Abstract
BACKGROUND
To test the hypothesis that short-term BP variability and abnormal patterns of diurnal BP variation, evaluated by ambulatory blood pressure (ABP), predicts risk of incident cardiovascular disease (CVD) in patients with type 2 diabetes (T2DM).
METHODS
ABP monitoring was performed in 300 patients with uncomplicated T2DM without known CVD and without BP medications, who were followed for 54 ± 20 months. The relationships of different measures of BP variability, the presence of abnormal patterns of diurnal BP variation (non-dipper, riser, or morning BP surge) and the standard deviations (SD) of awake and asleep ABP, were determined. Cox proportional hazards models were used to estimate hazard ratios (HR) and their 95% CI, before and after controlling for various covariates.
RESULTS
The mean age was 67.8±9.6 years, 48% were male, 253 (84%) had a diagnosis of hypertension, and the mean of the SDs of awake SBP/DBP were 18±6/11±4 mmHg, and those of sleep SBP/DBP were 13±5/9±3 mmHg. During follow-up, there were 29 cardiovascular events. In multivariable analyses, the SDs of sleep SBP (HR=1.08; 95%CI, 1.01–1.16, p<0.05) and sleep DBP (HR=1.13; 1.04–1.23, p<0.01) were independently associated with incident CVD. Neither the non-dipper and riser patterns, nor the morning BP surge were associated with incident CVD events independently of clinic and 24-h BP levels
CONCLUSIONS
Abnormal diurnal BP variation was not a predictor of CVD in patients with T2DM. Nighttime BP variability was an independent predictor of future incidence of CVD, suggesting that this measure could reflect pathophysiology of T2DM.
Keywords: Blood pressure variability, type 2 diabetes, ambulatory blood pressure, cardiovascular disease
INTRODUCTION
There is accumulating evidence that ambulatory BP (ABP) predicts cardiovascular disease (CVD) better than clinic BP, however, the use of ABP monitoring (ABPM) is not recommended in the current guidelines of American Diabetes Association.1 In addition to BP levels,2 BP variability can also be evaluated by ABP. Variability of BP, assessed by the standard deviation (SD) of ABP readings, has been reported to be associated with an adverse cardiovascular prognosis in hypertensive patients.3–6 In these reports, there is conflicting evidence as to which types of BP variability measures best predict future cardiovascular events. BP variability during the daytime,3 nighttime,5, 6 and both daytime and nighttime4 have all been reported to be associated with a higher incidence of CVD or CVD mortality. However, it has also been reported that SDs of 24-hour, daytime, and nighttime BP were not associated with CVD when adjusted by 24-hour average BP.7 Another measure of BP variability is the diurnal change of ABP, evaluated either as the dipping pattern, such as non-dipping, reverse dipping (i.e. rising), or as the morning BP surge,8 which have also been found to predict future cardiovascular events in subjects with hypertension 7, 9, 10 and type 2 diabetes.11, 12
There have been no studies comparing the associations of these different measures of BP variability with incident CVD in patients with type 2 diabetes. We performed this study to investigate the prognostic significance of these different measures of BP variability in an exclusively diabetic population, and thereby examine the generalizability of previous reports to diabetic patients.
METHODS
This prospective study was performed in a sample of 300 asymptomatic type 2 diabetes patients who were seen in clinics at 9 participating institutions in Japan: 3 clinics, 2 hospitals and one outpatient clinic of a university hospital (the Jichi Medical School-JMS ABPM Study Wave 1); and 1 clinic and 2 hospitals in the Karatsu-Nishiarita Study 13–15. The subjects in this study are recruited from the same population who met the criteria of diabetes mellitus in our recent publication.2
Subjects and definitions
During the period of recruitment, 1990–1998 for the JMS ABPM Study Wave 1 sample and 1996–2002 for the Karatsu-Nishiarita Study, subjects were enrolled consecutively while being treated or evaluated for hypertension in the clinic. The present study is restricted to those who had type 2 diabetes. At least two clinic BP readings were taken on each of two separate occasions after at least 5 min of rest in the sitting position, which were taken both before and after being fitted with an ABPM in subjects who stopped medication for ABPM. Hypertension was diagnosed when the clinic systolic BP (SBP) was ≥140 and/or diastolic BP (DBP) was ≥90 mmHg on at least two occasions according to current guidelines,16 or by a previous diagnosis of hypertension with current antihypertensive medication use. Subjects took no antihypertensive medications for a minimum of 7 days before the ABPM and more than 95% took no medications during the 14 days preceding the ABPM study. Type 2 diabetes was diagnosed according to the guidelines of the American Diabetes Association17 or a previous diagnosis and currently taking anti-diabetic medication. We excluded patients with type 1 or secondary diabetes, renal dysfunction (serum creatinine >1.9 mg/dl), active hepatitis (type B, type C, alcoholic), or liver scirrosis, ischemic heart disease or other cardiac diseases, congestive heart failure, arrhythmias (including atrial fibrillation), stroke (including transient ischemic attacks), or other major concomitant non-cardiovascular diseases. Left ventricular hypertrophy was not excluded, and non-cardiac exclusion criteria were screened by routine clinical practice procedure, such as blood test, X ray, abdominal echo, or gastric fiberscope. No shift workers existed in this population. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Smoking was defined as current smoking status. This study was approved by the Institutional Review Board of each participating hospital or clinic. All subjects were ambulatory and gave informed consent for the study.
Ambulatory BP monitoring
Noninvasive ABPM was performed on a weekday with an automatic system (either ABPM-630 {Nippon Colin. Co}, TM2421, or TM2425 {A&D, Tokyo}) which recorded BP, using the oscillometric method and pulse rate every 30 minutes for 24 hours. These devices have been previously validated.18, 19 Awake and sleep time were defined based on patients’ written diaries recorded during ABPM. Mean awake and sleep levels of SBP and DBP were computed and the nocturnal BP fall (%) was calculated as (awake SBP–sleep SBP)/awake SBP. Nocturnal BP fall was classified as follows: dipper if the fall was ≥10%, non-dipper if it was ≥0% but <10%, and riser if it was <0%,9, 10, 13 and these three categories were used as measures of diurnal BP variation. Morning BP was defined as the mean BP during the two hours after waking, and the morning BP surge as the morning BP minus the mean BP during the 1 hour that included the lowest BP at night.8 Short term BP variability was estimated by the standard deviation (SD) of the daytime and nighttime SBP and DBP.
Follow-up and events
The subjects’ medical records were reviewed once a year after ABPM for the purpose of identifying incident CVD. The 99 participants from JMS ABPM Study Wave 1 were followed from 1996 to 1998 for up to 5.7 years or until they moved, changed their telephone number or died; the 201 participants from the Karatsu-Nishiarita Study were similarly followed from March 2004 to October 2006 for up to 9.7 years. Participants who developed a malignant disease and/or died from non-cardiovascular causes were censored as of the time such events took place. The median follow-up period (5th and 95th percentiles) was 54.6 months (21 and 91 months). When subjects did not visit the clinics, we interviewed them by telephone. We defined three outcomes: stroke, fatal or non-fatal myocardial infarction (MI), and sudden cardiac death. Strokes and cardiac events were diagnosed by the physician caring for the patient at the time of the event, and independent neurologists or cardiologists reviewed the cases and confirmed the diagnosis by referrals or medical records. Stroke was diagnosed on the basis of sudden onset of a neurological deficit that persisted for >24 hours in the absence of any other disease process that could explain the symptoms. Stroke events included ischemic stroke (cerebral infarction and cerebral embolism), hemorrhagic stroke (cerebral hemorrhage and subarachnoid hemorrhage), and undefined types of stroke. We excluded transient ischemic attacks, in which the neurological deficit cleared completely in <24 hours.10 MI was diagnosed based on the AHA criterion of “definite” MI.20 Angina and congestive heart failure were not treated as endpoints.
Statistical analyses
All statistical analyses were carried out with SPSS/Windows, version 13.0 (SPSS Inc., Chicago, Illinois). The data are expressed as the mean (± SD) or percentage. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were based on multivariable Cox regression analysis in which age (years), sex (male=1, female=0), BMI (kg/m2) and either clinic SBP/DBP or 24-hour mean ambulatory SBP/DBP were controlled a priori, while current smoking status (yes or no), antihypertensive medication (yes or no), total cholesterol, and serum creatinine (mg/dl) were treated as potential additional covariates and controlled only if they significantly predicted the outcome. The predictive utility of each BP variability measures (non-dipper pattern and riser pattern, yes or no, morning SBP/DBP surge, SDs of awake, sleep SBP/DBP) was analyzed, controlling for age, current smoking, and reference BP levels. We performed the Kolmogorov supremum test, as implemented in the PROC PHREG procedure of the SAS package for each of the Cox regression models reported in our manuscript. For every model the result of the Kolmogorov-type supremum test was not significant (all ps>0.20). Therefore, the “proportional hazards assumption” was not violated. The null hypothesis was rejected when two-tailed P<0.05.
RESULTS
The baseline characteristics are shown in Table 1. The mean age was 67.8 ± 9.6 years; there were 145 men and 155 women; 57% of subjects were taking antihypertensive medication.
Table 1.
Baseline characteristics of subjects
| Variables | mean ±SD or percentage |
|---|---|
| n (male: female) | 300 (145:155) |
| Age (years) | 67.8 ± 9.6 |
| Body mass index (kg/m2) | 24.1 ± 3.6 |
| Current smoking (%) | 34 |
| Duration of diabetes* (years) | 10.8 ± 7.8 |
| Hemoglobin A1c* (%) | 7.4 ± 1.1 |
| Albuminuria* (%) | 37 |
| Antihypertensive medication (%) | 57 |
| Hematocrit (%) | 40.6 ± 4.7 |
| Triglycerides (mmol/l) | 1.61 ± 1.03 |
| Serum creatinine (μmol/l) | 69.8 ± 21.2 |
| Cockcroft -Gault GFR (ml/min) | 75.0 ± 30.4 |
| Clinic SBP (mmHg) | 154 ± 22 |
| Clinic DBP (mmHg) | 84 ± 14 |
| 24-hour SBP (mmHg) | 140 ± 17 |
| 24-hour DBP (mmHg) | 79 ± 9 |
| Awake SBP (mmHg) | 145 ± 18 |
| Awake DBP (mmHg) | 82 ± 10 |
| Sleep SBP (mmHg) | 130 ± 19 |
| Sleep DBP (mmHg) | 73 ± 10 |
| 24-hour PR (beats/min) | 71 ± 9 |
| Awake PR (beats/min) | 75 ± 10 |
| Sleep PR (beats/min) | 63 ± 9 |
| SD of awake SBP (mmHg) | 18.0 ± 5.6 |
| SD of awake DBP (mmHg) | 11.3 ± 3.7 |
| SD of sleep SBP (mmHg) | 13.0 ± 4.8 |
| SD of sleep DBP (mmHg) | 8.6 ± 3.2 |
| White-coat hypertension (%) | 43 (14.3%) |
| Non-dipping pattern† (%) | 48.3 |
| Risers (%) | 8.7 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; PR, pulse rates.
Data were available in 201 patients.
Non-dipping includes true non-dippers and risers.
Data are shown as means ± SD or percentages.
During the follow-up of 53.8 ± 20.6 months, 29 cardiovascular events occurred. The incidence of CVD was 2.2/100 person-years. In univariable analyses, in addition to clinic and ABP levels, SD of awake DBP, and SDs of sleep SBP and DBP, and a riser pattern were all significantly associated with incident CVD, but morning BP surge was not (Figure 1). Regarding pulse rates, the HR (95%CI) of awake PR was 1.03 (0.99–1.07), P=0.108, and sleep PR, HR= 1.05 (1.01–1.09), P=0.024, but 24-hr PR, HR 1.03 (0.99–1.07), P=0.18.
Figure 1.
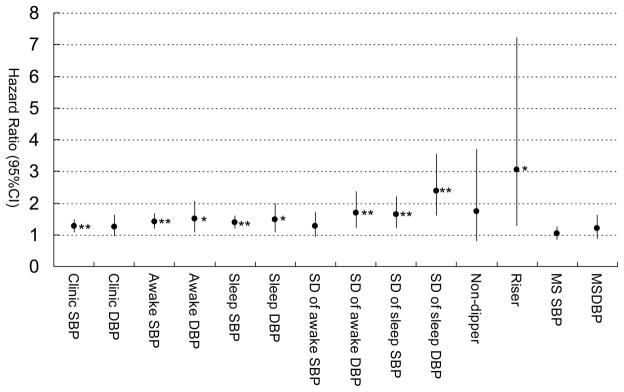
Univariable Cox regression analysis predicting cardiovascular events. Plots (bars) are shown as hazard ratio (95% CI) by 10-mmHg increase of each BP and 5-mmHg increase of each SD of BP values. MS indicates morning surge. *P<0.05, **P<0.01.
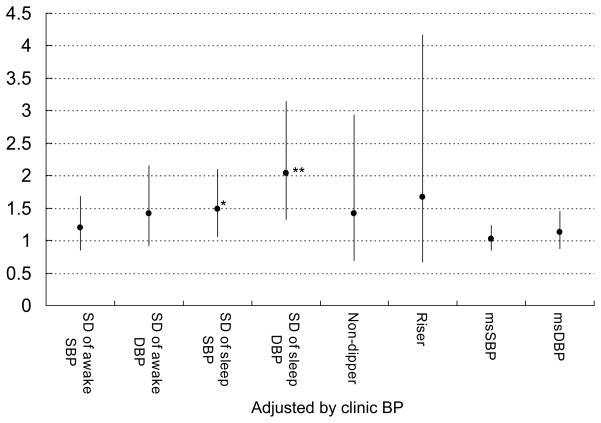
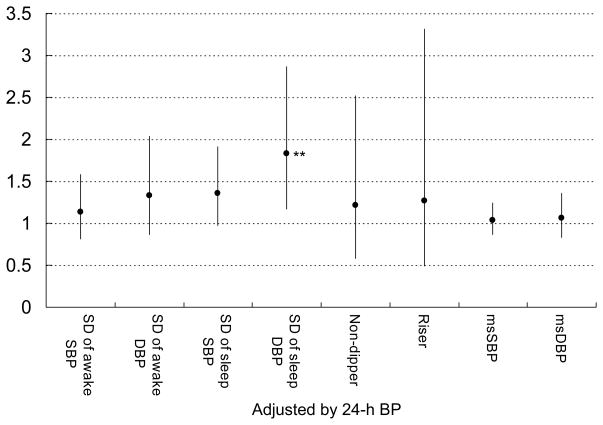
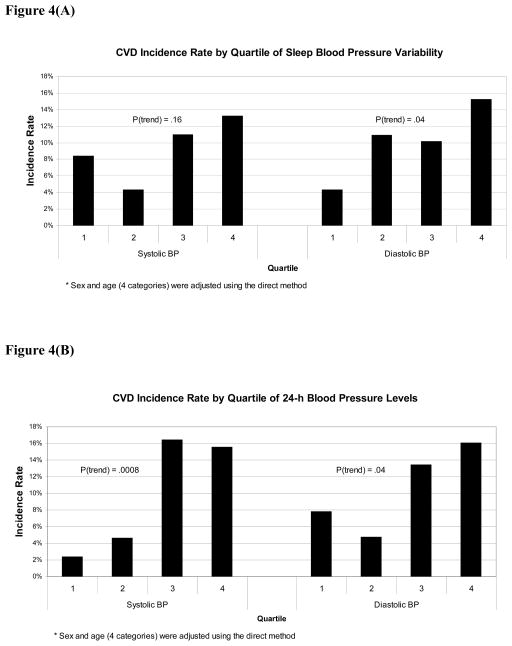
In our recent publication, the higher the ABP levels, the more CVD events occurred in diabetes.2 Based on the univariable analyses, we used clinic or ABP as a covariate for adjustment together with age, and current smoking, both of which were selected from a preliminary Cox regression analysis including essential and significant variables (age, sex, BMI, smoking, and serum creatinine). In multivariable Cox regression analyses that controlled for age, current smoking, and clinic SBP/DBP, neither the non-dipper or riser patterns, nor the morning BP surge were associated with CVD (Figure 2). The SDs of awake SBP/DBP were not associated with CVD, however, the SDs of sleep SBP/DBP were significantly associated with incident CVD (Figure 2). When the same analyses were repeated using 24-hour SBP/DBP in place of clinic SBP/DBP as a covariate (Figure 3), the association of the SDs of sleep SBP/DBP with CVD events continued to be significant, but none of the other measures of BP variability were significantly associated with incident CVD. The predictive value of sleep BP variability remained significant when the corresponding sleep BP levels were used instead of 24-hour SBP/DBP as a covariate. When the non-dipper/riser patterns were added in the same models based on our recent publication,2 the sleep BP variability also remained significant (data not shown). When we added sleep PR in multivariable models, the predictability of sleep SBP variability disappeared, but that of sleep DBP remained significant. There is a moderate, but not large, degree of collinearity (VIF<3 in all cases), our analyses showed that sleep BP variability has unique predictive utility while clinic/ambulatory BP level has predictive ability by itself, but not unique predictive ability if BP variability is also present. We have plotted the CVD incidence rate by quartile of blood pressure variability and by 24h BP level as shown in Figure 4. Consistent with our reported finding of the independent effect of diastolic BP variability on CVD incidence rate (Figure 3), there is a fairly clear dose-response pattern when comparing the two extreme quartiles against the middle 50% (the p-value for a linear dose-response pattern is .04).
Figure 2.
The predictive utility of each BP measures (non-dipper/riser pattern, yes or no, morning BP surge, and awake/sleep BP variability measures). Plots (bars) are shown as multivariable hazard ratio (95%CI) by 10-mmHg increase of each SBP/DBP and 5-mmHg increase of each SD of SBP/DBP values controlling for age, current smoking, and clinic SBP/DBP (each) as significant potential covariates. MS indicates morning surge. *P<0.05, **P<0.01.
Figure 3.
The predictive utility of each BP measures (non-dipper/riser pattern, yes or no, morning BP surge, and awake/sleep BP variability measures) adjusted by 24-h BP levels. Plots (bars) are shown as multivariable hazard ratio (95%CI) by 10-mmHg increase of each SBP/DBP and 5-mmHg increase of each SD of SBP/DBP values controlling for age, current smoking, and 24-hour SBP/DBP (each) as significant potential covariates. MS indicates morning surge. *P<0.05, **P<0.01.
Figure 4.
CVD incidence rates by quartiles of sleep BP variability (Figure 4A) and 24-h BP levels (Figure 4B). Sex and age (4 categories) were adjusted using the direct method.
DISCUSSION
In type 2 diabetes patients with or without hypertension, nighttime BP variability was a strong predictor for CVD events independent of other covariates, including 24-hour BP level and an abnormal circadian BP rhythm. This study is the first study showing the impact of BP variability on cardiovascular prognosis in type 2 diabetics. Although one abnormal diurnal BP variation, the riser pattern, was a significant predictor of incident CVD events,2 it was not independent of clinic or 24-hour SBP. Neither the non-dipper pattern nor the morning surge of BP was a predictor of incident CVD events.
Abnormal diurnal BP rhythm and morning BP surge in DM
In our study, neither an abnormal dipping pattern nor the morning BP surge was associated with incident CVD events independent of ABP level. The result with the dipping pattern was consistent with the finding of previous paper showing that ABP level was more important than the dipper/non-dipper patterns.2, 21 The hazard ratios for risers were about 1.5 to 2, but our data show that BP levels are more important than the diurnal pattern of BP variation in patients with type 2 diabetes.2 The lack of significance of the morning BP surge was not surprising because nearly 50 % of our diabetic patients showed a non-dipping pattern, and hence a diminished morning surge. Diabetic patients have some degree of neuropathy, which is usually diagnosed at the time of the diagnosis of diabetes. Our results suggest that an abnormal dipping pattern and the morning BP surge in diabetes have no additional impact on CVD over and above the average 24-hour BP. A study in diabetics has shown that morning BP evaluated at home was associated with micro- and macrovascular complications, but the study design was cross-sectional.22 For the management of BP in diabetics, the level of out-of-office BP such as ABP may be the most important measure,2 however, next to ABP levels, the home BP level may have additional impact on future cardiovascular events independent of clinic BP. This finding has important implications for the use of home BP monitoring, where the morning BP can easily be measured.
Nighttime BP variability in DM
We reported that ABP level were more important than clinic BP level, and confirmed the importance of abnormal circadian BP variation in predicting future CVD events in type 2 diabetes.2 In addition to, and independent of these, BP variability during sleep was shown to be a significant predictor of future CVD.
In our study, the SDs of sleep SBP and DBP were independent predictors for CVD. However, the SD of awake BP was not. This finding is consistent with a report of isolated systolic hypertension (ISH) showing that increased nighttime SBP variability was an independent risk factor for stroke.5 However, our findings were different from those of the other reports, in which either awake and sleep BP variability were both associated with cardiovascular outcomes 4, 6 or none of the SD measures were associated with CVD in a general population.7 The daytime BP variability in diabetics was reported to be higher than non-diabetics.23 The presence of advanced atherosclerosis 24 and impaired baroreflex sensitivity (BRS), which is associated with diabetic neuropathy,25 can lead to increased variability of BP in diabetics.26 The reason why only BP variability during sleep was associated with cardiovascular events is unclear. The significance of sleep SBP variability disappeared when adjusted by sleep PR, which indicates that increased SBP variability can be modulated by impairment of normal parasympathetic activity. In a previous report of diabetics, it was reported that parasympathetic nerve activity was relatively low during the night and resulting in nocturnal sympathetic predominance.27 Diminished vagal modulation of the heart is one of the earliest and most prominent features of autonomic neuropathy in diabetics,27 which is an independent predictor of CVD 28 and CVD mortality.29 The reason why our findings were in agreement with those in ISH 5 is not clear. However, the large fluctuations of awake BP in both diabetes and ISH could have obscured the predictability of awake BP variability because ISH in the elderly has sympathetic activation and impaired BRS,30 both of which are also seen in diabetics. The nighttime BP variability as a significant predictor for CVD could reflect early changes in the autonomic nervous system and could be an additional indicator of adverse cardiovascular events.
Limitations of the study
There are some limitations in this study. First, the small sample size is a major limitation of this study. The sample size for detecting predictability of the SDs of awake SBP/DBP and riser/non-dipper would be underpowered, which is a limitation to say that sleep BP variability is the only significant variable in predicting cardiovascular events. Because the study patients were recruited from the clinics of general internal medicine or community hospital, the number of diabetic patients for this ABPM study was limited. The small event number is also the limitation, however, the cardiovascular event rates 2.2/100 person-years were similar to the other outcome studies: major macrovascular events were 10.0% in the intense treatment group, and 10.6% in the control group in the ADVANCE trial 31; primary outcome (defined as either non-fatal MI, non-fatal stroke, or death from CV cause) was 2.11%per year in the intensive treatment group, and 2.29% per year in the standard therapy group in the ACCORD study 32. Second, the evaluation of BP variability was made by ABPM. Compared to the intra-arterial method, this method is probably less accurate; however, its non-invasive nature enabled us to study a larger number of patients than previous studies.4, 6 Withdrawal of medication for only 1–2 weeks before BP measurements does not exclude the possibility of a persistent, significant antihypertensive effect. Third, information on diabetic neuropathy, which could have influenced the BP variability, were not available in this study. Finally, the data on hemoglobin A1c and urinary albumin were limited to two thirds of the patients. Because BP variability may not be determined by recent glycemic control, the lack of hemoglobin A1c does not seem to be essential.
CONCLUSIONS
In our diabetic population, neither an abnormal dipping pattern nor the morning BP surge was a predictor of CVD events, whereas the nighttime BP variability appeared to be a strong predictor, independent of ABP level and other traditional risk factors. These results imply that in addition to ambulatory BP levels, evaluation of nighttime BP variability, which could reflect early change of diabetic autonomic neuropathy, is important for the prediction of cardiovascular events.
Acknowledgments
Supported in part by grants-in-aid (1998–1999, 2001–2002, 2004–2005) from the Foundation for the Development of the Community, Tochigi, Japan, and NHLBI (R24 HL76857 and P01 HL 47540).
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.American Diabetes Association. Hypertension management in adults with diabetes. Diabetes Care. 2004;27:65S–67S. doi: 10.2337/diacare.27.2007.s65. [DOI] [PubMed] [Google Scholar]
- 2.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443–450. doi: 10.1038/ajh.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickering TG, James GD. Ambulatory blood pressure and prognosis. J Hypertens. 1994;12 (suppl):S29–33. [PubMed] [Google Scholar]
- 4.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–906. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 5.Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH Syst-Eur investigators. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens. 2007;20:154–161. doi: 10.1016/j.amjhyper.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, Grassi G, Sega R. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265–1270. doi: 10.1161/HYPERTENSIONAHA.107.088708. [DOI] [PubMed] [Google Scholar]
- 8.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 9.Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, Kato J, Kikuchi N, Nishiyama A, Aihara A. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 10.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 11.Nakano S, Fukuda M, Hotta F, Ito T, Ishii T, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K. Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes. 1998;47:1501–1506. doi: 10.2337/diabetes.47.9.1501. [DOI] [PubMed] [Google Scholar]
- 12.Sturrock NDC, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med. 2000;17:360–364. doi: 10.1046/j.1464-5491.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 13.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients: advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 14.Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG. Silent and clinically overt stroke in older Japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol. 2001;38:238–245. doi: 10.1016/s0735-1097(01)01325-0. [DOI] [PubMed] [Google Scholar]
- 15.Eguchi K, Kario K, Shimada K. Greater impact of coexistence of hypertension and diabetes on silent cerebral infarcts. Stroke. 2003;34:2471–2474. doi: 10.1161/01.STR.0000089684.41902.CD. [DOI] [PubMed] [Google Scholar]
- 16.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 17.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 18.Imai Y, Sasaki S, Minami N, Munakata M, Hashimoto J, Sakuma H, Sakuma M, Watanabe N, Imai K, Sekino H, Abe K. The accuracy and performance of the A&D TM 2421, a new ambulatory blood pressure monitoring device based on the cuff-oscillometric method and the Korotkoff sound technique. Am J Hypertens. 1992;5:719–726. doi: 10.1093/ajh/5.10.719. [DOI] [PubMed] [Google Scholar]
- 19.White WB, Lund-Johansen P, McCabe EJ. Clinical evaluation of the Colin ABPM 630 at rest and during exercise: an ambulatory blood pressure monitor with gas-powered cuff inflation. J Hypertens. 1989;7:477–483. doi: 10.1097/00004872-198906000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 21.Nakano S, Ito T, Furuya K, Tsuda S, Konishi K, Nishizawa M, Nakagawa A, Kigoshi T, Uchida K. Ambulatory blood pressure level rather than dipper/nondipper status predicts vascular events in type 2 diabetic subjects. Hypertens Res. 2004;27:647–656. doi: 10.1291/hypres.27.647. [DOI] [PubMed] [Google Scholar]
- 22.Kamoi K, Miyakoshi M, Soda S, Kaneko S, Nakagawa O. Usefulness of home blood pressure measurement in the morning in type 2 diabetic patients. Diabetes Care. 2002;25:2218–2223. doi: 10.2337/diacare.25.12.2218. [DOI] [PubMed] [Google Scholar]
- 23.Chau NP, Bauduceau B, Chanudet X, Larroque P, Gautier D. Ambulatory blood pressure in diabetic subjects. Am J Hypertens. 1994;7:487–491. [PubMed] [Google Scholar]
- 24.Tamura K, Tsurumi Y, Sakai M, Tanaka Y, Okano Y, Yamauchi J, Ishigami T, Kihara M, Hirawa N, Toya Y, Yabana M, Tokita Y, Ohnishi T, Umemura S. A possible relationship of nocturnal blood pressure variability with coronary artery disease in diabetic nephropathy. Clin Exp Hypertens. 2007;29:31–42. doi: 10.1080/10641960601096760. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz J, Monbaron D, Parati G, Perret S, Haesler E, Danzeisen C, Hayoz D. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension. 2005;46:162–167. doi: 10.1161/01.HYP.0000169053.14440.7d. [DOI] [PubMed] [Google Scholar]
- 26.McKinlay S, Foster C, Clark A, Clark S, Kemp F, Denver E, Coats AJ. Increased blood pressure variability during 24h blood pressure monitoring as an early sign of autonomic dysfunction in non-insulin-dependent diabetics. J Hum Hypertens. 1994;8:887–890. [PubMed] [Google Scholar]
- 27.Vinik AI, Ziegler D. Diabetic Cardiovascular Autonomic Neuropathy. Circulation. 2007;115:387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 28.Valensi P, Sachs R-N, Harfouche B, Lormeau B, Paries J, Cosson E, Paycha F, Leutenegger M, Attali J-R. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care. 2001;24:339–343. doi: 10.2337/diacare.24.2.339. [DOI] [PubMed] [Google Scholar]
- 29.Toyry JP, Niskanen LK, Mantysaari MJ, Lansimies EA, Uusitupa MI. Occurrence, predictors, and clinical significance of autonomic neuropathy in NIDDM. Ten-year follow-up from the diagnosis. Diabetes Care. 1996;45:308–315. doi: 10.2337/diab.45.3.308. [DOI] [PubMed] [Google Scholar]
- 30.Grassi G, Seravalle G, Bertinieri G, Turri C, Dell’Oro R, Stella ML, Mancia G. Sympathetic and reflex alterations in systo-diastolic and systolic hypertension of the elderly. J Hypertens. 2000;18:587–593. doi: 10.1097/00004872-200018050-00012. [DOI] [PubMed] [Google Scholar]
- 31.The Advance Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 32.The Action to Control Cardiovascular Risk in Diabetes Study G. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]