Abstract
Higher left ventricular (LV) mass, wall thickness and internal dimension are associated with increased heart failure (HF) risk. Whether different LV hypertrophy patterns vary with respect to rates and types of HF incidence is unclear. We classified 4768 Framingham Heart Study participants (mean age 50 years; 56% women) into 4 mutually exclusive LV hypertrophy pattern groups (normal, concentric remodeling, concentric hypertrophy, eccentric hypertrophy) using American Society of Echocardiography recommended thresholds of echocardiographic LV mass/body surface area and relative wall thickness, and related them to HF incidence. We evaluated if risk for HF types (HF with reduced [<45%; HFREF] versus preserved [≥45%; HFPEF] ejection fraction) varied by hypertrophy pattern. On follow-up (mean 21 years), 458 participants (9.6%; 250 women) developed new-onset HF. The age-and-sex-adjusted 20-year HF incidence rose from 6.96% in normal LV group to 8.67%, 13.38% and 15.27% in the concentric remodeling, concentric hypertrophy and eccentric hypertrophy groups, respectively. After adjustment for co-morbidities and incident myocardial infarction, LV hypertrophy patterns were associated with higher HF incidence relative to normal LV (p=0.0002); eccentric hypertrophy carried the greatest risk (hazards ratio [HR] 1.89, 95% confidence interval [CI] 1.41-2.54), followed by concentric hypertrophy (HR [CI] 1.40 [1.04-1.87]). Participants with eccentric hypertrophy had a higher propensity for HFREF (HR 2.23; CI 1.48-3.37, whereas those with concentric hypertrophy were more prone to HFPEF (HR 1.66; CI 1.09-2.51). In conclusion, in our large community-based sample, HF risk varied by LV hypertrophy pattern, with eccentric and concentric hypertrophy predisposing to HFREF and HFPEF, respectively.
Keywords: Concentric hypertrophy, eccentric hypertrophy, left ventricular hypertrophy, heart failure, risk
Introduction
We evaluated the long-term prognosis of left ventricular (LV) hypertrophy patterns with respect to heart failure (HF) risk. We hypothesized an increased risk of HF in people with LV hypertrophy patterns, i.e., concentric remodeling, concentric hypertrophy and eccentric hypertrophy, compared to people with a normal LV. Further, we postulated a rising gradient of HF risk across LV remodeling patterns that varies by type of HF, i.e., risk of HF with reduced ejection fraction (HFREF) will increase from concentric remodeling to eccentric hypertrophy (intermediate incidence rates in those with concentric hypertrophy), whereas risk of HF with preserved ejection fraction (HFPEF) will rise from concentric remodeling to concentric hypertrophy (intermediate incidence rates in those with eccentric hypertrophy).
Methods
Attendees of the 16th examination cycle of Framingham Heart Study Original cohort1 (1978-1980) and 2nd examination cycle of the Offspring cohort2 (1979-1982) were eligible for our investigation (N = 6214). After excluding participants with prevalent myocardial infarction (MI) or HF (N=319), those missing follow-up (N=14) and those with missing or unavailable echocardiographic data (N=1113), 4768 individuals remained eligible. All participants were white of European descent, provided written informed consent, and the study protocol was approved by the Institutional Review Board of Boston University Medical Center.
At the baseline examinations, study participants underwent two-dimensionally-guided M-mode echocardiography. LV end-diastolic dimension (LVEDD), and the end-diastolic thicknesses of the interventricular septum (IVS) and LV posterior wall (LVPW) were measured according to American Society of Echocardiography (ASE) recommendations.3 LV mass (LVM)4 and relative wall thickness (RWT) were calculated using the formulae:
We calculated fractional shortening (FS; [LVEDD–LV systolic dimension]/LVEDD), and a value of ≤0.29 (corresponding to an EF of 0.50) indicated decreased LV systolic function.5
We used ASE recommended thresholds for identifying normal and elevated LVM (indexed to body surface area; ≤115 gm/m2 versus >115 gm/m2 for men, ≤95 gm/m2 versus >95 gm/m2 for women) and RWT (≤0.42 versus >0.42) to classify participants into 4 mutually exclusive LV hypertrophy patterns: normal (both LVM and RWT normal), concentric remodeling (LVM normal but RWT elevated), eccentric hypertrophy (LVM elevated but RWT normal), and concentric hypertrophy (both LVM and RWT elevated).
Covariates were defined at the baseline examination. Body mass index was calculated as the weight in kilograms divided by the square of height in meters. During the Heart Study clinic visit, a physician measured blood pressure twice on the left arm of the seated participants using a mercury-column sphygmomanometer and a cuff of appropriate size; the average of these 2 readings indicated the examination blood pressure. Serum lipids were measured using standardized assays. Diabetes mellitus was defined as fasting plasma glucose of 126 mg/dl or greater, a random plasma glucose of 200 mg/dl or greater, or use of insulin or other hypoglycemic therapy. Cardiac valve disease was defined as presence of a systolic murmur of grade three or louder, or any diastolic murmur at the Heart Study examination.
An endpoints committee reviews Heart Study clinic charts, hospitalization and physician office records for all suspected cardiovascular events, including HF, and adjudicates incident events using pre-specified criteria.6 We used Framingham criteria7 (Supplementary Table 1) to determine HF occurrence. We defined HF as “HFREF” if EF (at the time of HF event) was <45%, or “HFPEF” if EF was ≥45%.8
We estimated the age-and sex-adjusted 10-year cumulative and 20-year cumulative HF incidence for each LV pattern. We used Cox regression to compare HF hazards in each LV group (normal group serving as referent), after confirming that the assumption of proportionality of hazards was met. We constructed a multivariable model adjusting for age, sex, body mass index, systolic blood pressure, hypertension treatment, diabetes, total cholesterol/HDL ratio, smoking, valve disease, reduced baseline FS (FS ≤ versus >0.29), and MI occurrence on follow-up; all variables were entered simultaneously into the Cox models. As values of covariates (such as blood pressure) and proportions of participants who receive therapy that modifies HF risk (such as anti-hypertensive therapy) change over time, we updated the covariate profile at each subsequent examination attended by each participant (i.e., all variables, except for age, sex and LV hypertrophy patterns, were entered as time-dependent covariates in the Cox regression models).
To control for potential confounding in the relations of hypertrophy patterns to HF risk, we performed the following secondary analyses. Because LV hypertrophy patterns may be associated with a low FS, we repeated analyses excluding individuals with a reduced FS at baseline examination. To eliminate potential confounding by prevalent valve disease, we repeated our analysis excluding participants with clinical valve disease. To evaluate the impact of gender and age on the relations of hypertrophy patterns to HF risk, we repeated the analyses including appropriate interaction terms (hypertrophy pattern*sex and hypertrophy pattern*age dichotomized at median).
To evaluate if a differential gradient of HF risk existed across the LV hypertrophy patterns and if this gradient varied by type of HF, we related LV hypertrophy patterns to HFREF and HFPEF in separate Cox regression analyses using the statistical model described above. All analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC), and a p-value <0.05 was considered statistically significant. All authors had full access to the data and take responsibility for the integrity of the data.
Results
The baseline clinical and echocardiographic characteristics of participants are presented in Tables 1 and 2. Participants excluded for unavailable echocardiographic data were older, had a higher mean systolic blood pressure, higher total/HDL cholesterol ratio and higher prevalence of diabetes and hypertension treatment, compared to the study sample as a whole. In the study sample, mean LV mass, LV dimensions and wall thicknesses were higher in men compared to women.
Table 1.
Baseline characteristics of study sample by left ventricular hypertrophy patterns.
| Variable | All participants (N = 4768) | Normal LV (N = 3384) | Concentric Remodeling (N = 686) | Concentric Hypertrophy (N = 373) | Eccentric Hypertrophy (N = 325) |
|---|---|---|---|---|---|
| Age (years) | 50±14 | 47±13 | 59±13 | 64±14 | 55±15 |
| Women | 56% | 57% | 50% | 57% | 58% |
| Body mass index(kg/m2) | 25.7±4.3 | 25.2±4.1 | 26.7±4.5 | 27.8±4.4 | 26.4±4.6 |
| Systolic Blood Pressure (mm Hg) | 126±19 | 122±17 | 134±19 | 142±21 | 134±21 |
| Total cholesterol (mg/dl) | 210±41 | 205±41 | 223±41 | 234±8.9 | 213±39 |
| High density lipoprotein cholesterol (mg/dl) | 50±14 | 50±14 | 48±14 | 47±15 | 50±14 |
| Diabetes mellitus | 3.9% | 2.5% | 6.0% | 11.5% | 5.7% |
| Lung disease | 11.9% | 5.4% | 7.1% | 8.0% | 10.2% |
| Kidney disease | 6.1% | 6.4% | 5.3% | 5.4% | 5.3% |
| Hypertension treatment | 15.6% | 10.4% | 24.6% | 39.7% | 23.7% |
| Valve Disease | 1.4% | 0.5% | 0.6% | 8.7% | 4.0% |
| Smoking | 33% | 34% | 30% | 28% | 34% |
| Interim myocardial infarction | 9.7% | 8.0% | 14.6% | 13.4% | 13.5% |
| Stroke | 7.8% | 6.2% | 11.8% | 14.3% | 8.7% |
Values are means ± standard deviation or %.
Interim myocardial infarction = myocardial infarction occurrence between baseline examination and diagnosis of heart failure or end of follow-up (whichever occurred earlier).
Table 2.
Echocardiographic characteristics of study sample according to left ventricular hypertrophy patterns.
| Men (N = 2098) | Women (N = 2670) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Normal LV | Concentric Remodeling | Concentric Hypertrophy | Eccentric Hypertrophy | Normal LV | Concentric Remodeling | Concentric Hypertrophy | Eccentric Hypertrophy |
| Left ventricular mass/body surface area (gm/m2) | 88±13 | 96±12 | 141±34 | 130±18 | 73±11 | 79±10 | 117±26 | 109±18 |
| Interventricular septum thickness (cm) | 0.92±0.08 | 1.10±0.10 | 1.31±0.18 | 1.06±0.08 | 0.81±0.08 | 0.97±0.09 | 1.23±0.22 | 0.94±0.07 |
| Left ventricular posterior wall thickness (cm) | 0.91±0.08 | 1.09±0.09 | 1.27±0.16 | 1.06±0.08 | 0.80±0.08 | 0.97±0.08 | 1.17±0.16 | 0.93±0.07 |
| Left ventricular wall thickness (cm) | 1.83±0.16 | 2.19±0.18 | 2.58±0.32 | 2.12±0.15 | 1.61±0.16 | 1.94±0.17 | 2.41±0.34 | 1.87±0.14 |
| Left ventricular end-diastolic dimension (cm) | 5.10±0.35 | 4.74±0.32 | 5.15±0.44 | 5.79±0.39 | 4.60±0.30 | 4.15±0.29 | 4.46±0.45 | 5.14±0.39 |
| Relative wall thickness | 0.36±0.04 | 0.46±0.05 | 0.51±0.09 | 0.37±0.04 | 0.35±0.04 | 0.47±0.05 | 0.55±0.11 | 0.37±0.04 |
| Fractional shortening | 0.36±0.04 | 0.38±0.04 | 0.37±0.04 | 0.35±0.05 | 0.38±0.04 | 0.41±0.04 | 0.40±0.05 | 0.37±0.05 |
| Fractional shortening ≤0.29 (%) | 2.0 | 0.9 | 4.3 | 7.3 | 0.4 | 0.3 | 2.4 | 3.2 |
Values are mean ± standard deviation or %.
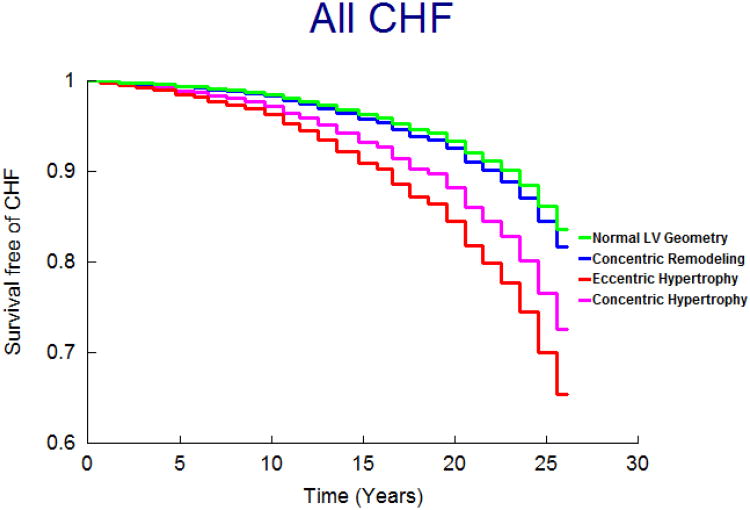
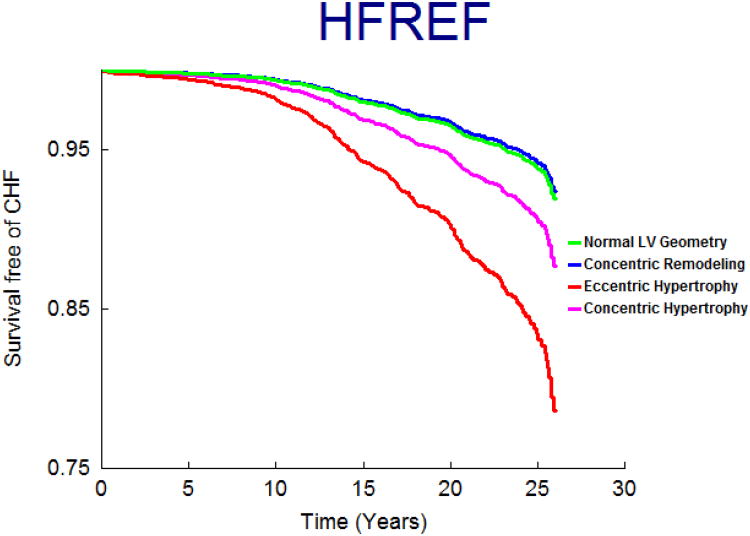
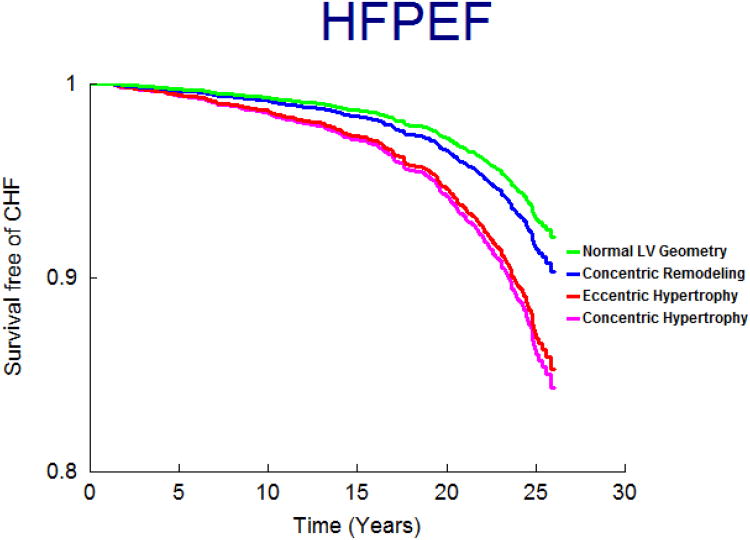
On follow-up (mean 21 years; maximum 28 years), 458 participants (9.6%; 250 women) developed new-onset HF. Figure 1 displays the age- and sex-adjusted survival free of HF in the 4 groups and demonstrates the highest incidence of HF in those with eccentric, followed by concentric hypertrophy, with participants with concentric remodeling having a risk intermediate between the normal group and these groups; Figures 2 and 3 display analogous data for the HFREF and HFPEF. Age-and-sex adjusted incidence was highest for those with eccentric hypertrophy (Table 3).
Figure 1.
Age- and sex-adjusted survival free of HF according to LV hypertrophy patterns.
Figure 2.
Age- and sex-adjusted survival free of HFREF according to LV hypertrophy patterns.
Figure 3.
Age- and sex-adjusted survival free of HFPEF according to LV hypertrophy patterns.
Table 3.
Age- and sex adjusted heart failure incidence by left ventricular hypertrophy patterns.
| Normal LV | Concentric Remodeling | Concentric Hypertrophy | Eccentric Hypertrophy | |
|---|---|---|---|---|
| No. of events/No. at risk | 216/3384 | 97/686 | 81/373 | 64/325 |
| Age- sex-adjusted 10-year incidence* | 1.58 (0.98-2.17) | 1.99 (1.08-2.86) | 3.91 (2.45-5.28) | 5.19 (3.00-7.20) |
| Age- sex-adjusted 20-year incidence* | 6.96 (5.45-8.36) | 8.67 (6.45-10.70) | 13.38 (9.90-16.43) | 15.27 (11.01-18.90) |
Heart failure incidence are age- and sex-adjusted per 100 persons; 95% confidence intervals in parenthesis.
In multivariable-adjusted Cox regression models, LV hypertrophy patterns were associated with increased HF incidence compared to the normal LV group (Table 4.A). Eccentric hypertrophy and concentric hypertrophy were associated with increased risk, whereas concentric remodeling was not independently associated with HF risk. Qualitatively similar results were obtained when analyses were repeated in the subgroup of participants with a normal FS (>0.29) (Table 4.B) and in analyses excluding participants with valve disease (Table 4.C). We did not observe any statistically significant sex interaction (p-value for interaction term = 0.53). The interaction term for age was statistically significant (p = 0.02), suggesting the relations of hypertrophy patterns to HF risk may vary by age. However, when our main multivariable model relating hypertrophy pattern to HF risk was repeated separately in participants above and below median age, the results were similar (data not shown). Of note, most of the covariates were also statistically significantly associated with incident HF, and the results are provided in Supplementary Table 2.
Table 4.
Multivariable analyses relating left ventricular hypertrophy patterns to incident heart failure.
| Adjusted Hazards Ratio* for HF (95% CI) | ||||
|---|---|---|---|---|
| Normal LV | Concentric Remodeling | Concentric Hypertrophy | Eccentric Hypertrophy | p-value† |
| A. All participants (n = 4768) | ||||
| Referent | 1.09 (0.85-1.40) | 1.40 (1.04-1.87) | 1.89 (1.41-2.54) | 0.0002 |
| B. Participants with normal FS (FS >0.29; n = 4700) | ||||
| Referent | 1.21 (0.94-1.57) | 1.52 (1.13-2.05) | 1.78 (1.31-2.41) | 0.0009 |
| C. Participants without clinical valve disease (n = 4678) | ||||
| Referent | 1.16 (0.90-1.50) | 1.38 (1.02-1.87) | 1.70 (1.25-2.32) | 0.005 |
Hazard ratios indicate HF risk associated with individual hypertrophy patterns compared to the group with normal LV (referent), and adjusted for the following covariates: age, sex, body mass index, systolic blood pressure, valve disease, hypertension treatment, diabetes, total cholesterol/high density lipoprotein cholesterol ratio, smoking status, fractional shortening and interim MI.
P-value indicates statistical significance (p<0.05) for the global test for differences among the 4 LV patterns with respect to HF risk.
EF at the time of HF diagnosis was available for 404 of the 458 participants (88%) who developed the incident HF event. HFREF was most common in the group with eccentric hypertrophy (Table 5.A), whereas HFPEF incidence was highest in those with concentric hypertrophy (Table 5.B).
Table 5.
Age- and sex-adjusted heart failure incidence by left ventricular hypertrophy patterns and heart failure type.
| A. HF with Reduced EF† | |||||
|---|---|---|---|---|---|
| Normal LV | Concentric Remodeling | Concentric Hypertrophy | Eccentric Hypertrophy | p-value* | |
| No. of events/No. at risk | 101/3384 | 36/686 | 28/373 | 33/325 | |
| Age- sex-adjusted 10-year incidence§ | 0.63 (0.25-1.01) | 0.62 (0.10-1.13) | 1.38 (0.41-2.31) | 2.70 (0.93-4.34) | |
| Hazards ratio (95% CI)¶ | Referent | 0.96 (0.64-1.44) | 1.32 (0.84-2.07) | 2.23 (1.48-3.37) | 0.02 |
| B. HF with Preserved EF‡ | |||||
| Normal LV | Concentric Remodeling | Eccentric Hypertrophy | Concentric Hypertrophy | p-value* | |
| No. of events/No. at risk | 93/3384 | 46/686 | 23/325 | 44/373 | |
| Age- sex-adjusted 10-year incidence rates§ | 0.77 (0.33-1.21) | 1.17 (0.43-1.87) | 1.57 (0.37-2.72) | 2.11 (1.03-3.14) | |
| Hazards ratio (95% CI)¶ | Referent | 1.25 (0.86-1.81) | 1.63 (1.02-2.61) | 1.66 (1.09-2.51) | 0.007 |
P-value indicates statistical significance (p<0.05) for trend test across the 4 LV patterns with respect to HF risk.
Participants who developed HF with preserved EF on follow-up were censored at the time of HF event.
Participants who developed HF with reduced EF on follow-up were censored at the time of the HF event.
Incidences are age and sex adjusted calculated per 100 persons.
Hazard Ratios are from multivariable models adjusting for age, sex, BMI, SBP, valve disease, HT treatment, diabetes, TC/HDL ratio, smoking status and fractional shortening, and indicate HF risk associated with individual hypertrophy pattern compared to the group with normal LV (referent).
CI=confidence interval.
In multivariable analyses, we observed an increasing gradient of risk for HFREF from concentric remodeling to concentric hypertrophy to eccentric hypertrophy (Table 5.A). Specifically, eccentric hypertrophy was associated with over 2-fold higher risk of HFREF relative to those with normal LV. In contrast, we observed an increasing gradient of risk for HFPEF from concentric remodeling to concentric hypertrophy (Table 5.B) with eccentric hypertrophy and concentric hypertrophy both associated with a statistically significant increase in HFPEF risk.
Discussion
In our community-based sample free of prevalent MI or HF, LV hypertrophy patterns were associated with increased HF risk. Individuals with eccentric hypertrophy experienced an approximately 90% higher HF risk whereas concentric hypertrophy was associated with a statistically significant 40% increased risk for HF, findings that remained robust in the subgroup with normal FS, and in those without clinical valve disease. Although HF rates were higher in participants with concentric remodeling, the association of this pattern with HF was attenuated in multivariable analyses, suggesting that a greater burden of risk factors (including interim MI) may have contributed to higher HF incidence in this group. Previous investigations from Framingham reported the strong effect of age on longitudinal change in measures of cardiac structure over the adult life course.9-11 However, the results of our main analysis were similar in participants above and below median age. In analyses of the subset of individuals with available echocardiographic EF after the onset of HF, participants with eccentric hypertrophy were more likely to develop HFREF and those with concentric hypertrophy were at higher risk for HFPEF, and there was an increasing gradient of risk by HF type across the 4 LV groups, consistent with our hypothesis.
Our investigation is strengthened by several design elements. We demonstrate prospectively the relationship between pattern of LV hypertrophy and HF risk and the relative preponderance of type of HF between concentric and eccentric hypertrophy, utilizing a large sample size from 2 cohorts uniformly followed over a long period. The echocardiographic data were derived from routine studies performed on a community-based sample, thereby removing selection bias; however, this may limit generalizability of our findings as similar patients in a primary care setting may not have undergone echocardiography due to lack of a clinical indication. The baseline examinations for our investigation were performed in 1978-1982, which permitted long-term follow-up and accrual of large number of HF cases to have adequate statistical power to analyze incident HF. In addition, information about co-morbid conditions and other cardiovascular outcomes were available at serial time-points thus enabling us to fit Cox regression models with time-dependent clinical covariates. We were thus able to identify the prognosis of LV hypertrophy patterns over the adult life course independent of changing co-morbidity profiles, and assess the relative contributions of LV remodeling versus associated burden of cardiovascular risk factors to the propensity for overall HF and types of HF.
Several prior reports related the individual components of LV hypertrophy, i.e., LVM, wall thickness and internal dimensions, to HF risk. We previously reported that higher LV internal dimensions were positively related to HF risk in people without a prior MI12. Data from the Cardiovascular Health Study also demonstrated that higher LV mass and wall thickness predict HF incidence.13,14 In the latter investigation, Gardin et al also reported increased risk for HF in participants with LV hypertrophy patterns but the sample was modest-sized and elderly, and the analyses were limited by very few HF events (n=23). Similarly, a recent investigation from the Multiethnic Study of Atherosclerosis reported associations between cardiac magnetic resonance imaging derived LV mass and concentricity index to incident CHD, stroke and HF.15 However, previous investigations were limited by low rates of HF events,14,16 were confined to specific subgroups (based on age,17 hypertension status,18 post-MI16 etc) and did not specifically address the their relation to HF risk, but rather focused on the relations of these patterns to overall cardiovascular outcomes and death.14,15,17-23
One interpretation of these findings is that elevated LVM on the basis of increased LV internal dimensions (the substrate for eccentric LV hypertrophy) is more strongly associated with HF risk relative to elevated LVM due to increased wall thickness (evident in concentric hypertrophy), or compared with greater wall thickness alone (with normal LVM, as in concentric remodeling). According to the Laplace law, the tension in the LV wall is directly proportional to transmural pressure and chamber radius and inversely proportional to wall thickness. A greater amount of tension must be developed in the wall of a dilated LV to generate the same amount of forward flow compared to a normal LV, requiring that wall thickness increase in proportion to the increased chamber diameter. Because LV wall thickness (an adaptive response to reduce wall stress) does not increase in proportion to LV dilation in eccentric hypertrophy (but does so in concentric hypertrophy),24 this hypertrophy pattern is likely associated with greater LV wall stress, which may contribute to a greater propensity for overt HF overall.
An alternative interpretation is that a dilated LV hypertrophy pattern confers higher HF risk due to associated changes in LV shape, i.e., increased sphericity. Evidence from experimental and clinical studies also is consistent with the premise that reduction of LV sphericity (the pattern noted in eccentric hypertrophy) ameliorates LV systolic function.25,26 Experimental evidence27 and observations in humans28 suggest that concentric hypertrophy is associated with abnormal diastolic function, and abnormalities of active relaxation, passive stiffness or both have been shown to be key correlates of HFPEF.29,30 These observations serve to explain the association between concentric hypertrophy and HFPEF. Thus both eccentric hypertrophy and concentric hypertrophy predict HF occurrence, but differ with respect to magnitude of risk, type of incident HF and pathophysiological mechanisms.
Our choice of baseline examinations limited us to the use of M-mode echocardiograms, the available imaging technology at that time. Our investigation is therefore limited by lack of adjustment for LV wall motion abnormalities, baseline LVEF and indices of baseline LV diastolic function. As valve disease was assessed based on physical examination, it is possible that participants with clinically important valve disease but without a significant murmur were misclassified in our investigation. Although we accounted for intervening MI, confounding of our results by occult coronary disease is possible. Our study sample was comprised of middle-aged white individuals and so our results may not be generalizable to other ethnicities or age groups.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the National Heart, Lung and Blood Institute's contract No. N01-HC-25195 and NIH grants RO1 HL67288.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 3.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 4.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, Levy D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, Wolf PA, Garrison RJ. Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements Section 34 The Framingham Heart Study: 30 Year Follow-Up. National Institute of Health; Bethesda, MD: 1987. NIH publication 87-2703. [Google Scholar]
- 7.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left atrial diameter over the adult life course: Clinical correlates in the community. Circulation. 2010;121:667–674. doi: 10.1161/CIRCULATIONAHA.109.885806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085–3092. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 13.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29(6):741–747. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 14.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 15.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carluccio E, Tommasi S, Bentivoglio M, Buccolieri M, Filippucci L, Prosciutti L, Corea L. Prognostic value of left ventricular hypertrophy and geometry in patients with a first, uncomplicated myocardial infarction. Int J Cardiol. 2000;74:177–183. doi: 10.1016/s0167-5273(00)00264-3. [DOI] [PubMed] [Google Scholar]
- 17.Lavie CJ, Milani RV, Ventura HO, Messerli FH. Left ventricular geometry and mortality in patients >70 years of age with normal ejection fraction. Am J Cardiol. 2006;98:1396–1399. doi: 10.1016/j.amjcard.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Gerdts E, Cramariuc D, de Simone G, Wachtell K, Dahlof B, Devereux RB. Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the LIFE study) Eur J Echocardiogr. 2008;9:809–815. doi: 10.1093/ejechocard/jen155. [DOI] [PubMed] [Google Scholar]
- 19.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 20.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 21.Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, Poisa P, Rizzoni D, Castellano M, Agabiti-Rosei E. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004;43:731–738. doi: 10.1161/01.HYP.0000121223.44837.de. [DOI] [PubMed] [Google Scholar]
- 22.Taylor HA, Penman AD, Han H, Dele-Michael A, Skelton TN, Fox ER, Benjamin EJ, Arnett DK, Mosley TH., Jr Left ventricular architecture and survival in African-Americans free of coronary heart disease (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2007;99:1413–1420. doi: 10.1016/j.amjcard.2006.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, Velazquez EJ, McMurray JJ, Kober L, Pfeffer MA, Califf RM, Solomon SD. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008;1:582–591. doi: 10.1016/j.jcmg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy PM, Takagaki M, Ochiai Y, Young JB, Tabata T, Shiota T, Qin JX, Thomas JD, Mortier TJ, Schroeder RF, Schweich CJ, Jr, Fukamachi K. Device-based change in left ventricular shape: a new concept for the treatment of dilated cardiomyopathy. J Thorac Cardiovasc Surg. 2001;122:482–490. doi: 10.1067/mtc.2001.115240. [DOI] [PubMed] [Google Scholar]
- 26.Tulner SA, Steendijk P, Klautz RJ, Bax JJ, Schalij MJ, van der Wall EE, Dion RA. Surgical ventricular restoration in patients with ischemic dilated cardiomyopathy: evaluation of systolic and diastolic ventricular function, wall stress, dyssynchrony, and mechanical efficiency by pressure-volume loops. J Thorac Cardiovasc Surg. 2006;132:610–620. doi: 10.1016/j.jtcvs.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Cantor EJ, Babick AP, Vasanji Z, Dhalla NS, Netticadan T. A comparative serial echocardiographic analysis of cardiac structure and function in rats subjected to pressure or volume overload. J Mol Cell Cardiol. 2005;38:777–786. doi: 10.1016/j.yjmcc.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Fox ER, Taylor J, Taylor H, Han H, Samdarshi T, Arnett D, Myerson M. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) Study: clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007;153:238–244. doi: 10.1016/j.ahj.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 30.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.