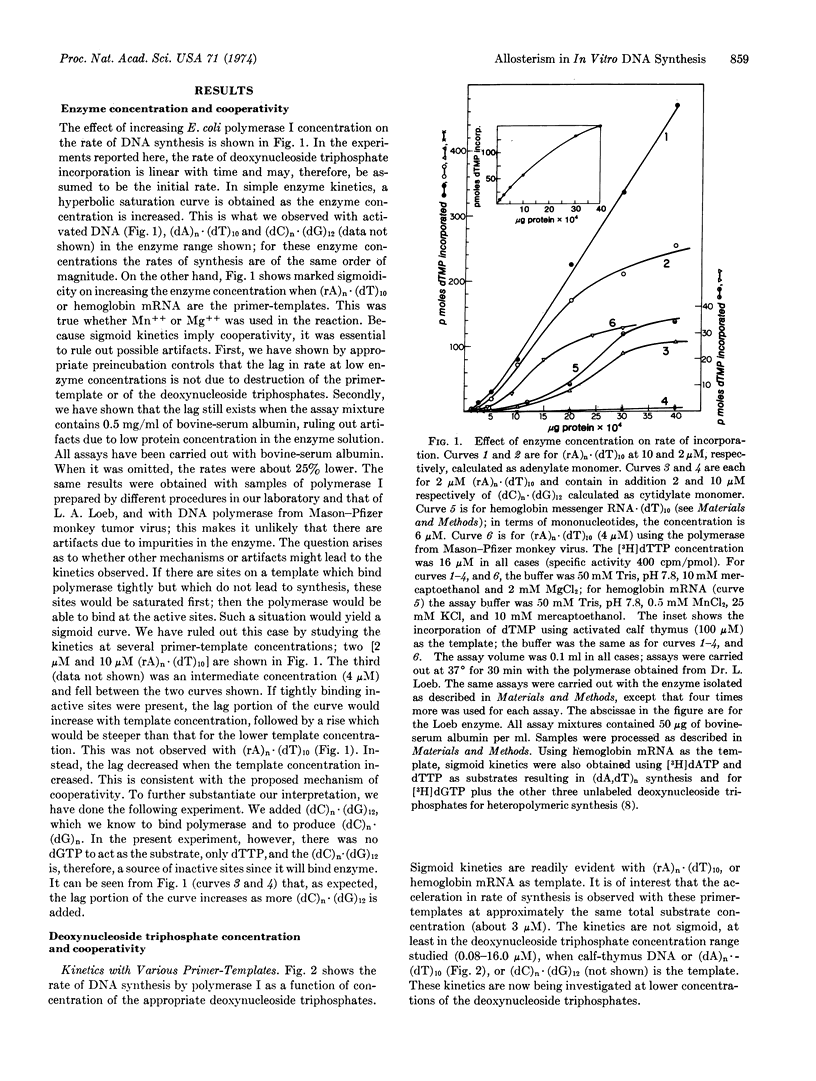
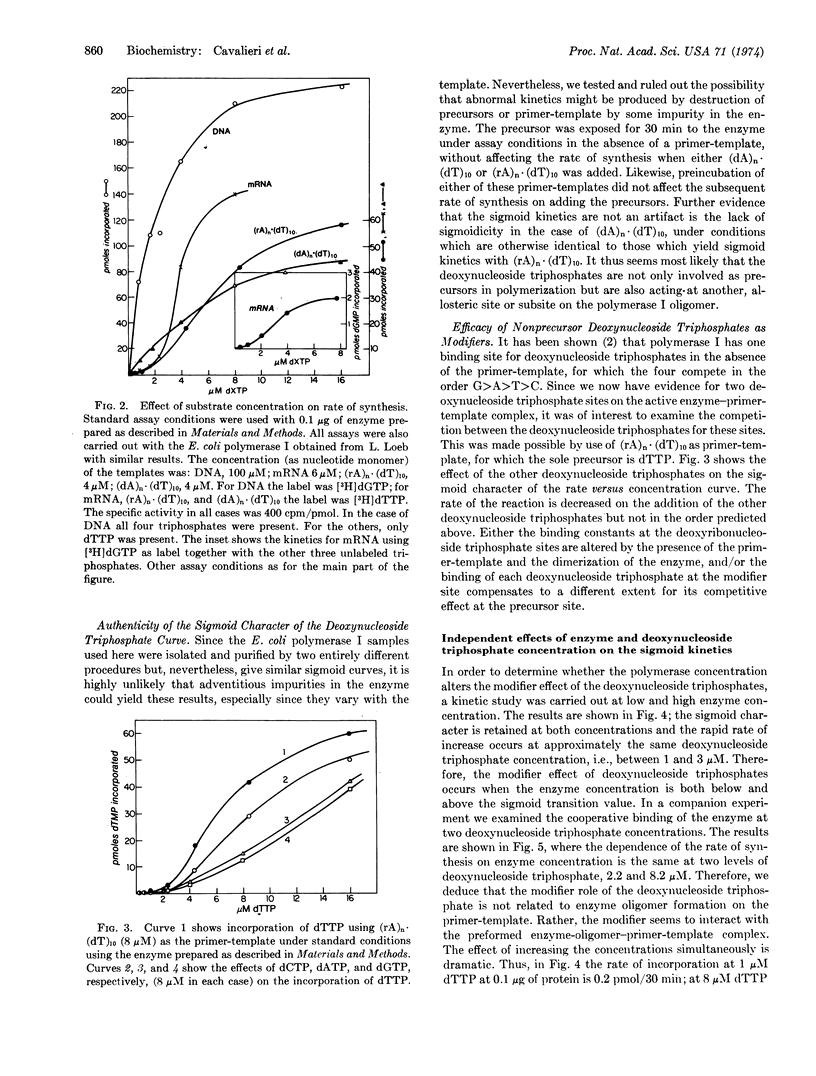
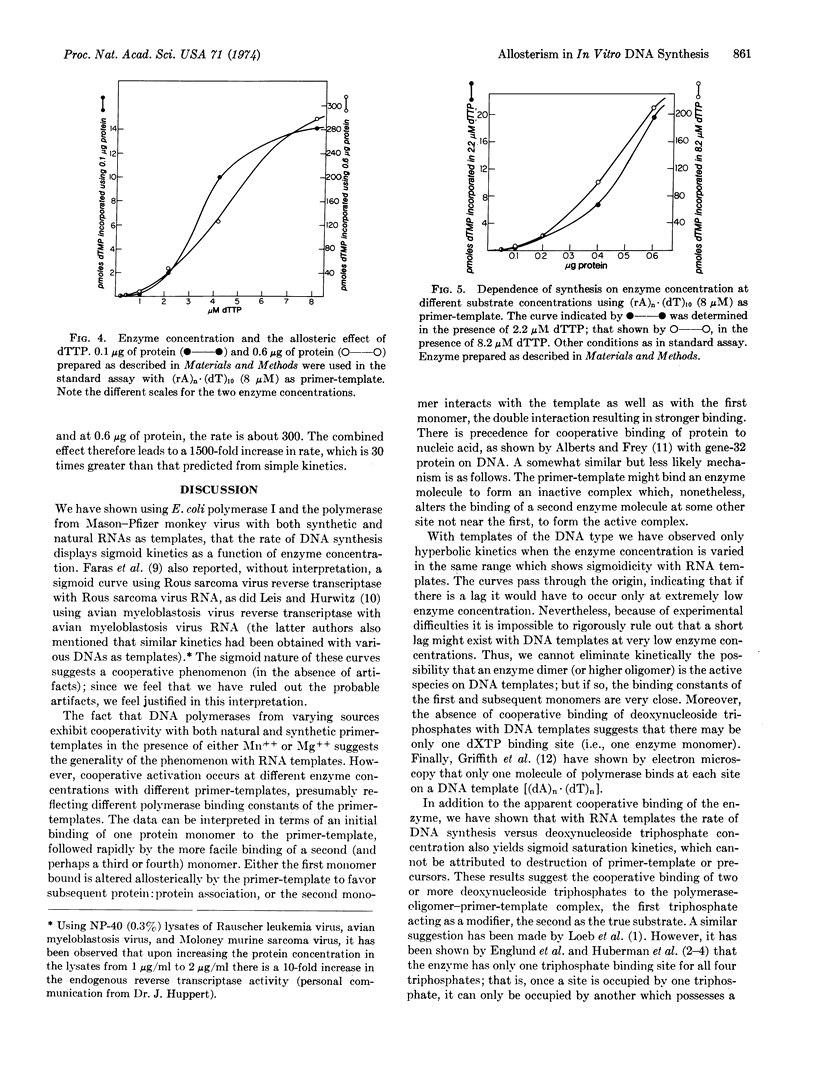
Abstract
Hemoglobin mRNA and (rA)n·(dT)10 have been used as primer-templates in a kinetic study of DNA synthesis with Escherichia coli DNA polymerase I (DNA nucleotidyl transferase, EC 2.7.7.7) and Mason-Pfizer monkey virus reverse transcriptase (RNA-directed DNA polymerase). The rate versus enzyme concentration curve is sigmoidal and is consistent with a cooperative phenomenon. The results could be interpreted in terms of the formation of an active complex containing enzyme dimers (or oligomers) on the primer-template. We have also observed sigmoidal kinetics in rate versus deoxynucleotide triphosphate concentration. These results are consistent with an allosteric mechanism in which the triphosphates act as both modifiers and DNA precursors. In the critical range, a 6- to 8-fold increase in both enzyme and triphosphate concentrations can lead to a 1500-fold increase in the rate of synthesis on an RNA template. Thus, small changes in enzyme and precursor concentrations could play a regulatory role in vivo.
Keywords: DNA polymerase, reverse transcriptase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Cavalieri L. F., Carroll E. DNA polymerase: evidence for multiple molecular species. Proc Natl Acad Sci U S A. 1968 Mar;59(3):951–958. doi: 10.1073/pnas.59.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri L. F., Deutsch J. F., Rosenberg B. H. The Molecular Weight and Aggregation of DNA. Biophys J. 1961 Mar;1(4):301–315. doi: 10.1016/s0006-3495(61)86890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- Englund P. T., Kelly R. B., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXI. Binding of deoxyribonucleic acid to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3045–3052. [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Griffith J., Huberman J. A., Kornberg A. Electron microscopy of DNA polymerase bound to DNA. J Mol Biol. 1971 Jan 28;55(2):209–214. doi: 10.1016/0022-2836(71)90192-6. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXV. A 3'-hydroxylribonucleotide binding site of Escherichia coli deoxyribonucleic acid polymerase. J Biol Chem. 1970 Oct 25;245(20):5326–5334. [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater J. P., Tamir I., Loeb L. A., Mildvan A. S. The mechanism of Escherichia coli deoxyribonucleic acid polymerase I. Magnetic resonance and kinetic studies of the role of metals. J Biol Chem. 1972 Nov 10;247(21):6784–6794. [PubMed] [Google Scholar]


